News – NIH Director's Blog (original) (raw)
NIH’s CARE for Health Primary Care Research Network: Connecting the Lab, the Clinic, and the Community
Posted on October 8th, 2024 by Dr. Monica M. Bertagnolli

Credit: Donny Bliss
Since I became NIH Director last year, one key principle has guided my vision and approach: Our work is not finished when we deliver scientific discoveries; our work is finished when all people are living long and healthy lives.
But unfortunately, we’re seeing some alarming trends in the health of the U.S. population. It’s a bit of a puzzle. On one hand, significant advances in biomedical research over the last several decades have led to lifesaving interventions for a range of diseases and conditions. At the same time, the overall health of the people in this country appears to be stalled and even getting worse.
Looking at one measure of health, U.S. life expectancy is no longer steadily increasing—in fact, we’ve had a decline in life expectancy over the last decade. And though this included a dramatic drop because of the COVID-19 pandemic, the rate was declining before that. Life expectancy in the U.S. is also low compared to peer nations, even though we spend much more money on our health system. Disparities in mortality also persist among certain racial and ethnic groups and geographic regions. Our health is determined not only by the genes we inherit from our parents, but by our environment and social and economic factors. We know that in the U.S. today, your zip code can significantly impact your health.
I believe that biomedical research can play a key role in reversing these trends. In my first blog post, I explained how one of my goals as NIH Director is to ensure that the biomedical research enterprise is more inclusive to people from all walks of life, and I noted we can engage more communities as our research partners by meeting people where they are. Despite having the knowledge and technology to do so, our research and advances are not reaching everyone they should. Many people are not adequately represented in clinical research, and research data is especially lacking for people who are older, uninsured, belong to minority groups, or live in rural locations. Many people also face barriers to participating in clinical research, such as arranging and paying for transportation, getting time off from work and coordinating childcare, or lack of trust in medical institutions. To address these concerning trends in health, we need to do a better job of connecting the lab, the clinic, and the community.
In September, we moved closer to this goal by announcing awards as part of a new NIH primary care clinical research network that aims to improve access to and involve communities in the clinical research that informs medical care. The Communities Advancing Research Equity for Health™ or CARE for Health™ program will actively engage communities historically underrepresented in clinical research. This effort is very close to my heart, as I was born and raised in a rural community, and I’d like to tell you more about how it will work.
In this program, NIH will connect with primary care providers and their patients, giving them access to research and the opportunity to participate in clinical trials. By engaging people on the front lines of health care—in the primary care clinician’s office—we will build an infrastructure that leads to sustained relationships with primary care providers and patients and earns people’s trust. Many of the areas of the country where we want to focus do not have medical specialists, and primary care providers are often the only practitioners available for every health challenge. Through CARE for Health, we want to integrate clinical care with research to support knowledge generation that meets the needs of people in all communities.
The awards we’ve announced, totaling over 5millioninfundingforthefirstyear,willsupportthreeNetworkResearchHubstoestablishtheprogram’sinitialinfrastructure.ThefirstawardeeinstitutionsareOregonHealthandScienceUniversity,theUniversityofWisconsin−Madison,andWestVirginiaUniversity.TheseinstitutionswillparticipateinthreeongoingNIH−fundedclinicaltrialsthatcoverarangeoftopicsimportanttoprimaryhealthcare,includingpainmanagement,opioidandpolysubstanceuse,andgout,withmanymorestudiesonareasimportanttodiversecommunitiestocomeinthefuture.Overall,NIHis[investingapproximately5 million in funding for the first year, will support three Network Research Hubs to establish the program’s initial infrastructure. The first awardee institutions are Oregon Health and Science University, the University of Wisconsin-Madison, and West Virginia University. These institutions will participate in three ongoing NIH-funded clinical trials that cover a range of topics important to primary health care, including pain management, opioid and polysubstance use, and gout, with many more studies on areas important to diverse communities to come in the future. Overall, NIH is [investing approximately 5millioninfundingforthefirstyear,willsupportthreeNetworkResearchHubstoestablishtheprogram’sinitialinfrastructure.ThefirstawardeeinstitutionsareOregonHealthandScienceUniversity,theUniversityofWisconsin−Madison,andWestVirginiaUniversity.TheseinstitutionswillparticipateinthreeongoingNIH−fundedclinicaltrialsthatcoverarangeoftopicsimportanttoprimaryhealthcare,includingpainmanagement,opioidandpolysubstanceuse,andgout,withmanymorestudiesonareasimportanttodiversecommunitiestocomeinthefuture.Overall,NIHisinvestingapproximately30 million in total over fiscal years 2024 and 2025 to pilot the program, which is supported through the NIH Common Fund. After the first year, we will aim to broaden the program so communities throughout the country can participate.
CARE for Health is a new paradigm for biomedical research. NIH has never had a network for the primary care medical environment that works across all 27 Institutes and Centers of NIH. We are starting it with a pilot program because we know that for a program of this scale, we first need to learn from our research teams and from the primary care clinicians who are going to help us bring this kind of research to their communities. We will also ask community members what their health priorities are and allow them to select the research studies that are most meaningful to them. In the future, CARE for Health partners will have a long menu of studies to pick from based on local interests and needs.
Ultimately, we believe that this program will have a meaningful impact on health outcomes, especially among those who have been previously underrepresented and underserved in medical research.
