Comparative effects of microvascular and macrovascular disease on the risk of major outcomes in patients with type 2 diabetes (original) (raw)
- Original investigation
- Open access
- Published: 27 July 2017
- Mark Woodward1,4,5,
- Michel Marre2,3,6,
- Stephen Colagiuri7,
- Mark Cooper8,
- Stephen Harrap9,
- Giuseppe Mancia10,
- Neil Poulter11,
- Bryan Williams12,
- Sophia Zoungas1,13 &
- …
- John Chalmers1
Cardiovascular Diabetology volume 16, Article number: 95 (2017)Cite this article
- 7948 Accesses
- 68 Citations
- 10 Altmetric
- Metrics details
Abstract
Background
Microvascular disease is associated with a high risk of macrovascular events in patients with type 2 diabetes, but the impact of macrovascular disease on the risk of microvascular events remains unknown. We sought to evaluate the respective effects of prior microvascular and macrovascular disease on the risk of major outcomes, including microvascular events, in these patients.
Methods
Participants in the Action in Diabetes and Vascular Disease: PreterAx and DiamicroN Modified-Release Controlled Evaluation (ADVANCE) trial (n = 11,140) and the ADVANCE-ON post-trial study (n = 8494) were categorized into 4 groups at baseline: dual absence of microvascular or macrovascular disease (n = 6789), presence of microvascular disease alone (n = 761), macrovascular disease alone (n = 3196), and both (n = 394). Outcomes were all-cause mortality, major macrovascular events (MACE), and major clinical microvascular events.
Results
All-cause mortality, MACE, and major clinical microvascular events occurred in 2265 (20%), 2166 (19%), and 807 (7%) participants respectively, during a median follow-up of 9.9 (inter-quartile interval 5.6–10.9) years. The adjusted hazard ratios [95% CI] of death, MACE, and major clinical microvascular events were each greater in patients with baseline microvascular disease (1.43 [1.20–1.71], 1.64 [1.37–1.97], and 4.74 [3.86–5.82], respectively), macrovascular disease (1.43 [1.30–1.57], 2.04 [1.86–2.25], and 1.26 [1.06–1.51]) or both (2.01 [1.65–2.45], 2.92 [2.40–3.55], and 6.30 [4.93–8.06]) compared with those without these conditions. No interaction was observed between baseline microvascular and macrovascular disease for these events. The addition of microvascular disease (change in c-statistic [95% CI] 0.005 [0.002–0.008], p = 0.02) or macrovascular disease (0.005 [0.002–0.007], p < 0.0001) considered separately or together (0.011 [0.007–0.014], p < 0.0001) improved the discrimination and the classification (integrated discrimination improvement (IDI): 0.013 [0.010–0.016], p < 0.001; net reclassification improvement (NRI): 0.021 [0.011–0.032], p < 0.001) of the risk of all-cause mortality. Microvascular disease improved discrimination (0.009 [0.003–0.014]) and classification (IDI: 0.008 [0.006–0.010]; NRI: 0.011 [0.001–0.020]) of MACE. Baseline macrovascular disease modestly enhanced IDI (0.002 [0.001–0.002]) and NRI (0.041 [0.002–0.087]), but not discrimination, of major clinical microvascular events.
Conclusions
Microvascular and macrovascular disease are independently associated with the 10-year risk of death, MACE, and major clinical microvascular events in patients with type 2 diabetes. The coexistence of these conditions was associated with the highest risks.
Background
Type 2 diabetes is a leading cause of microvascular complications and confers an excess risk of cardiovascular disease and death [1, 2]. Microvascular and macrovascular complications often occur concomitantly, and share similar risk factors and pathological pathways [3,4,5]. The presence of microvascular disease increases the risk of cardiovascular morbidity and mortality in people with type 2 diabetes, independent of the major established cardiovascular risk factors [6]. However, the impact of macrovascular disease on the risk of microvascular events has not been fully investigated. We sought to evaluate the impact of microvascular and macrovascular disease, considered individually, and together, on the risk of death and major macrovascular and microvascular events in patients with type 2 diabetes participating in the Action in Diabetes and Vascular Disease: PreterAx and DiamicroN Modified-Release Controlled Evaluation (ADVANCE) trial (ClinicalTrials.gov Number, NCT00145925) and ADVANCE-ON post-trial study (ClinicalTrials.gov Number, NCT00949286).
