Air and Steam Mixtures (original) (raw)
Air in the steam will lower the surface temperatures in heat exchangers - and less heat will be transferred.
Air in the steam will cause the temperatures on heat-exchangers surfaces to be lower than expected due to the saturation temperatures in the steam tables. The heat transfer will be reduced and the system efficiency will be decreased.
Air in steam can be described with the "Daltons Law of Partial Pressure".
Daltons Law of Partial Pressure
The total pressure of a mixture of gases is made up by the sum of the partial pressures of the components in the mixture as known from Dalton's Law of Partial Pressures:
The total pressure exerted by a mixture of gases is the sum of the partial pressures of the individual gases!
The total pressure in a mixture of steam and air can be expressed as:
p = pa + ps (1)
where
pa = partial pressure of air (Pa, bar, psi)
ps = partial pressure of steam (Pa, bar, psi)
The Partial Pressure in a Mixture
The partial pressure is the pressure exerted by each component as if it was occupying the same volume of the mixture. The effective partial pressure of the steam can be expressed as:
ps_effective = vs / V p (2)
where
ps_ effective = effective steam pressure, absolute (Pa, bar, psi)
vs = volume of steam (m3, in3)
V = volume of mixture (m3, in3)
p = absolute pressure (Pa, bar, psi)
Reducing the part of steam reduces the effective steam pressure. Increasing the part of steam (until 100%) increases the effective pressure.
Example - Mix of Air and Steam
The effective pressure in a steam/air mixture made up by 3 parts steam and 1 part air, with total pressure 5 bar absolute, can be expressed as:
p s_effective = (3 parts) / (3 parts + 1 part) (5 bar abs)
= 3.75 bar absolute
Important! Since the steam has an effective pressure of 3.75 bar instead of the pressure of 5 bar a, the mixture would have a temperature of approximately 139 oC rather than the expected saturation temperature of 152 oC. This has a major effect on the heat transfer capability of an heat exchanger.
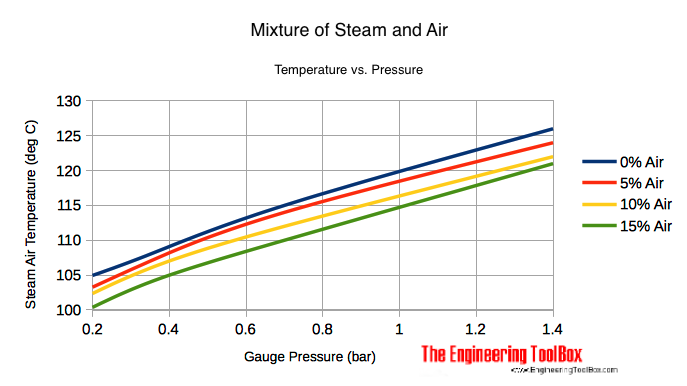
Mixing Air and Steam - Result Temperature
Mixing Air and Steam - Resulting Temperatures
| Mixture Pressure | 0% Air (Pure Steam) | 5% Air | 10% Air | 15% Air | |||||
|---|---|---|---|---|---|---|---|---|---|
| (psig) | (bar) | (oF) | (oC) | (oF) | (oC) | (oF) | (oC) | (oF) | (oC) |
| 2 | 0.15 | 219 | 104 | 216 | 102 | 213 | 101 | 210 | 99 |
| 5 | 0.35 | 227 | 108 | 225 | 107 | 222 | 106 | 219 | 104 |
| 10 | 0.7 | 239 | 115 | 237 | 114 | 233 | 112 | 230 | 110 |
| 20 | 1.4 | 259 | 126 | 256 | 124 | 252 | 122 | 249 | 121 |
Related Topics
Design of steam & condensate systems with properties, capacities, sizing of pipe lines, system configuration and more.
Calculate heat, work, temperature and energy. The thermodynamics of steam and condensate systems. Water and Ice properties.
Related Documents
Basic air changing state processes - heating, cooling, mixing, humidifying and dehumidifying by adding steam or water - psychometric diagrams and the Mollier charts.
Air can be humidified by adding water or steam.
Estimate the amount of steam required (lb/h in 100 cfm) in humid air.
Using steam to humidify air.
The moisture holding capacity of air increases with temperature.
Gibbs' Dalton's law of the total pressure of a mixture of gases.
Sprayed coils, spinning discs and steam humidifiers.
The change in state wwhen mixing moist air - enthalpy, heat, temperature and specific humidity.
Density of the mix of dry air and water vapor - moist humid air.
The pressure in a mixture of dry air and water vapor - humid or moist air - can be estimated by using Daltons Law of partial pressures.
Calculate steam heated air systems.
Calculating the amount of steam in non-flow batch and continuous flow heating processes.
An introduction to vapor and steam.
About the Engineering ToolBox!
Privacy Policy
We don't collect information from our users. More about
We use a third-party to provide monetization technologies for our site. You can review their privacy and cookie policy here.
You can change your privacy settings by clicking the following button: .
Citation
This page can be cited as
- The Engineering ToolBox (2003). Air and Steam Mixtures . [online] Available at: https://www.engineeringtoolbox.com/steam-air-mixture-d\_427.html [Accessed Day Month Year].
Modify the access date according your visit.