Reference:
Bertagnolli MM. Connecting lab, clinic, and community. Science. DOI:10.1126/science.adq2140 (2024).
NIH Support: NIH Common Fund
Welcoming Senator Reed and Congressional Staff to the NIH Campus
Posted on September 24th, 2024 by Dr. Monica M. Bertagnolli

Senator Jack Reed (right) visited the NIH Clinical Center, which included a tour of the National Cancer Institute’s pediatric oncology lab. Credit (all photos): Chia-Chi Charlie Chang, NIH
On September 13, I was delighted to welcome Senator Jack Reed to the NIH campus. His tour included a visit to the NIH Clinical Center, where he heard about recent findings in RNA sequencing from experts from the National Institute of Environmental Health Sciences and the National Human Genome Research Institute. We then joined several senior leaders and scientists from the National Cancer Institute to discuss advances in childhood cancer research, and to visit a pediatric oncology lab and meet with a patient who received treatment there. He also met with leaders of NIH’s Fogarty International Center. Later in the day, I welcomed a group of new Congressional Staff from the House Democratic Caucus. They, too, got a chance to visit NIH labs and talk to researchers. We then had a roundtable discussion where I introduced myself and my background, talked about current trends in health, and answered questions about the work we do here and my vision and priorities for NIH. I’d like to thank Sen. Reed and Congressional Staff for coming to NIH and joining in such informative conversations.

NIH Director Monica Bertagnolli (left) on a tour of the NIH Clinical Center, talking with Sen. Reed (center) and Dr. Brigitte Widemann (right), Chief of the National Cancer Institute’s Pediatric Oncology Branch.

Dr. Bertagnolli speaking at a roundtable with Congressional Staff.
Study of Protective Gene Variant Provides Insight into Delaying Onset of Alzheimer’s Dementia
Posted on July 18th, 2024 by Dr. Monica M. Bertagnolli

Credit: Donny Bliss/NIH
Alzheimer’s disease is currently the seventh leading cause of death in the U.S. While your likelihood of developing Alzheimer’s-related cognitive impairment increases with age, risk for this disease and age of its onset depend on many factors, including the genes you carry. An intriguing new study suggests that having just one copy of a protective gene variant may be enough to delay cognitive impairment from this devastating disease in individuals who are otherwise genetically predisposed to developing early-onset Alzheimer’s dementia.
The findings, from a study supported in part by NIH and reported in The New England Journal of Medicine, offer important insights into the genetic factors and underlying pathways involved in Alzheimer’s dementia.1 While much more study is needed, the findings have potential implications for treatments that could one day work like this gene variant does to delay or perhaps even prevent Alzheimer’s dementia.
This research comes from an international team including Yakeel Quiroz, Massachusetts General Hospital, Boston; Joseph Arboleda-Velasquez, Mass Eye and Ear, Boston; and Francisco Lopera, University of Antioquia, Colombia. For the last 40 years, Lopera has been studying a Colombian family of about 6,000 blood relatives, 1,200 of whom carry a mutation known as Paisa (or Presenilin-1 E280A) that predisposes them to developing early-onset Alzheimer’s dementia. Those who carry a single copy of this gene variant typically show signs of cognitive decline in their early 40s, progressing to dementia by age 50. They frequently die from dementia-related complications in their 60s.
In 2019, the researchers reported on an extraordinary individual who was an exception to this prognosis.2 Even though she carried the Paisa mutation, she didn’t develop any notable cognitive decline until her late 70s—30 years later than expected. The researchers traced her protection against dementia to two copies of a rare variant of the APOE gene dubbed Christchurch. Further study of her brain after death also found lower levels of inflammation and tau protein, which forms damaging tangles inside neurons in the Alzheimer’s brain.
Christchurch is a rare variant, and it’s far more common for people to carry one copy of the protective variant versus two. Would a single copy of the Christchurch variant offer some protection against Alzheimer’s dementia, too? To find out in the new study, the researchers analyzed data from 27 members of this family carrying a single copy of the Christchurch variant among 1,077 carriers of the Paisa mutation.
The researchers compared Christchurch carriers to those without the protective variant and found the variant did delay the age of onset of Alzheimer’s-related cognitive decline and dementia. The median age at the onset of mild cognitive impairment was 52 in family members with the Christchurch variant, compared to approximately age 47 in a matched group without the variant. Similarly, the median age at the onset of dementia was 54, compared to the median age of 50 in noncarriers.
To learn more, the researchers imaged the brains of two of the individuals who had one copy of Christchurch. The brain scans showed lower levels of tau and more normal metabolic activity in brain areas that are known to play a role in Alzheimer’s. Interestingly, their brains still showed accumulations of amyloid proteins, which form plaques that are another hallmark of Alzheimer’s. The team also analyzed autopsy samples from four deceased individuals with one copy of the Christchurch variant and found that blood vessels in their brains appeared healthier, which may help to explain the protective effects of Christchurch. The findings suggest a significant role for blood vessel health in protecting the brain from cognitive decline, as well as a role for disease of the brain blood vessels in contributing to cognitive decline and dementia.