Materials and methods
Participants
ADVANCE was a multi-national randomized trial testing the effect of intensive glucose control (using a gliclazide-MR) and routine blood pressure treatment (using a fixed-dose combination of perindopril and indapamide) on the risk of major microvascular and macrovascular events in 11,140 patients with type 2 diabetes and at least one other cardiovascular risk factor or pre-existing cardiovascular disease [7,8,9]. Subsequently, 8494 of the surviving participants were enrolled in the post-trial observational study (ADVANCE-ON). The design and characteristics of participants have been previously described [8,9,10]. The Institutional Ethics Committee of each participating centre approved the ADVANCE and ADVANCE-ON protocols, and all participants provided written informed consent.
Definition of microvascular and macrovascular disease at baseline
At baseline, microvascular disease was defined as the presence of macroalbuminuria (urinary albumin to creatinine ratio (ACR) >300 mg/g), requirement of retinal photocoagulation therapy, proliferative retinopathy, macular oedema, or diabetes-related blindness. Macrovascular disease was defined as the presence, at baseline, of myocardial infarction, stroke, coronary artery bypass graft, percutaneous transluminal coronary angioplasty, hospital admission for unstable angina or transient ischaemic attack, lower-extremity amputation of at least one digit secondary to arterial insufficiency, or a peripheral revascularisation procedure. Participants were categorized into four baseline groups: absence of both microvascular and macrovascular disease, presence of microvascular disease alone, presence of macrovascular disease alone, and presence of both microvascular and macrovascular disease.
Definition of outcomes
The primary outcomes were all-cause mortality, major macrovascular events (MACE: a composite of nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death), and major clinical microvascular events (a composite of end-stage renal disease (ESRD), defined as requirement for renal-replacement therapy; death induced by renal disease; requirement for retinal photocoagulation; or diabetes-related blindness in either eye). The secondary outcomes were cardiovascular death, fatal or nonfatal myocardial infarction, fatal or nonfatal stroke, ESRD or renal death, and requirement for retinal photocoagulation or blindness. Outcomes were adjudicated by an independent End Point Adjudication Committee in the ADVANCE trial, through to the end of randomized allocation, and were reported by investigators without adjudication in the ADVANCE-ON study, in accordance with its pre-specified protocol [10].
Statistical analyses
Continuous variables were summarized as mean (SD) or, for those with a skewed distribution, median (interquartile range). Categorical variables were summarized as the number of patients with corresponding percentage. Characteristics of participants according to status of microvascular and macrovascular disease at baseline were compared using Chi squared, ANOVA, or Kruskal–Wallis tests.
Cumulative incidence curves were used to plot survival (outcome-free) rates during follow-up, and compared using the log-rank test. Cox proportional hazards regression models were fitted to estimate hazard ratios (HRs), with associated 95% confidence intervals (CI), for outcomes by joint status of microvascular and macrovascular disease at baseline. Analyses were adjusted for randomized study allocations plus every potential confounding variable that was significantly different between microvascular and macrovascular disease at baseline: sex, age, region of origin (Asia: Philippines, China, Malaysia, and India; established market economies: Australia, Canada, France, Germany, Ireland, Italy, Netherlands, New Zealand, United Kingdom; and Eastern Europe: Czech Republic, Estonia, Hungary, Lithuania, Poland, Russia, Slovakia), body mass index, duration of diabetes, HbA1c, systolic blood pressure, antihypertensive treatment, estimated glomerular filtration rate (eGFR; computed by the Chronic Kidney Disease–Epidemiology Collaboration equation [11]) and its square, urinary ACR, LDL- and HDL-cholesterol, and history of ever smoking (basic model). The proportional hazards assumption was checked using the Schoenfeld residuals method.
We tested multiplicative interaction between baseline history of microvascular disease and macrovascular disease on the risk of each outcome by including these two individual variables and their product within Cox models.
Harrell’s c-statistic [12], net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were used to compare discrimination and classification of primary outcomes, assessed using survival methodology, between two prognostic models: basic model and basic model plus baseline history of microvascular or macrovascular disease (as appropriate). Since we assessed the additive value of microvascular disease (including macroalbuminuria) on the risk of all-cause mortality and MACE, urinary ACR was not included in the basic model in these analyses. We also evaluated the individual and joint prognostic value of urinary ACR (as a continuous variable) and diabetic retinopathy, added to the basic model, on the discrimination of all-cause mortality and MACE.
