Pressure (original) (raw)
The pressure in a fluid is defined as
"the normal force per unit area exerted on a imaginary or real plane surface in a fluid or a gas"
The equation for pressure can be expressed as:
p = F / A (1)
where
p = pressure (lb/in2 (psi), lb/ft2 (psf), N/m2, kg/ms2 (Pa))
F = force (N)1)
A = area (in2, ft2, m2)
- In the Imperial - English Engineering System special care must be taken for the force unit. The basic unit for mass is slug and the unit for force is pound (lb) or pound force (lbf).
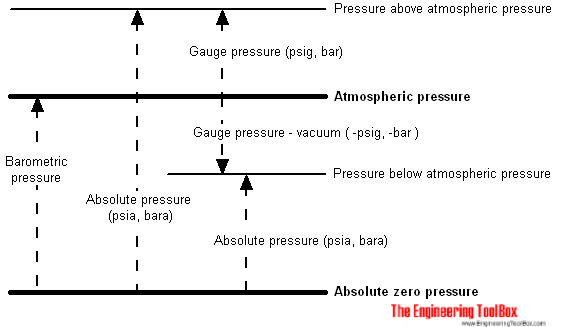
Absolute Pressure
The absolute pressure - pabs - is measured relative to the absolute zero pressure - the pressure that would occur at absolute vacuum. All calculations involving the gas law requires pressure (and temperature) to be in absolute units.
Gauge Pressure
A gauge is often used to measure the pressure difference between a system and the surrounding atmosphere. This pressure is often called the gauge pressure and can be expressed as
pg = ps - patm (2)
where
pg = gauge pressure (Pa, psi)
ps = system pressure (Pa, psi)
patm = atmospheric pressure (Pa, psi)
Atmospheric Pressure
Atmospheric pressure is the pressure in the surrounding air at - or "close" to - the surface of the earth. The atmospheric pressure varies with temperature and altitude above sea level.
Standard Atmospheric Pressure
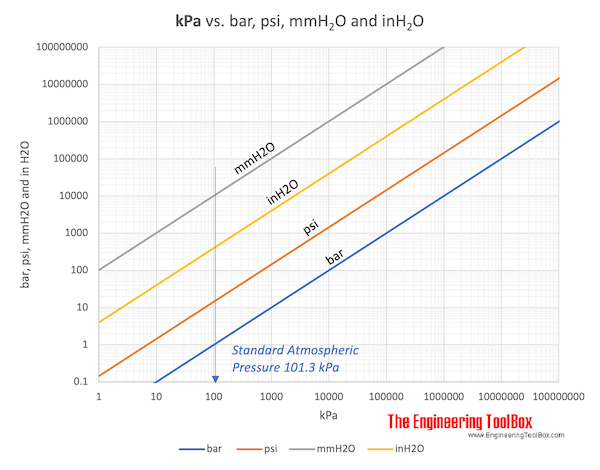
The Standard Atmospheric Pressure (atm) is normally used as the reference when listing gas densities and volumes. The Standard Atmospheric Pressure is defined at sea-level at 273 K (0 oC) and is 1.01325 bar or 101325 Pa (absolute). The temperature of 293 K (20 oC) is sometimes used.
In imperial units the Standard Atmospheric Pressure is 14.696 psi.
- 1 atm = 1.01325 bar = 101.3 kPa = 1.013×105 Pa = 14.696 psi (lbf/in2)= 760 mmHg =10.33 mH2O = 760 torr = 29.92 inHg = 1013 mbar = 1.0332 kgf/cm2 = 33.90 ftH2O
Pressure Units
Since 1 Pa is a small pressure unit the unit hectoPascal (hPa) is widely used, especially in meteorology. The unit kiloPascal (kPa) is commonly used in the design of technical applications - like HVAC systems, piping systems and similar.
- 1 hectoPascal = 100 Pascal = 1 millibar
- 1 kiloPascal = 1000 Pascal
Some Pressure Levels
- 10 Pa - the pressure below 1 mm of water
- 1 kPa - approximately the pressure exerted by a 10 g of mass on a 1 cm2 area
- 10 kPa - the pressure below 1 m of water, or the drop in air pressure when moving from sea level to 1000 m elevation
- 10 MPa - nozzle pressure in a "high pressure" washer
- 10 GPa - pressure enough to form diamonds
Some Alternative Units of Pressure
- 1 bar - 100,000 Pa
- 1 millibar - 100 Pa
- 1 atmosphere - 101325 Pa
- 1 mm Hg - 133 Pa
- 1 inch Hg - 3386 Pa
A torr (often used in vacuum applications) is named after Torricelli and is the pressure produced by a column of mercury 1 mm high - equals to 1 / 760th of an atmosphere.
- 1 atm = 760 torr = 14.696 psi = 1.013 bar
Pounds per square inch (psi) was commonly used in the U.K. but is now replaced in almost every country except in the U.S. by SI units. Since atmospheric pressure is 14.696 psi - a column of air on a area of one square inch area from the Earth's surface to the space - weights 14.696 pounds.
The bar (bar) is commonly used in the industry. One bar is 100,000 Pa , and for most practical purposes can be approximated to one atmosphere even if
1 bar = 0.9869 atm = 14.5 psi
There are 1000 millibar (mbar) in one bar , a unit common in meteorology and weather applications.
1 millibar = 0.001 bar = 0.750 torr = 100 Pa
Download kPa to bar, psi, mmH2O and inH2O chart
Related Mobile Apps from The Engineering ToolBox 
- free apps for offline use on mobile devices.
Related Documents
Acetone - Thermophysical Properties
Chemical, physical and thermal properties of acetone, also called 2-propanone, dimethyl ketone and pyroacetic acid. Phase diagram included.
International Standard Atmosphere
International standard atmosphere in elevation -2000 to 30000 metre - pressure, temperature, density, viscosity, thermal conductivity and velocity of sound.
Pressure Energy
Calculate the potential of pressure energy in a incompressible fluid.
SI System
An introduction to the SI metric system.
Unit Converter with commonly used Units
Common converting units for Acceleration, Area, Density, Energy, Energy per unit mass, Force, Heat flow rate, Heat flux, Heat generation per unit volume and many more.