The researchers note this study is limited to a relatively small number of people with both the Paisa and Christchurch variants in one group of related individuals. Further studies involving larger and more diverse samples are needed to learn more about this protective gene variant and its effects on the brain in the general population. The hope is these findings may one day yield new approaches to delaying the onset of Alzheimer’s or slowing its progression in millions more people around the world at risk of developing this devastating disease.
References:
[1] Quiroz YT, et al. APOE3 Christchurch Heterozygosity and Autosomal Dominant Alzheimer’s Disease. The New England Journal of Medicine. DOI: 10.1056/NEJMoa2308583 (2024).
[2] Arboleda-Velasquez JF, et al. Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nature Medicine. DOI: 10.1038/s41591-019-0611-3 (2019).
NIH Support: National Institute on Aging, National Institute of Neurological Disorders and Stroke
Posted In: Health, News, Science
Tags: aging, Alzheimer’s disease, brain, cognitive decline, dementia, DNA, gene variants, genetics, neurological disease
Sequencing Technique Detects Earliest Signs of Genetic Mutations Underlying Cancer, Aging, and More
Posted on July 11th, 2024 by Dr. Monica M. Bertagnolli

Every day, billions of cells in your body divide, each producing two daughter cells. It’s an essential process for your tissues and organs to renew themselves and remain healthy. To do it, cells must first duplicate their DNA to ensure that each daughter cell gets an accurate copy. In this process, mistakes are inevitably made. Most DNA errors are accurately fixed and do not lead to mutations. But when small errors akin to single-letter typos aren’t corrected, they can become permanent in a cell and multiplied with each subsequent cell division. Even cells that don’t divide, such as neurons in your brain, acquire damage and mutations in their DNA with age. As a result, your tissues contain collections of cells with distinct mutations that accumulate over time.
While many of these small errors will show no obvious consequences, others can lead to cancer and other health conditions. Now, a new DNA sequencing technique, described in Natureand developed through research supported by NIH, promises to detect early DNA changes before they become permanent mutations in a cell’s genome. The method, called Hairpin Duplex Enhanced Fidelity Sequencing (HiDEF-seq), could advance our understanding of how and why mutations arise, with potentially important implications for our health. For example, the ability to identify signs that precede mutations may help predict a person’s health risks based on genetic predispositions, environmental exposures, or other factors.
The HiDEF-seq technique comes from an international team led by Gilad Evrony at NYU Grossman School of Medicine’s Center for Human Genetics and Genomics in New York City. To understand how the method works, it helps to remember that each DNA molecule stores genetic information in the form of two complementary strands made up of four molecular “letters,” or chemical bases. Those bases are adenine (A), thymine (T), guanine (G), and cytosine (C). The sequence of about three billion As, Ts, Gs, and Cs in human DNA’s two strands generally should match up, such that As pair with Ts and Gs with Cs.
The first step in which DNA mutations arise usually involves a change in only one of the two DNA strands. Those single-strand errors only become permanent mutations in both strands when a cell’s copying machinery fails to detect the mistake before the cell divides again, or when the cell’s DNA repair machinery makes a mistake in the correction process. However, because other methods to sequence DNA can’t accurately detect changes that are in only one of the DNA strands, it hasn’t been possible for researchers to study this process in detail. This is where HiDEF-seq comes in.
The researchers wanted to develop an approach for directly sequencing single DNA molecules to detect these early-stage DNA errors. Detecting changes that are in only one of the two DNA strands requires an extremely high degree of sequencing accuracy, with less than one error per one billion bases, so the team devised a method to read DNA with higher precision than was previously possible. To put HiDEF-seq to the test, they profiled 134 samples from various human tissues, including those from people with syndromes that predisposed them to cancer due to an unusually high number of new mutations.
The research team found they could use HiDEF-seq to identify changes present in only one of the two DNA strands that were the precursors to mutational events. For example, they identified places where a C was mistakenly paired with a T instead of a G. As expected, those early changes in DNA turned up more often in people with syndromes that increase their risk of cancer than in those without. The patterns of those single-strand DNA changes also looked a lot like the patterns of double-strand DNA mutations seen in people with these syndromes, suggesting that the HiDEF-seq method was indeed seeing the precursors to mutations.
The method can also detect a common form of DNA damage called cytosine deamination, in which cytosine is converted to a different base called uracil (U), which is another source of mutations. Experiments in human sperm, which rarely pick up mutations compared to other cell types, showed a pattern of cytosine deamination that closely matched damage caused by heating healthy DNA in the lab. This led the researchers to suggest that the damage to DNA happens similarly in both situations.
The researchers have already begun to produce a catalog of the various single-strand DNA errors they’ve uncovered. They suggest that HiDEF-seq may allow new ways to monitor the everyday effects of environmental exposures or other insults on our DNA and shed light on the balance in cells between DNA damage, repair, and replication. Along the way, this new technique will enable the continued study of DNA damage and the origins of mutations in a way that hasn’t been possible before.
Reference:
Liu MH, et al. Single-strand mismatch and damage patterns revealed by single-molecule DNA sequencing. Nature. DOI: 10.1038/s41586-024-07532-8 (2024).