Sensitivity analyses were conducted after including further components of microvascular disease at baseline: (i) chronic kidney disease (CKD defined as eGFR <60 ml/min/1.73 m2) or (ii) diabetic peripheral neuropathy (defined as a disturbance of 10-g monofilament sensation or absence of ankle reflex in both feet). We also evaluated the associations of microvascular and macrovascular disease at baseline on the risk of MACE and major clinical microvascular events after treating non-renal and non-cardiovascular death as a competing risk using the Fine and Gray method [13].
Statistical analyses were performed using SAS software, version 9.3 (SAS Institute, www.sas.com), and Stata software version 13 (StataCorp, www.stata.com). A P value less than 0.05 was considered significant.
Results
Clinical characteristics at baseline
Among 11,140 participants enrolled in ADVANCE trial, 761 (6.8%) had microvascular disease alone, 3196 (28.7%) had macrovascular disease alone, and 394 (3. 5%) had both at baseline (Additional file 1: Table S1). Patients with microvascular disease alone at baseline had a longer duration of diabetes and higher HbA1c, systolic blood pressure, and ACR levels, whereas those with macrovascular disease alone were more frequently men, from established market economies, ever smokers, and treated with antihypertensive, lipid lowering and antiplatelet drugs (Table 1).
Table 1 Clinical characteristics according to microvascular or macrovascular disease at baseline
Incidence of major outcomes during follow-up according to status of microvascular and macrovascular disease at baseline
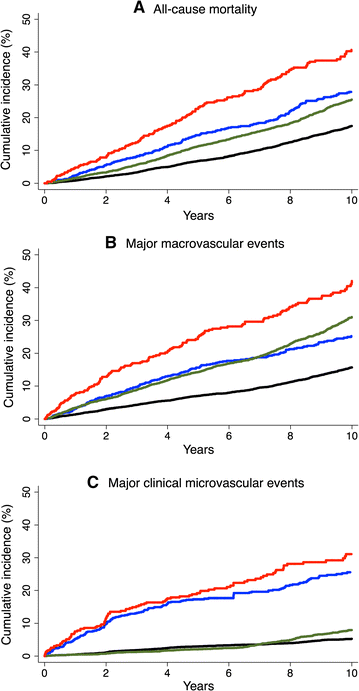
All-cause mortality, MACE, and major clinical microvascular events occurred in 2265 (20.3%), 2166 (19.4%), and 807 (7.2%) participants, respectively, during a median overall follow-up of 9.9 (inter-quartile interval 5.6–10.9) years. Their incidence rates were 2.4, 2.4, and 0.9 per 100 person-years, respectively. The risks of these respective outcomes were higher in patients with baseline history of only microvascular (HRs [95% CI] 1.43 [1.20–1.71], p < 0.0001; 1.64 [1.37–1.97], p < 0.0001; and 4.74 [3.86–5.82], < 0.0001) and of only macrovascular disease (1.43 [1.30–1.57], p < 0.0001; 2.04 [1.86–2.25], p < 0.0001; and 1.26 [1.06–1.51], p = 0.01), compared with patients without either of these conditions (Fig. 1; Table 2). The highest risks were observed in patients with both conditions at baseline (2.01 [1.65–2.45], p < 0.0001; 2.92 [2.40–3.55], p < 0.0001; and 6.30 [4.93–8.06], p < 0.0001). There was no evidence of interaction between microvascular and macrovascular disease at baseline on the risks of all-cause mortality (p = 0.89), MACE (p = 0.25), or major clinical microvascular events (p = 0.75).
Fig. 1
Cumulative incidence of outcomes during follow-up according to status of microvascular and macrovascular disease at baseline. a All-cause mortality. b Major macrovascular events. c Major clinical microvascular disease (p < 0.0001 for all). Black line absence of both macrovascular and microvascular disease. Blue line presence of microvascular disease alone. Green line presence of macrovascular disease alone. Red line presence of both microvascular and macrovascular disease
Table 2 Distibution of patients, event rate (per 100 person years) and hazard ratios (HRs) for outcomes during follow-up, according to the history of microvascular or macrovascular disease at baseline
Comparable results were observed with secondary endpoints, except for the absence of association of baseline macrovascular disease with the risk of ESRD or renal death (Table 2).
Additive values of microvascular or macrovascular disease at baseline in discrimination and classification of outcomes during follow-up
The addition of microvascular disease (change in c-statistic [95% CI] 0.005 [0.002–0.008], p = 0.02) or macrovascular disease (0.005 [0.002–0.007], p < 0.0001) considered separately or together (0.011 [0.007–0.014], p < 0.0001) improved the discrimination, as well as the classification (IDI: 0.013 [0.010–0.016], p < 0.001; NRI: 0.021 [0.011–0.032], p < 0.001) of the risk of all-cause mortality. The addition of ACR (0.010 [0.006–0.014], p < 0.0001) displayed a higher improvement of death discrimination than diabetic retinopathy (0.002 [0.001–0.004], p = 0.01).
