Methanol - Dynamic and Kinematic Viscosity vs. Temperature and Pressure (original) (raw)
The viscosity of a fluid is a measure of its resistance to gradual deformation by shear stress or tensile stress .
For further definitions, go to Absolute (dynamic) and kinematic viscosity . Absolute or dynamic viscosity is used to calculate Reynold's Number to determine if a fluid flow is laminar, transient or turbulent.
Tabulated values of methanol viscosity and viscosity units conversion are given below the figures.
Online Methanol Viscosity Calculator
The calculator below can be used to estimate dynamic or kinematic viscosity of liquid methanol at given temperatures. The atmospheric boiling point of methanol is 64.15 °C (147.47 °F), and methanol must be pressurized to be present as liquid above that temperature.
The output dynamic viscosity is given as Pas, Ns/m2, cP, mPas, lbfs/ft2 and lbm/fth, while the kinematic viscosity is given as cSt, m2/s and ft2/s.
Temperature
Choose the actual unit of temperature:
°C °F K °R
See also other properties of Methanol at varying temperature and pressure: Density and specific weight and Specific Heat (Heat Capacity), and Thermophysical properties at standard conditions,
as well as dynamic and kinematic viscosity of air, ammonia, benzene, butane, carbon dioxide, ethane, ethanol, ethylene, methane, nitrogen, oxygen, propane and water.
Dynamic and kinematic viscosity of liquid methanol at varying temperature:

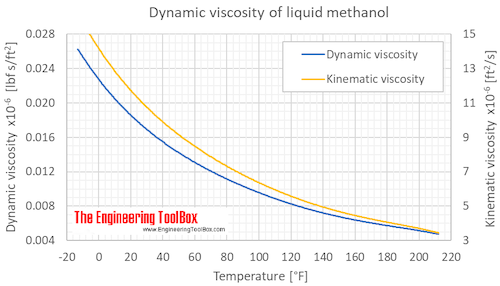
The atmospheric boiling point of methanol is 64.15 °C (147.47 °F), and methanol must therefore be pressurized to be present as liquid above that temperature.
Dynamic (absolute) and kinematic viscosity of liquid methanol at given temperatures and pressures, SI and Imperial units:
For full table with Dynamic and Kinematic Viscosity - rotate the screen!
Methanol - Dynamic and Kinematic Viscosity vs. Temperature and Pressure
| State | Temperature | Dynamic (Absolute) Viscosity | Kinematic viscosity | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (K) | (°C) | (°F) | (μPa s) | (cP), (mPa s) | (×10-6 lbf s/ft2) | (×10-6 lbm s/ft s) | (lbm/ft h) | (cSt), (×10-6 m2/s) | (×10-6 ft2/s) | |
| Liquid | 248.2 | -25 | -13 | 1258 | 1.258 | 0.02627 | 0.8453 | 0.003043 | 1.508 | 16.23 |
| 273.2 | 0.0 | 32 | 795.0 | 0.7950 | 0.01660 | 0.5342 | 0.001923 | 0.9815 | 10.56 | |
| 293.2 | 20 | 68 | 594.0 | 0.5940 | 0.01241 | 0.3991 | 0.001437 | 0.7500 | 8.073 | |
| 298.2 | 25 | 77 | 543.5 | 0.5435 | 0.01135 | 0.3652 | 0.001315 | 0.6906 | 7.434 | |
| 303.2 | 30 | 86 | 507.0 | 0.5070 | 0.01059 | 0.3407 | 0.001226 | 0.6488 | 6.983 | |
| 323.2 | 50 | 122 | 392.0 | 0.3920 | 0.00819 | 0.2634 | 0.000948 | 0.5142 | 5.535 | |
| 348.2 | 75* | 167* | 294.0 | 0.2940 | 0.00614 | 0.1976 | 0.000711 | 0.4000 | 4.306 | |
| 373.2 | 100* | 212* | 227.0 | 0.2270 | 0.00474 | 0.1525 | 0.000549 | 0.3206 | 3.451 |
* The atmospheric boiling point of methanol is 64.15 °C (147.47 °F), and methanol must therefore be pressurized to be present as liquid above that temperature.
