WebElements Periodic Table » Americium (original) (raw)
Americium - 95Am: the essentials
Name: americium
Symbol: Am
Atomic number: 95
Relative atomic mass (_A_r): [ 243 ] (longest lived isotope)
Standard state: solid at 298 K
Appearance: silvery white
Classification: Metallic
Group name: Actinoid
Period in periodic table: 7 (actinoid)
Shell structure: 2.8.18.32.25.8.2
CAS Registry: 7440-35-9
Americium atoms have 95 electrons and the shell structure is 2.8.18.32.25.8.2. The ground state electronic configuration of neutral americium is [Rn].5f7.7s2 and the term symbol of americium is 8S7/2.
Americium: description
Your user agent does not support the HTML5 Audio element.
The lustre of freshly prepared americium metal is whiter and more silvery than plutonium or neptunium prepared in the same manner. Americium is a component of the smoke detector above.
Americium appears to be more malleable than uranium or neptunium and americium tarnishes slowly in dry air at room temperature. Americium is a radioactive rare earth metal which must be handled with care to avoid contact, since it is a heavy α and γ emitter. It is named after America. The α activity of 241Am is about three times that of radium. Americium is available to qualified users in the UK and in the USA.
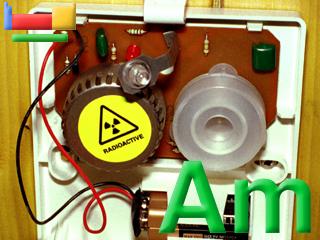
Americium is a key component of some smoke detectors.
Americium: binary compounds
Binary compounds with halogens (known as halides), oxygen (known as oxides), hydrogen (known as hydrides), and other compounds of americium where known.
Americium: compound properties
Bond strengths; lattice energies of americium halides, hydrides, oxides (where known); and reduction potentials where known.