NIH Support: Common Fund, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute on Aging, National Institute of Neurological Disorders and Stroke, National Cancer Institute
Posted In: Health, News, Science
Tags: aging, cancer, cell division, DNA, DNA damage, DNA sequencing, genetics, genomics, mutations
Molecular Portrait of Key Driver of Pancreatic Cancer Offers Hope for Continued Treatment Advances
Posted on June 27th, 2024 by Dr. Monica M. Bertagnolli

Credit: magicmine/Adobe Stock
Cancer arises when changes in genes that normally control cell division lead to unchecked growth at the expense of healthy tissues. One of the most common genetic alterations across human cancers—occurring in 95% of pancreatic cancers but also many non-small cell lung cancers, colorectal cancers, and others—is in a gene known as KRAS. While promising new treatments targeting KRAS to shrink cancerous tumors have recently gained approval, less than 40% of pancreatic cancers respond to treatment with KRAS inhibitors for reasons that aren’t well understood.
There’s much more to learn about how KRAS spurs cancer growth—and how KRAS-mutant cancers resist treatment with existing KRAS inhibitors. To address this need, researchers behind two studies in Science have established the most comprehensive molecular portrait yet of the workings of KRAS and how its many downstream impacts may influence outcomes for people with pancreatic cancer.1,2 The findings could lead to new treatment approaches, including ways to potentially guide treatment for individuals with pancreatic cancer, the third leading cause of cancer-related death in the U.S.
These studies, supported in part by NIH, come from a team led by Channing Der and Adrienne Cox, together with Jeffrey Klomp, Clint Stalnecker, and Jennifer Klomp, at the Lineberger Comprehensive Cancer Center, University of North Carolina at Chapel Hill. The researchers were inspired in part by the Food and Drug Administration’s recent approval of treatments that block a mutated version of KRAS that drives many pancreatic cancers. The team was also motivated by the realization that many patients whose cancers initially respond to the new treatments relapse rather quickly as the cancers find ways to reactivate underlying growth pathways.
The researchers wanted to know more about KRAS and its influence on another essential pathway, including a protein called ERK, by defining all the genes that are actively transcribed into proteins in KRAS-mutant cancer cell lines and tumors. Their findings show that changes in KRAS signaling drive cancer growth mainly though the ERK network. In turn, ERK regulates many other genes to determine which ones are switched on or off while also influencing the activity of many other proteins. This shows that the effect of mutant KRAS on the ERK protein alone leads to widespread effects on the activity of thousands of other genes and proteins. The study uncovered underlying mechanisms that affected multiple stages of the cell cycle that leads to cell division.
Kinases, including ERK, alter the activity of other proteins by the addition of a chemical phosphate group. One of the things that makes ERK unique is that it activates a wide range of functionally distinct proteins, both directly and indirectly. To learn more about its influence, the team explored the many proteins ERK chemically modifies in pancreatic cancers that rely on mutant KRAS. Altogether, they found more than 2,100 proteins that were modified by activated ERK, more than half of which had not been associated with this protein before. Because activation of this pathway is so important for the response to KRAS inhibitors, the findings promise to help elucidate the underlying mechanisms involved in treatment responses as well as drug resistance.
Importantly, the researchers found that the molecular signatures they’ve uncovered may predict tumor responses in patients treated with KRAS inhibitors or ERK inhibitors. Based on their findings, they suspect that the reason so many pancreatic cancers don’t respond to KRAS inhibitors may be because the drugs simply don’t block KRAS well enough—and not because the cancers no longer depend on KRAS signals for their growth. The researchers suggest it may be beneficial to monitor these underlying molecular pathways in patients to better understand treatment outcomes and guide treatment decisions.
The team plans to continue exploring the role of these and other important drivers of cancer growth and treatment resistance. Ultimately, their goal is to help advance the development of the next generation of KRAS inhibitors that will work even better for many more people with pancreatic or other KRAS-driven cancers.
References:
[1] Klomp JA, et al. Defining the KRAS- and ERK-dependent transcriptome in KRAS-mutant cancers. Science. DOI: 10.1126/science.adk0775 (2024).
[2] Klomp JE, et al. Determining the ERK-regulated phosphoproteome driving KRAS-mutant cancer. Science. DOI: 10.1126/science.adk0850 (2024).