The addition of baseline microvascular disease to established cardiovascular risk factors improved the discrimination (0.009 [0.003–0.014], p = 0.002) and classification (IDI: 0.008 [0.006–0.010], p < 0.001; NRI: 0.011 [0.001–0.020], p = 0.02) of MACE. The improvement in discrimination of MACE was similar after addition of either urinary ACR (0.008 [0.003–0.013], p = 0.002) or diabetic retinopathy (0.007 [0.002–0.011], p = 0.005). Adding both urinary ACR and diabetic retinopathy together yielded the highest increase in discrimination of MACE (0.014 [0.007–0.021], p < 0.0001).
Baseline macrovascular disease modestly enhanced IDI (0.002 [0.001–0.002]), p < 0.001 and NRI (0.041 [0.002–0.087], p < 0.0001), but not discrimination (c-statistic), of major clinical microvascular events (Table 3).
Table 3 Harrell’s c-statistics, NRI, IDI for risk of major outcomes according to traditional risk factors without and with history of microvascular or macrovascular disease at baseline
Sensitivity analyses
Comparable associations were observed between baseline microvascular disease, including CKD or peripheral diabetic neuropathy, and the risk of outcomes, except for myocardial infarction or stroke (Additional file 1: Tables S2, S3). Baseline microvascular or macrovascular disease remained significantly associated with MACE and major clinical microvascular events when we corrected for competing risk of non-renal and non-cardiovascular death (Additional file 1: Table S4).
Discussion
This study demonstrates the independent association of microvascular or macrovascular disease at baseline with excess risks of all-cause mortality, MACE, and major clinical microvascular events in patients with type 2 diabetes followed for a median duration of 9.9 years. The presence of both conditions led to the highest risks. Baseline microvascular disease enhanced discrimination and classification of MACE, while baseline macrovascular disease modestly improved classification, but not discrimination, of major clinical microvascular events. Overall, the improvement in discrimination and classification of outcomes was quiet modest, but statistically significant. Interestingly, urinary ACR and diabetic retinopathy yielded together the highest improvement of MACE supporting their additive value in the prediction of macrovascular disease. Of note, both ACR and retinopathy were independent components of the 10-year prognostic vascular risk score recently reported by our group in the ADVANCE-ON stud [14].
Microvascular disease and risk of MACE
Our findings support previous studies showing that microvascular disease is an important predictor of future macrovascular disease and death [6, 15]. ADVANCE participants with microvascular disease alone at baseline displayed similar hazard ratios for all-cause mortality or MACE, compared to those with macrovascular disease alone, suggesting that microvascular impairment plays an important role in the development of diabetic angiopathy. A recent population-based cohort study has shown that retinal and skin microvascular abnormalities occurred early in prediabetes, were more severe in type 2 diabetes, and may contribute to the development of cardiovascular disease [16]. Other studies reported a high prevalence of coronary microvascular dysfunction in patients with type 2 diabetes free for known cardiovascular disease [17, 18].
Macrovascular disease and risk of major clinical microvascular events
We evaluated, for the first time, the impact of macrovascular disease at baseline on the 9.9-year risk of major clinical microvascular events in patients with type 2 diabetes. Macrovascular disease at baseline was significantly associated with an elevated risk of the composite major microvascular endpoint, as well as retinal photocoagulation or blindness, but not ESRD or renal death. We recently reported that the history of peripheral arterial disease was associated with the risk of severe diabetic retinopathy, but not renal endpoints in ADVANCE and ADVANCE-ON studies [19]. Based on their poor prognosis, patients with chronic macrovascular disease at baseline may have died before experiencing ESRD during the follow-up. The impact of macrovasular disease on the risk of major clinical microvascular events seems to be weaker than the effect of microvascular disease on MACE risk, but there was no evidence of an interaction between the two conditions at baseline on the risk of any measured outcome. Despite their common background, these conditions had independent effects, suggesting that different mechanisms may be involved in the impact of microvascular and macrovascular disease on future vascular events. Hence there is a need to evaluate new predictors for both microvascular and macrovascular events. For instance, a high index of microcirculatory resistance and low coronary flow reserve were recently found associated with poor cardiovascular prognosis in patients with intermediate coronary stenosis (29% with type 2 diabetes) [20].
