Ethanol Freeze Protected Water Solutions (original) (raw)
Freezing temperature and flash points for ethanol based water solutions or brines.
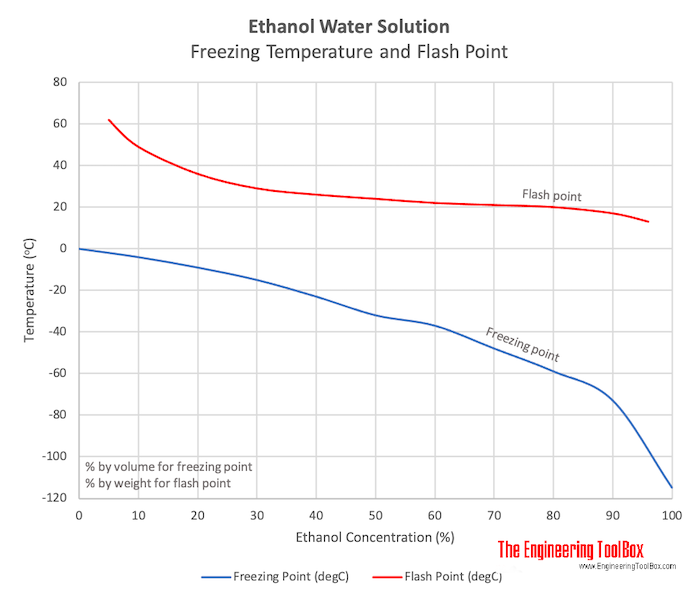
Ethanol based Water Solutions Freezing Point
Ethanol Freeze Protected Water Solutions - Freezing Points
| Ethanol Concentration (% by volume) | 0 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Freezing Point | (oF) | 32 | 25 | 15 | 5 | -10 | -25 | -35 | -55 | -75 | -110 | -175 |
| (oC) | 0 | -4 | -9 | -15 | -23 | -32 | -37 | -48 | -59 | -73 | -115 |
Flash Points of Ethanol based Water Solutions
The flash point of a chemical is the lowest temperature where it will evaporate enough fluid to form a combustible concentration of gas. The flash point is an indication of how easy a chemical may burn.
Ethanol Freeze Protected Water Solutions - Flash Points
| Ethanol Concentration (% by weight) | 5 | 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 96 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flash Point | (oF) | 144 | 120 | 97 | 84 | 79 | 75 | 72 | 70 | 68 | 63 | 55 |
| (oC) | 62 | 49 | 36 | 29 | 26 | 24 | 22 | 21 | 20 | 17 | 13 |
Warning
- HIGH flammability if pure
Download and print Ethanol Water Solution - Freezing Temperature and Flash Point chart.
Alternatives to Ethanol based Water Solutions
- Ethylene glycol based water solutions
- Propylene glycol based water solutions
- Methanol based water solutions
Example - Ethanol Concentration at Freezing Point at -20oC
By using linear interpolation between two known concentrations and their freezing points - the concentration at a third freezing point can be calculated as
CC = [(CB - CA) / (tB - tA)] (tC - tA) + CA (1)
where
C = concentration in ethanol - water solution
t = freezing point (oC, oF)
A, B = known freezing points
C = calculated freezing point
The ethanol concentration with freezing point at -20oC can be calculated by interpolating the concentration between freezing point -15oC and -23oC in the table above.
Cc = [((40 %) - (30 %)) / ((-23 oC) - (-15 oC))] ((-20 oC) - (-15 oC)) + (30 %) = 36.25 %
Note that this calculation is simplified by assuming that the concentration vs. freezing point follows a straight line. This not necessary correct.
If a 90% ethanol-water solution shall be mixed with clean water to achieve a freezing point of -20oC (ethanol concentration 36.25% (0.3625)) - the amount of added water can be calculated with volume balance - the amount of ethanol before mix is the same as after the mix:
C Vs = Cm (Vs + Vw) (2)
where
Vs = volume of the ethanol - water solution (liter, gallon)
Cm = concentration in mix
Vm = volume of mix (liter, gallon)
Vw = volume of the added clean water (liter, gallon)
Rearranging the equation to express the volume of water added to the mixture
Vw = (C - Cm) Vs / Cm (2b)
Substituting with values
Vw = (0.9 - 0.3625) Vs / 0.3625
= 1.48 Vs
- for every liter 90% ethanol-water solution 1.48 liter of clean water must be mixed in to achieve an ethanol concentration of 36.25 % and freezing point -20oC.
Related Topics
Properties of gases, fluids and solids. Densities, specific heats, viscosities and more.
Melting and freezing points of elements and chemical species at varying conditions.
Related Documents
Freezing point, density, specific heat and dynamic viscosity of Calcium Chloride Water coolants.
Online calculator, figures and tables showing dynamic and kinematic viscosity of ethanol, C2H5OH, at varying temperature and pressure - Imperial and SI Units.
Online calculator, figures and tables showing density and specific weight of ethanol at temperatures ranging from -25 to 325 °C (-10 to 620 °F) at atmospheric and higher pressure - Imperial and SI Units.
Online calculators, figures and tables showing specific heat , Cp and Cv, of gasous and liquid ethanol at temperatures ranging from -25 to 325 °C (-10 to 620 °F) at atmospheric and higher pressure - Imperial and SI Units.
Density of Ethyl Alcohol aqueous solutions.
Properties like freezing point, viscosity, specific gravity and specific heat of ethylene glycol based heat-transfer fluids, or brines.
Comparing antifreezes used in water based heat transfer fluids or brines.
Freezing mixtures, cooling agents and freezing points.
The flash point of a chemical indicates how easy it may ignite and burn.
Boiling temperatures (°C and °F) with varying carbon numbers up to C33.
Freezing and flash points of isopropanol (2-Propanol) based water solutions or brines.
Common fluids and their freezing and melting points.
Melting temperature (°C and °F) with carbon number up to C33.
Freezing and flash points for methanol or methyl based heat-transfer fluids or brines.
Freezing points of propylene glycol based heat-transfer fluids suitable for the food processing industry.
Freezing point, density, specific heat and dynamic viscosity of Sodium Chloride and Water coolant.
About the Engineering ToolBox!
Privacy Policy
We don't collect information from our users. More about
We use a third-party to provide monetization technologies for our site. You can review their privacy and cookie policy here.
You can change your privacy settings by clicking the following button: .
Citation
This page can be cited as
- The Engineering ToolBox (2005). Ethanol Freeze Protected Water Solutions. [online] Available at: https://www.engineeringtoolbox.com/ethanol-water-d\_989.html [Accessed Day Month Year].
Modify the access date according your visit.