NIH Support: National Cancer Institute, National Institute of General Medical Sciences
Antibiotic Compound Kills Hard-to-Treat, Infectious Bacteria While Sparing Healthy Bacteria in the Gut
Posted on June 20th, 2024 by Dr. Monica M. Bertagnolli
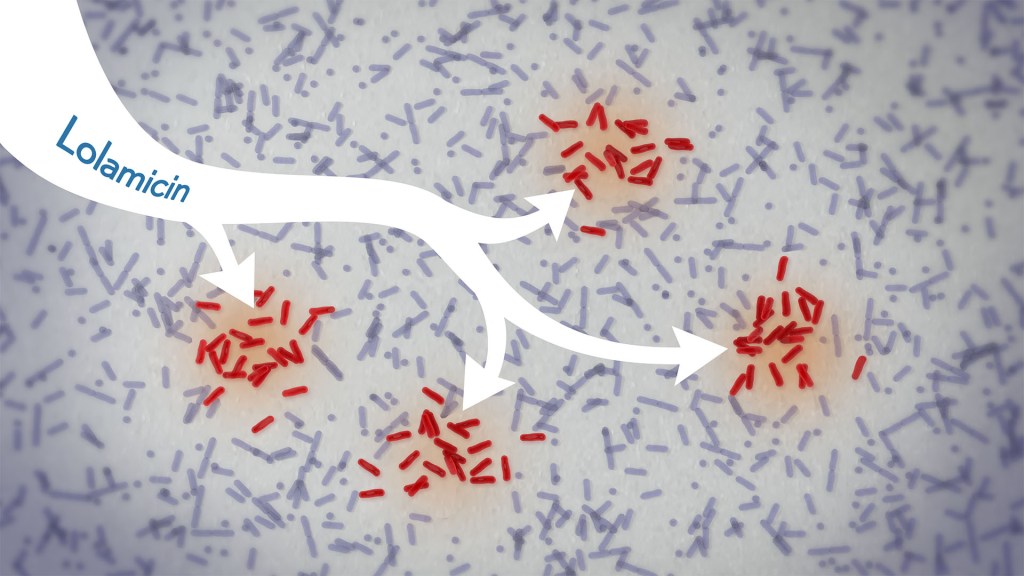
In a new study, an antibiotic compound, lolamicin, targeted infectious, gram-negative bacteria without harming the gut microbiome. Credit: Donny Bliss/NIH
Drug-resistant bacteria are responsible for a rise in serious, hospital-acquired infections, including pneumonia and sepsis. Many of these bacteria are classified as “gram-negative,” and are harder to kill than “gram-positive” bacteria. Unfortunately, the limited number of antibiotics that can help combat these dangerous infections can also damage healthy microbes in the gut, leaving people at risk for other, potentially life-threatening infections. Such antibiotic-induced disruption has also been linked in studies to irritable bowel syndrome, colon cancer, and many other health conditions.
There’s a great need for more targeted antibiotics capable of fending off infectious gram-negative bacteria while sparing the community of microbes in the gut, collectively known as the gut microbiome. Now, in findings reported in the journal Nature, a research team has demonstrated a promising candidate for the job. While the antibiotic hasn’t yet been tested in people, the findings in cell cultures suggest it could work against more than 130 drug-resistant bacterial strains. What’s more, the study, supported in part by NIH, shows that this compound, when given to infected mice, thwarts potentially life-threatening bacteria while leaving the animals’ gut microbiomes intact.
One reason it’s been difficult to find antibiotics that work against gram-negative bacteria without killing too many benign bacteria is that most promising targets for gram-negative bacteria are shared by gram-positive bacteria. But the team, led by Paul Hergenrother and Kristen Muñoz at the University of Illinois Urbana-Champaign, recognized an intriguing target for more specific microbiome-sparing antibiotics in a collection of proteins that gram-negative bacteria depend on to transport lipoproteins between their inner and outer cell membranes. Gram-positive bacteria, with only one cell membrane, don’t require lipoprotein transport and therefore lack these proteins.
The researchers knew that this lipoprotein-transport system, known as the Lol system, is required for infectious E. coli bacteria to live and grow. It’s also found in many other infectious gram-negative bacteria. They thought that compounds aimed at this system might be doubly selective—specifically targeting hard-to-treat gram-negative bacteria and leaving the gut microbiome relatively unscathed. They also knew other drugs targeting the Lol system had showed some promise in selectively targeting gram-negative bacteria. However, these antibiotics didn’t work well enough on their own to fight infections.
In the new study, the researchers tinkered with the design of those compounds in search of one that might work better. It led them to a compound, which they call lolamicin, that they found could selectively target three different types of infectious gram-negative bacteria (E. coli, Klebsiella pneumoniae, and Enterobacter cloacae) in the lab. They also found that the antibiotic at high doses killed up to 90% of multidrug-resistant strains of those infectious bacteria in cell cultures.
In additional experiments, the researchers wanted to see how well lolamicin would treat infected mice. They found that treatment with the antibiotic was well tolerated by the animals. They went on to show in mouse models of acute pneumonia and sepsis that oral treatment with lolamicin reduced the number of infectious bacteria. When mice with sepsis were treated with lolamicin, all of them survived. Lolamicin treatment also rescued 70% of mice with pneumonia infection.
While treatment with amoxicillin, a broad-spectrum antibiotic, and clindamycin, an antibiotic that only targets gram-positive bacteria, disrupted the assemblage of healthy microbes living in the mouse gut, the researchers found that treatment with lolamicin did not. They saw no big changes in the microbial community present in the mouse gut after three days of lolamicin treatment. As a result, unlike mice treated with the other two antibiotics, mice treated with lolamicin were protected from secondary infection by Clostridioides difficile, a bacterium that can infect the colon to cause diarrhea and life-threatening tissue damage.
These new findings, while promising, are at an early stage of drug discovery and development, and much more study is needed before this compound could be tested in people. It will also be important to learn how rapidly infectious gram-negative bacteria may develop resistance to lolamicin. Nevertheless, these findings suggest it may be possible to further develop lolamicin or related antibiotic compounds targeting the Lol system to treat dangerous gram-negative infections without harming the microbiome.
Reference:
Muñoz KA, et al. A Gram-negative-selective antibiotic that spares the gut microbiome. Nature. DOI: 10.1038/s41586-024-07502-0 (2024).