Physiopathological mechanisms linking microvascular and macrovascular disease
Several pathways may explain the relationship between microvascular and macrovascular disease in patients with diabetes. Diabetic microvascular complications are mainly caused by prolonged exposure to high glucose levels. Diabetes is also associated with accelerated atherosclerosis affecting large vessels [21, 22]. Atherosclerosis is more prevalent in diabetic patients with microvascular disease compared to those without [21, 22]. However, it is still unknown why intensive glucose control does not yield the same benefit on macrovascular events as observed for microvascular outcomes [23]. Chronic hyperglycaemia causes vascular damage through activation of major biochemical paths including polyol pathway flux, increased formation of advanced glycation end products (AGEs), increased expression of AGEs receptor and its activating ligands, activation of protein kinase C isoforms, and overactivity of the hexosamine pathway [24]. Numerous lines of evidence suggest that these biochemical abnormalities may be activated by mitochondrial overproduction of reactive oxygen species (ROS) induced by hyperglycaemia [25]. The excess of ROS production with decreased in antioxidant capacity lead to oxidative stress, which plays an important role in the premature vascular morbidity and mortality in patients with diabetes [26]. Oxidative stress impairs endothelial function and endothelium-dependent vasodilation by inactivation of NO, and induces cell proliferation, hypertrophy, cardiac remodeling, apoptosis, and low-grade inflammation in endothelial and smooth cells of the vascular wall [27,28,29,30]. Moreover, oxidative stress is associated with many other accelerating conditions including insulin resistance, metabolic syndrome, hypertension, dyslipidaemia, and obesity, leading to both microvascular and macrovascular disease [25, 31]. The depletion of circulating stem cells may also explain the association between microvascular and macrovascular disease. A recent study has shown lower CD34+ and CD34+CD133+ cells in type 2 diabetic patients with cardiovascular events compared to those without [32]. The reduced levels of circulating progenitor cells improve the prediction of both macrovascular and microvascular events [32, 33]. The remodeling of bone marrow involves neurovascular changes and vascular abnormalities comparable to the known microangiopathy seen in the kidney and the retina [34].
Strengths and limitations
The main strength of our study is the evaluation of the individual and the combined impact of microvascular and macrovascular disease at baseline on the risk of death and major microvascular and macrovascular outcomes in a large international cohort of patients with type 2 diabetes followed for a median duration of 9.9 years. The main limitation is the absence of biochemical renal assessment during the ADVANCE-ON follow-up, which may underestimate the association between baseline macrovascular disease and kidney disease. Furthermore, peripheral diabetic neuropathy was not investigated as an outcome in the post-trial, ADVANCE-ON, observational study. Although comparable findings were observed when we evaluated the associations of microvascular disease at baseline, including the history of peripheral diabetic neuropathy, with the main outcomes.
Conclusion
The presence of microvascular or macrovascular disease at baseline is independently associated with increased risk of death, MACE, and major clinical microvascular events in people with type 2 diabetes followed for a median duration of 9.9 years, and their coexistence has an additive prognostic value. These findings encourage consideration of prior microvascular disease including retinal status, in addition to traditional cardiovascular risk factors, in selecting participants for forthcoming clinical trials aiming to assess vascular endpoints in patients with type 2 diabetes.
Abbreviations
AGEs:
advanced glycation end products
ACR:
albumin to creatinine ratio
ADVANCE:
Action in Diabetes and Vascular Disease: PreterAx and DiamicroN Modified-Release Controlled Evaluation trial
CKD:
chronic kidney disease
CI:
confidence interval
eGFR:
estimated glomerular filtration rate
ESRD:
end stage renal disease
HR:
hazard ratio
IDI:
integrated discrimination improvement
MACE:
major macrovascular events
NRI:
net reclassification improvement
ROS:
reactive oxygen species
SD:
standard deviation
References
- Cordero A, Lopez-Palop R, Carrillo P, Moreno-Arribas J, Bertomeu-Gonzalez V, Frutos A, Garcia-Carrilero M, Gunturiz C, Bertomeu-Martinez V. Comparison of long-term mortality for cardiac diseases in patients with versus without diabetes mellitus. Am J Cardiol. 2016;117(7):1088–94.
Article PubMed Google Scholar - Bots SH, van der Graaf Y, Nathoe HM, de Borst GJ, Kappelle JL, Visseren FL, Westerink J. The influence of baseline risk on the relation between HbA1c and risk for new cardiovascular events and mortality in patients with type 2 diabetes and symptomatic cardiovascular disease. Cardiovasc Diabetol. 2016;15(1I):101.