Unit conversion:
Viscosity Dynamic, Absolute
centipoise (cP), gram/(centimeter second) (g/(cm s)) = poise (P), kilogram/meter second (kg/m s)=newton second/square meter (N s/m2)= pascal second (Pa s), pound/(foot hour) (lb/(ft h)), pound/(foot second) (lb/(ft s)), reyn (reyn)
- 1 cP = 0.001 Pa s = 0.01 P = 0.01 g/(cm sec) = 6.72197×10-4 lb/(ft s) = 2.4191 lb/(ft h)
- 1 lb/(ft h) = 0.00027778 lb/(ft s) = 0.00041338 Pa s = 0.0041338 P = 0.41338 cP
- 1 lb/(ft s) = 3600 lb/(ft h) = 1.48816 Pa s = 14.8816 P = 1488.16 cP
- 1 kg/(m s) = 1 (N s)/m2= 1 Pa s = 10 P = 1000 cP = 0.672197 lb/(ft s) = 2419.09 lb/(ft h)
- 1 (N s)/m2= 1 kg/(m s) = 1 Pa s = 10 P = 1000 cP = 0.672197 lb/(ft s) = 2419.09 lb/(ft h)
- 1 P = 1 g/(cm s) = 0.1 Pa s = 100 cP = 0.067197 lb/(ft s) = 241.909 lb/(ft h)
- 1 Pa s = 1 kg/(m s) = 1 (N s)/m2= 10 P = 1000 cP = 0.672197 lb/(ft s) = 2419.08 lb/(ft h) = 0.00014504 reyn
- 1 reyn = 6894.76 Pa s
Absolute or Dynamic Viscosity Unit Converter
Viscosity Kinematic
centistoke (cSt) = square millimeter/second (mm2/s), square foot/hour (ft2/h), square foot/second (ft2/s), square inch/second (in2/s), square meter/hour (m2/h), square meter/second (m2/s), stoke (St) = square centimeter/second (cm2/s)
- 1 cm2/s = 1 St = 100 mm2/s = 100 cSt = 1×10-4 m2/s = 0.36 m2/h = 1.07639×10-3 ft2/s = 3.875008 ft2/h = 0.1550003 in2/s
- 1 cSt = 1 mm2/s = 0.01 St = 1×10-6 m2/s = 0.0036 m2/h = 1.07639×10-5ft2/s = 0.03875008 ft2/h = 0.001550003 in2/s
- 1 ft2/h = 2.7778×10-4 ft2/s = 0.04 in2/s = 2.58064×10-5m2/s = 0.09290304 m2/h = 25.8064 cS = 0.258064 St
- 1 ft2/s = 3600 ft2/h = 144 in2/s = 0.09290304 m2/s = 334.451 m2/h = 92903.04 cSt = 929.0304 St
- 1 in2/s = 0.0069444 ft2/s = 25 ft2/h = 0.00064516 m2/s = 2.322576 m2/h = 645.16 cSt = 6.4516 St
- 1 m2/h = 1/3600 m2/s = 2.7778×10-4 m2/s = 2.7778 cm2/s = 277.78 mm2/s = 277.78 cSt = 2.7778 St = 0.00298998 ft2/s = 10.7639 ft2/h = 0.430556 in2/s
- 1 m2/s = 3600 m2/h = 1×104 cm2/s = 1×104 St = 1×106 mm2/s = 1×106 cSt = 10.7639 ft2/s = 38750.08 ft2/h = 1550003 in2/s
- 1 mm2/s = 1 cSt = 1×10-6 m2/s = 0.0036 m2/h = 0.01 cm2/s = 0.01 St = 1.07639×10-5ft2/s = 0.03875008 ft2/h = 0.001550003 in2/s
- 1 St = 1 cm2/s = 100 cSt = 100 mm2/s = 1×10-4 m2/s = 0.36 m2/h = 1.076×10-3 ft2/s = 3.875008 ft2/h = 0.1550003 in2/s
Kinematic Viscosity Unit Converter
Related Documents
Methanol - Thermophysical Properties
Chemical, physical and thermal properties of methanol, CH3OH (also called carbinol, wood alcohol, hydroxy methyl and methyl alcohol). Phase diagram included.