NIH Support: National Institute of Allergy and Infectious Diseases
Insights into Molecular Basis of PTSD and Major Depression Could One Day Aid in Diagnosis and Treatment
Posted on June 13th, 2024 by Dr. Monica M. Bertagnolli

Credit: S. Thomas Carmichael/UCLA, Yuliia/Adobe Stock
We know stress can take a toll on our mental health. Yet, it’s unclear why some people develop stress-related mental health disorders and others don’t. The risk for developing a stress-related mental health disorder such as post-traumatic stress disorder (PTSD) or major depressive disorder (MDD) depends on a complex interplay between the genetic vulnerabilities we are born with and the impact of traumatic stress we experience over our lifetimes.
Given this complexity, it’s been difficult for researchers to pinpoint the underlying biological pathways in the body that ultimately produce changes associated with PTSD, major depression, or other mental health conditions. Now, a study reported in a special issue of Science on decoding the brain uses a comprehensive approach to examine multiple biological processes across brain regions, cell types, and blood to elucidate this complexity. It’s an unprecedented effort to understand in a more holistic way the essential biological networks involved in PTSD and MDD.
While earlier studies looked at stress hormones, the immune system, and other molecular signatures of stress in blood samples, what had been largely missing from the picture of PTSD and MDD were links between those changes in the body and changes in the brain. To get a more complete picture, a multisite research team led by Nikolaos P. Daskalakis and Kerry Ressler of McLean Hospital, Belmont, MA, developed a vast molecular dataset including DNA variants, RNA, proteins, and chemical modifications to DNA. This “multi-omic” dataset was generated by the NIH-supported PTSD Brainomics Project of the PsychENCODE Consortium, and included postmortem data from 231 individuals with PTSD and/or MDD, as well as from individuals who didn’t have known mental health conditions.
In the study, the researchers looked at three essential brain regions: the medial prefrontal cortex (mPFC), the hippocampal dentate gyrus, and the central nucleus of the amygdala. They conducted single-cell RNA sequencing analysis of 118 dorsolateral prefrontal cortex (dlPFC) samples to look at cell-type-specific patterns and evaluated protein changes in the blood of more than 50,000 UK Biobank samples to look for biomarkers of stress-related disorders. After identifying key brain-based genes whose expression was altered in PTSD and/or MDD, the researchers compared them to genes linked to increased risk for these conditions.
Among many findings, the study results show an important role for the mPFC in both stress-related conditions, which is interesting, as the mPFC is essential for integrating signals from other brain areas and is known to play a role in cognitive processes, emotional regulation, motivation, and sociability. The findings also highlight important roles for molecular pathways known to play a role in immune function, the regulation of neurons and neural connections, and stress hormones. The single-cell RNA sequencing in the dlPFC also uncovered dysregulated stress-related signals in neurons and other brain cell types.
Furthermore, the findings reveal shared changes in gene activity between PTSD and MDD, as well as notable differences in the patterns of methyl marks on the DNA, suggesting changes in the way genes are switched on or off, and at the level of cell-type-specific gene activity. The researchers also found that history of childhood trauma and suicide were drivers of molecular changes in both disorders.
The data point to a short list of proteins that may be important in regulating key genetic pathways underlying these disorders. They also reveal links to gene networks related to aging, inflammation, stress, and more. Similarities in disease signals in the brain and blood suggest that blood-based tests might one day offer an additional avenue for assessing these disorders. Interestingly, there was little overlap between PTSD and MDD risk genes and those involved in the underlying molecular-level changes in the brains of people with one or both conditions. This shows that there’s a need for more research into how genetic risk factors are related to molecular-level disease processes.
There’s clearly much more to discover in the years ahead. But these insights already point to important roles for known stress-related pathways in fundamental brain changes underlying PTSD and MDD, while also revealing more novel pathways as potentially promising new treatment targets. With further study, the researchers hope these findings can also begin to answer vexing questions, such as why some people develop PTSD or major depression after stressful events and others don’t.
Reference:
Daskalakis NP, et al. Systems biology dissection of PTSD and MDD across brain regions, cell types, and blood. Science. DOI: 10.1126/science.adh3707 (2024).
This paper is part of a larger collection of studies from the PsychENCODE Consortium looking at the underlying mechanisms of neuropsychiatric diseases.
NIH Support: National Institute of Mental Health
Posted In: Health, News, Science
Tags: biomarkers, brain, depression, mental health, mental health disorders, molecular signature, multiomics, neuroscience, post-traumatic stress disorder, PTSD, stress
Study Suggests Computerized Brain Implant Could One Day Decode Internal Speech for Those Who Can No Longer Speak
Posted on June 6th, 2024 by Dr. Monica M. Bertagnolli

Credit: Tom Merton/KOTO/Adobe Stock
The ability to communicate using only your thoughts might sound like the stuff of science fiction. But for people who don’t have the ability to speak or move due to injury or disease, there’s great hope that this may one day be possible using brain-computer interfaces (BCIs) that can “read” relevant brain signals and translate them into written or spoken words. A research team has made a preliminary advance in this direction by showing for the first time that a computerized brain implant can decode internal speech with minimal training.
In the new NIH-supported study, researchers implanted such a device in a brain area known to be important for representing spoken words called the supramarginal gyrus in two people with tetraplegia, a condition marked by full body paralysis from the neck down due to cervical spinal cord injury. The researchers found that the device could decode several words the participants “spoke” only in their minds. While we are far from using such a device to decode whole sentences or even phrases, and the exact mechanisms of internal speech are still under study, the findings, reported in Nature Human Behavior, are notable because it had been unclear whether the brain signals involved in thinking words could be reproducibly translated.