Article PubMed PubMed Central Google Scholar - Krentz AJ, Clough G, Byrne CD. Interactions between microvascular and macrovascular disease in diabetes: pathophysiology and therapeutic implications. Diabetes Obes Metab. 2007;9(6I):781–91.
Article CAS PubMed Google Scholar - Bramlage P, Gitt AK, Schneider S, Deeg E, Tschope D. Clinical course and outcomes of type-2 diabetic patients after treatment intensification for insufficient glycaemic control—results of the 2 year prospective DiaRegis follow-up. BMC Cardiovasc Disord. 2014;14:162.
Article PubMed PubMed Central Google Scholar - Kontopantelis E, Springate DA, Reeves D, Ashcroft DM, Rutter MK, Buchan I, Doran T. Glucose, blood pressure and cholesterol levels and their relationships to clinical outcomes in type 2 diabetes: a retrospective cohort study. Diabetologia. 2015;58(3I):505–18.
Article CAS PubMed Google Scholar - Brownrigg JR, Hughes CO, Burleigh D, Karthikesalingam A, Patterson BO, Holt PJ, Thompson MM, de Lusignan S, Ray KK, Hinchliffe RJ. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: a population-level cohort study. Lancet Diabetes Endocrinol. 2016;4(7I):588–97.
Article PubMed Google Scholar - ADVANCE Management Committee. Study rationale and design of ADVANCE: action in diabetes and vascular disease–preterax and diamicron MR controlled evaluation. Diabetologia. 2001;44(9I):1118–20.
Google Scholar - Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24I):2560–72.
CAS PubMed Google Scholar - Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590I):829–40.
Article CAS PubMed Google Scholar - Zoungas S, Chalmers J, Neal B, Billot L, Li Q, Hirakawa Y, Arima H, Monaghan H, Joshi R, Colagiuri S, et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371(15I):1392–406.
Article PubMed Google Scholar - KDIGO Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Inter. 2013;3(1):1–150.
Article Google Scholar - Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23(13I):2109–23.
Article PubMed Google Scholar - Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
Article Google Scholar - Woodward M, Hirakawa Y, Kengne AP, Matthews DR, Zoungas S, Patel A, Poulter N, Grobbee R, Cooper M, Jardine M, et al. Prediction of 10-year vascular risk in patients with diabetes: the AD-ON risk score. Diabetes Obes Metab. 2016;18(3I):289–94.
Article CAS PubMed Google Scholar - Mohammedi K, Woodward M, Hirakawa Y, Zoungas S, Williams B, Lisheng L, Rodgers A, Mancia G, Neal B, Harrap S, et al. Microvascular and macrovascular disease and risk for major peripheral arterial disease in patients with type 2 diabetes. Diabetes Care. 2016;39(10I):1796–803.
Article PubMed Google Scholar - Sorensen BM, Houben AJ, Berendschot TT, Schouten JS, Kroon AA, van der Kallen CJ, Henry RM, Koster A, Sep SJ, Dagnelie PC, et al. Prediabetes and type 2 diabetes are associated with generalized microvascular dysfunction: the maastricht study. Circulation. 2016;134(18I):1339–52.
Article PubMed Google Scholar - von Scholten BJ, Hasbak P, Christensen TE, Ghotbi AA, Kjaer A, Rossing P, Hansen TW. Cardiac (82)Rb PET/CT for fast and non-invasive assessment of microvascular function and structure in asymptomatic patients with type 2 diabetes. Diabetologia. 2016;59(2I):371–8.
Article Google Scholar - von Scholten BJ, Hansen CS, Hasbak P, Kjaer A, Rossing P, Hansen TW. Cardiac autonomic function is associated with the coronary microcirculatory function in patients with type 2 diabetes. Diabetes. 2016;65(10I):3129–38.
Article Google Scholar - Mohammedi K, Woodward M, Hirakawa Y, Zoungas S, Colagiuri S, Hamet P, Harrap S, Poulter N, Matthews DR, Marre M, et al. Presentations of major peripheral arterial disease and risk of major outcomes in patients with type 2 diabetes: results from the ADVANCE-ON study. Cardiovasc Diabetol. 2016;15(1I):129.
Article PubMed PubMed Central Google Scholar - Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES, Koo BK. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol. 2016;67(10I):1158–69.
Article PubMed Google Scholar - Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus—mechanisms, management, and clinical considerations. Circulation. 2016;133(24I):2459–502.