The findings come from a team led by Richard Andersen at the California Institute of Technology, Pasadena, CA, and Sarah Wandelt, now at the Feinstein Institutes for Medical Research in Manhasset, NY, and the study was supported by the NIH _Brain Research Through Advancing Innovative Neurotechnologies_® (BRAIN) Initiative Research Opportunities in Humans program. Though earlier research had shown that brain implants could decode vocalized, attempted, and mimed speech, it had yet to be seen whether internal speech could be similarly decoded.
An earlier advance in decoding speech signals from the brain came in 2022, when the researchers reported they could accurately predict the words that a person with tetraplegia was thinking using a BCI. In the new study, they’ve shown that the device works in a second person with tetraplegia. The finding is an indication that the approach can work in different individuals and doesn’t depend on the brain characteristics of a particular person or the precise orientation of the implant in their brain.
In the study, the researchers trained their device to recognize brain patterns associated with certain internal “spoken” words including six actual words (battlefield, cowboy, python, spoon, swimming, and telephone) and two nonsense words (nifzig and bindip). During three sessions, the researchers flashed words on a screen and asked each participant to think about “saying” the words without speaking or moving. The BCI then used measurements of brain activity during the sessions and a computer model to predict the words being “spoken” internally.
The researchers found that in this task the device could decode the words with an average accuracy of 79% with the first participant and 23% with the second participant. They noted that the second participant had fewer unique patterns of brain activity associated with the different words, which may explain the lower results. Nevertheless, the findings show that the brain region in question generally contains signals for internal speech, although there is likely also variation among people in how thoughts of particular words are represented in patterns of brain activity. Furthermore, the device’s ability to decode nonsense words suggests that the words are represented in this part of the brain phonetically and not necessarily based on their meanings.
While there is much more to learn about how to decode internal speech more reliably across individuals, the findings offer proof-of-concept for a high-performance internal speech BCI. The new research adds to a growing portfolio of rapidly advancing technologies supported by the BRAIN Initiative that could one day routinely restore the ability to communicate for those who can no longer speak or even move, including people with brain injuries, paralysis, or diseases such as amyotrophic lateral sclerosis (ALS).
Reference:
Wandelt SK, et al. Representation of internal speech by single neurons in human supramarginal gyrus. Nature Human Behaviour. DOI: 10.1038/s41562-024-01867-y (2024).
NIH Support: NIH BRAIN Initiative
Posted In: Health, News, Science
Tags: amyotrophic lateral sclerosis, brain, BRAIN Initiative, brain-computer interfaces, internal speech decoder, neuroscience, paralysis, speech, tetraplegia, thoughts
Most Detailed 3D Reconstruction of Human Brain Tissue Ever Produced Yields Surprising Insights
Posted on May 30th, 2024 by Dr. Monica M. Bertagnolli
Researchers have developed a detailed 3D reconstruction of a cubic millimeter of brain tissue. Credit: Images in video from Google Research & Lichtman Lab, Harvard University. Renderings by D. Berger, Harvard. Video compiled by Donny Bliss/NIH
The NIH _Brain Research Through Advancing Innovative Neurotechnologies_® (BRAIN) Initiative has expanded scientists’ understanding of the human brain in recent years, offering fascinating insights into the ways that individual cells and complex neural circuits interact dynamically to enable us to think, feel, and act. But neuroscientists still have much more to learn about how our brains are put together at the most fundamental, subcellular level.
As a step in that direction, in a new study supported in part by the NIH BRAIN Initiative and reported in the journal Science, researchers have created the most detailed nanoscale resolution map ever produced of a cubic millimeter of brain tissue, about the size of half a grain of rice.
Despite its small size, this fragment of healthy brain contained about 57,000 cells of various types, 230 millimeters of blood vessels, 150 million neural connections, or synapses, and the protective myelin that insulates neurons. To capture it all in vivid detail, the researchers relied on electron microscopy to amass an impressive 1,400 terabytes of imaging data. For perspective, one terabyte of data is enough to store 100,000 photos on your smartphone.
While there are many more details yet to analyze given the sheer quantity of data, this impressively detailed subcellular map has already revealed multiple brain structures that have never been seen before. This includes a class of triangular neurons in deep brain layers being described for the first time. The map also revealed axons, the long extensions of nerve cells that carry electrical impulses, with as many as 50 synapses and other unusual structures, including axons arranged into extensive spiraling patterns that now warrant further study.
The findings come from a team led by Jeff W. Lichtman, Harvard University, Cambridge, MA, and Viren Jain, Google Research, Mountain View, CA. They recognized that fully understanding the human brain requires knowledge of its most basic construction. While the imaging technologies needed to produce this kind of map were available, there were other barriers, including a limited availability of healthy and high-quality human brain tissue samples for study.
Most biopsies of the brain are done to examine or take out abnormal growths of cells or tissues, making them unsuitable for understanding the normal makeup of the brain. In this case, the researchers were able to obtain a tiny sample from the brain tissue removed and destined for disposal during the normal course of surgery for a patient with epilepsy. The researchers first stained the preserved sample to make the cells easier to trace individually before slicing it into 5,000 thin layers for microscopic imaging.