Article CAS PubMed PubMed Central Google Scholar - Reinhard H, Wiinberg N, Hansen PR, Kjaer A, Petersen CL, Winther K, Parving HH, Rossing P, Jacobsen PK. NT-proBNP levels, atherosclerosis and vascular function in asymptomatic type 2 diabetic patients with microalbuminuria: peripheral reactive hyperaemia index but not NT-proBNP is an independent predictor of coronary atherosclerosis. Cardiovasc Diabetol. 2011;10:71.
Article CAS PubMed PubMed Central Google Scholar - Avogaro A, Fadini GP, Sesti G, Bonora E, Del Prato S. Continued efforts to translate diabetes cardiovascular outcome trials into clinical practice. Cardiovasc Diabetol. 2016;15(1I):111.
Article PubMed PubMed Central Google Scholar - Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6I):1615–25.
Article CAS PubMed Google Scholar - Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9I):1058–70.
Article CAS PubMed PubMed Central Google Scholar - Doney AS, Lee S, Leese GP, Morris AD, Palmer CN. Increased cardiovascular morbidity and mortality in type 2 diabetes is associated with the glutathione S transferase theta-null genotype: a Go-DARTS study. Circulation. 2005;111(22I):2927–34.
Article CAS PubMed Google Scholar - Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73(3I):411–8.
Article CAS PubMed Google Scholar - Rosa CM, Xavier NP, Henrique Campos D, Fernandes AA, Cezar MD, Martinez PF, Cicogna AC, Gimenes C, Gimenes R, Okoshi MP, et al. Diabetes mellitus activates fetal gene program and intensifies cardiac remodeling and oxidative stress in aged spontaneously hypertensive rats. Cardiovasc Diabetol. 2013;12:152.
Article PubMed PubMed Central Google Scholar - Patel H, Chen J, Das KC, Kavdia M. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc Diabetol. 2013;12:142.
Article PubMed PubMed Central Google Scholar - Odegaard AO, Jacobs DR Jr, Sanchez OA, Goff DC Jr, Reiner AP, Gross MD. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc Diabetol. 2016;15:51.
Article PubMed PubMed Central Google Scholar - Hopps E, Noto D, Caimi G, Averna MR. A novel component of the metabolic syndrome: the oxidative stress. Nutr Metab Cardiovasc Dis. 2010;20(1I):72–7.
Article CAS PubMed Google Scholar - Fadini GP, Rigato M, Cappellari R, Bonora BM, Avogaro A. Long-term prediction of cardiovascular outcomes by circulating CD34+ and CD34+ CD133+ stem cells in patients with type 2 diabetes. Diabetes Care. 2017;40(1I):125–31.
Article PubMed Google Scholar - Rigato M, Bittante C, Albiero M, Avogaro A, Fadini GP. Circulating progenitor cell count predicts microvascular outcomes in type 2 diabetic patients. J Clin Endocrinol Metab. 2015;100(7I):2666–72.
Article CAS PubMed Google Scholar - Oikawa A, Siragusa M, Quaini F, Mangialardi G, Katare RG, Caporali A, van Buul JD, van Alphen FP, Graiani G, Spinetti G, et al. Diabetes mellitus induces bone marrow microangiopathy. Arterioscler Thromb Vasc Biol. 2010;30(3I):498–508.
Article CAS PubMed Google Scholar
Authors’ contributions
KM wrote the manuscript with assistance from MW and JC; KM and JC designed the study; MM, SC, MC, SH, GM, NP, BW, and SZ contributed to the discussion and reviewed the manuscript. JC and KM are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Acknowledgements
KM was supported by grants from the Société Francophone du Diabète (SFD) and the Association Diabète Risque Vasculaire (ADRV), and travel support from the Association pour le Développement de l’Enseignement et des Recherches auprès des universités, des centres de recherches et des entreprises d’Aquitaine (ADERA).
Competing interests
Dr. Kamel Mohammedi reports personal fees from Novo-Nordisk, outside the submitted work; Prof. Marc Woodward reports personal fees from Amgen, outside the submitted work; Prof. Michel Marre reports grants and personal fees from Novo Nordisk, grants and personal fees from Sanofi, grants and personal fees from Eli Lilly, personal fees from Servier, grants and personal fees from Merck Sharp and Dohme, personal fees from Abbott, grants and personal fees from Novartis, personal fees from Astra Zeneca, outside the submitted work; Prof. Stephen Harrap reports grants from National Health and Medical Research Council of Australia, grants from The George Institute for Global Health, during the conduct of the study; other from Servier, outside the submitted work; Prof. Neil Poulter reports grants from The George Institute, grants from British Heart Foundation/Diabetes UK/The George Institute, during the conduct of the study; grants from The George Institute, grants from BHF/DUK/The George Institute, outside the submitted work; Prof. Bryan Williams reports lecture fees from Servier, Novartis, Beoringher Ingelheim and Diachi Sankyo, outside the submitted work; and Prof. John Chalmers reports grants from National Health and Medical Research Council of Australia, grants and personal fees from Servier, outside the submitted work. No other potential conflict of interest relevant to this article was reported.
Availability of data and materials
The datasets analysed during the current study are not publicly available due to consideration of intellectual property, due to many ongoing active collaborations worldwide, and to continuing analyses by the study investigators, but may be available from the principal investigator on reasonable request.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The Institutional Ethics Committee of each participating centre approved the ADVANCE and ADVANCE-ON protocols, and all participants provided written informed consent.
Funding
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
- The George Institute for Global Health, University of Sydney, Sydney, NSW, Australia
Kamel Mohammedi, Mark Woodward, Sophia Zoungas & John Chalmers - INSERM, UMRS 1138, Centre de Recherche des Cordeliers, Paris, France
Kamel Mohammedi & Michel Marre - Department of Diabetology, Endocrinology and Nutrition, Assistance Publique Hôpitaux de Paris, Bichat Hospital, DHU FIRE, Paris, France
Kamel Mohammedi & Michel Marre - The George Institute for Global Health, University of Oxford, Oxford, UK
Mark Woodward - Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA
Mark Woodward - University Paris Diderot, Sorbonne Paris Cité, UFR de Médecine, Paris, France
Michel Marre - Boden Institute of Obesity, Nutrition, Exercise & Eating Disorders, Sydney Medical School, University of Sydney, Sydney, NSW, Australia
Stephen Colagiuri - Baker IDI Heart and Diabetes Institute, Melbourne, VIC, Australia
Mark Cooper - The University of Melbourne and Royal Melbourne Hospital, Melbourne, VIC, Australia
Stephen Harrap - The University of Milan-Bicocca and Istituto Auxologico Italiano, Milan, Italy
Giuseppe Mancia - The International Centre for Circulatory Health, National Heart and Lung Institute, Imperial College, London, UK
Neil Poulter - Institute of Cardiovascular Sciences, University College London (UCL) and NIHR UCL Hospitals Biomedical Research Centre, London, UK
Bryan Williams - Monash Centre for Health Research and Implementation, School of Public Health and Preventive Medicine, Monash University, Clayton, VIC, Australia
Sophia Zoungas
Authors
- Kamel Mohammedi
You can also search for this author inPubMed Google Scholar - Mark Woodward
You can also search for this author inPubMed Google Scholar - Michel Marre
You can also search for this author inPubMed Google Scholar - Stephen Colagiuri
You can also search for this author inPubMed Google Scholar - Mark Cooper
You can also search for this author inPubMed Google Scholar - Stephen Harrap
You can also search for this author inPubMed Google Scholar - Giuseppe Mancia
You can also search for this author inPubMed Google Scholar - Neil Poulter
You can also search for this author inPubMed Google Scholar - Bryan Williams
You can also search for this author inPubMed Google Scholar - Sophia Zoungas
You can also search for this author inPubMed Google Scholar - John Chalmers
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toKamel Mohammedi.
Additional file
12933_2017_574_MOESM1_ESM.docx
Additional file 1: Table S1. Components of microvascular and macrovascular disease at baseline. Table S2. Distibution of patients, event rate (per 100 person years) and hazard ratios (HRs) for outcomes during follow-up, according to the history of microvascular (including chronic kidney disease) or macrovascular disease at baseline. Table S3. Distibution of patients, event rate (per 100 person years) and hazard ratios (HRs) for outcomes during follow-up, according to the history of microvascular (including peripheral neuropathy) or macrovascular disease at baseline. Table S4. Subdistribution hazard ratios for outcomes during follow-up according to the history of microvascular or macrovascular disease at baseline with correction for competing risk of non-renal and non-cardiovascular death.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Mohammedi, K., Woodward, M., Marre, M. et al. Comparative effects of microvascular and macrovascular disease on the risk of major outcomes in patients with type 2 diabetes.Cardiovasc Diabetol 16, 95 (2017). https://doi.org/10.1186/s12933-017-0574-y
- Received: 08 May 2017
- Accepted: 21 July 2017
- Published: 27 July 2017
- DOI: https://doi.org/10.1186/s12933-017-0574-y