To put those slices back together into a complete 3D reconstruction, the researchers relied on artificial intelligence (AI) models. Because the dataset is too large for any one group to fully analyze, they’ve made it all freely available to the research community in an online resource. They’ve also provided tools for its further analysis and proofreading.
While there is plenty still left to uncover, the findings offer proof-of-principle that it’s possible to visualize the brain at this very detailed level. This is crucial groundwork for new research now supported by the BRAIN Initiative Connectivity Across Scales (BRAIN CONNECTS) program. BRAIN CONNECTS will develop and scale up tools to produce an equally detailed map of a complete mouse brain, which is about 1,000 times larger than the human brain fragment. The researchers now hope their 3D map and others like it will be put to work to understand both normal and disordered brain function more fully.
Reference:
[1] Shapson-Coe A, et al. A petavoxel fragment of human cerebral cortex reconstructed at nanoscale resolution. Science. DOI: 10.1126/science.adk4858 (2024).
NIH Support: NIH BRAIN Initiative, National Institute of Mental Health
Posted In: News, Science, technology
Tags: 3d reconstruction, brain, BRAIN Initiative, brain tissue, data, imaging, neural circuitry, neuroscience, research tools
Speeding the Diagnosis of Rare Genetic Disorders with the Help of Artificial Intelligence
Posted on May 16th, 2024 by Dr. Monica M. Bertagnolli

Credit: Donny Bliss/NIH, Qpt/Shutterstock, taka/Adobe Stock
Millions of children around the world are born each year with severe genetic disorders. Many of these are Mendelian disorders, which are rare genetic conditions caused by mutations in a single gene. But pinpointing the specific gene responsible for a disorder to get a clear diagnosis for an individual can be labor-intensive, and reanalysis of undiagnosed cases is also difficult. As a result, only about 30% of people with a rare genetic disorder get a definitive diagnosis, and on average, it takes 6 years from symptom onset to diagnosis.
Progress is needed to get accurate diagnoses to individuals and families more often and faster, and to create more efficient ways to update genetic diagnoses as new discoveries are made. As an important step in this direction, a team funded in part by NIH has developed a new artificial intelligence (AI) system called AI-MARRVEL (AI-Model organism Aggregated Resources for Rare Variant ExpLoration).1
As reported in NEJM AI by a research team led by Pengfei Liu, Hugo Bellen, and Zhandong Liu at the Baylor College of Medicine and the Jan and Dan Duncan Neurological Research Institute at Texas Children’s Hospital in Houston, AI-MARRVEL relies on a machine learning approach. Machine learning involves using vast quantities of data to train computer systems to become increasingly better at recognizing patterns.
The AI-MARRVEL system was trained using a compendium of data called MARRVEL, previously developed by the research team. MARRVEL integrates genetic information from six human databases and seven model organism databases into one site and includes more than 3.5 million known genetic variants from thousands of healthy individuals as well as those with diagnosed cases of genetic disorders. Using what it has learned from that compendium of data, AI-MARRVEL uses a person’s symptoms and protein-coding genome sequences to narrow down the most likely variants responsible for that person’s genetic condition.
To find out how well it works, the researchers compared the results from AI-MARRVEL to other previously published tools for genetic diagnosis based on three different databases containing established molecular diagnoses from a clinical diagnostic laboratory: Baylor Genetics, the NIH-funded Undiagnosed Diseases Network (UDN), and the Deciphering Developmental Disorders (DDD) project. Overall, the researchers found that AI-MARRVEL consistently made accurate diagnoses in twice as many cases as these other tools.
While hundreds of new disease-causing variants are discovered each year, there’s currently no streamlined way to determine which cases should be reanalyzed when previous sequencing and interpretation failed to identify the cause.2 To see how well AI-MARRVEL does at identifying diagnosable cases from pools of unsolved cases, the researchers designed a confidence metric and found the tool achieved a precision rate of 98% and correctly identified 57% of diagnosable cases out of a collection of 871 cases. The researchers also suggest that AI-MARRVEL could help identify short lists of possible gene candidates in even more potentially solvable cases and then send them on to a panel of experts for follow-up review.
There is some early evidence that AI-MARRVEL could also be put to work in making new discoveries that link novel gene variants to diseases for the first time. In fact, the model already correctly identified two recently reported disease genes in a list of top candidates.
These findings suggest a promising path forward where machine learning could one day make diagnostic decisions in a way that’s comparable to experts, only more efficiently. What’s especially exciting is AI-MARRVEL could have the potential for solving rare disease cases, including those that have remained a mystery for years. The hope is that, by combining the power of AI tools together with the latest sequencing data in the years to come, doctors will be able to get faster diagnoses to many more people with rare genetic disorders.
References:
[1] Mao, D, et al. AI-MARRVEL: A Knowledge-Driven Artificial Intelligence for Molecular Diagnostics of Mendelian Disorders. NEJM AI. DOI: 10.1056/AIoa2300009 (2024).
[2] Liu, P, et al. Reanalysis of Clinical Exome Sequencing Data. N Engl J Med. DOI: 10.1056/NEJMc1812033 (2019).
NIH Support: NIH Common Fund, National Human Genome Research Institute, National Institute of Neurological Disorders and Stroke, Eunice Kennedy Shriver National Institute of Child Health and Human Development