Uncategorized – NIH Director's Blog (original) (raw)
Maternal Brain Hormone Key to Strengthening Bones Could Help Treat Osteoporosis, Bone Fractures
Posted on August 1st, 2024 by Dr. Monica M. Bertagnolli

Credit: Donny Bliss/NIH
More than 200 million people around the world have osteoporosis, a condition that weakens bones to the point that they break easily. Women are at especially high risk after menopause due to declining levels of the hormone estrogen, which helps keep bones strong. While osteoporosis rarely has noticeable symptoms, it can lead to serious injuries when otherwise minor slips and falls cause broken bones that in turn can lead to further fracture risk and fracture-related mortality. So, I’m pleased to share NIH-supported research suggesting a surprising candidate for strengthening bones: a maternal hormone produced in the brain.
The study in mice reported in Nature shows that this newly discovered hormone maintains and rebuilds bone strength in lactating females, even as estrogen levels dip and calcium is lost to the demands of milk production. 1 The findings suggest this hormone—or a drug that acts similarly—could be key to treating osteoporosis and preventing and healing broken bones.
The findings come from a team led by Holly Ingraham, University of California, San Francisco. The researchers knew from studies in mice and humans that a protein related to parathyroid hormone, which is made in the mammary glands, is the main driver for stripping calcium from maternal bones for milk production. As a result of this process, nursing mothers tend to lose a lot of bone. In humans, this bone loss is 10% on average, compared to nearly 30% in mice. Fortunately, these losses are reversed after lactation ends, suggesting to the researchers there must be some other bone-strengthening factor in play.
Previous work in Ingraham’s lab, also supported by NIH, offered other clues. The researchers found that in female mice, blocking a certain estrogen receptor in select neurons in a small area of the brain led to the development of bones that were exceptionally dense and strong. 2 This was an early hint that an unidentified hormone might have a role. The team’s search in this latest study led them to brain-derived communication network factor 3 (CCN3).
The new findings showed that, in lactating female mice, CCN3 is produced in the same brain area identified in the previous study. When the researchers prevented the brain from making CCN3, lactating female mice rapidly lost bone. The researchers also found that male and female young adult and older mice gained a considerable amount of bone mass and strength when their levels of circulating CCN3 were boosted over a two-week period. In fact, in some female mice that were very old or completely lacked estrogen, the hormone more than doubled their bone mass. Tests showed that the animals’ bones weren’t just denser, but also stronger.
Further studies conducted by co-author Thomas Ambrosi, University of California, Davis, revealed that bone stem cells were responsible for receiving signals and generating the new bone. When those cells were exposed to CCN3, they ramped up bone production even more. When the researchers applied a hydrogel patch containing CCN3 to the sites of bone breaks, this spurred the formation of new bone. As a result, the researchers saw rapid bone healing in older mice comparable to what would be expected in much younger mice.
In future studies, the researchers want to gain insight into the underlying mechanisms of CCN3. They also plan to explore the hormone’s potential for treating bone loss in people at increased risk, including postmenopausal women, breast cancer survivors taking estrogen blockers, and those with other conditions leading to unhealthy bone mass, such as genetic bone disorders, chronic kidney disease, or premature ovarian failure. They suggest that more immediate local uses for CCN3 include fracture repair, cartilage regeneration, and bone improvements for anchoring dental implants. It’s a great example of how finding an answer to a scientific puzzle—like how maternal bones stay strong during breastfeeding—can potentially lead to advances that help many more people.
References:
[1] Babey ME, et al. A maternal brain hormone that builds bone. Nature. DOI: 10.1038/s41586-024-07634-3 (2024).
[2] Herber CB, et al. Estrogen signaling in arcuate Kiss1 neurons suppresses a sex-dependent female circuit promoting dense strong bones. Nature Communications. DOI: 10.1038/s41467-018-08046-4 (2019).
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases, National Institute on Aging, National Institute of General Medical Sciences, National Institute of Arthritis and Musculoskeletal and Skin Diseases
Posted In: Health, Science, Uncategorized
Tags: aging, basic research, Bone, bone fracture, breastfeeding, calcium, estrogen, hormone, maternal health, menopause, osteoporosis
Celebrating New Clinical Center Exhibit for Nobel Laureate Dr. Harvey Alter
Posted on February 27th, 2024 by Dr. Monica M. Bertagnolli

On Feb. 14, 2024, NIH hosted an event to open an exhibit on display in the NIH Clinical Center in Bethesda, Maryland, titled, “Harvey Alter and the Discovery of Hepatitis C: Making Our Blood Supply Safe.” Left to right: Anthony Fauci, distinguished professor at the Georgetown University School of Medicine and the McCourt School of Public Policy; Diane Dowling (Harvey Alter’s wife); honoree Harvey Alter; James Gilman, Chief Executive Officer of the NIH Clinical Center; NIH Director Monica Bertagnolli; and NIH Principal Deputy Director Lawrence Tabak. Credit: Chia-Chi Charlie Chang, NIH
Earlier this month, I had the great honor of attending the opening of an exhibit at the NIH Clinical Center commemorating the distinguished career of Dr. Harvey Alter. Harvey’s collaborators, colleagues, and family members joined him to celebrate this display dedicated to his groundbreaking hepatitis C work developed by the Office of NIH History and Stetten Museum.
As I remarked at the event, we at NIH are proud to be able to claim Harvey as our own. He has spent almost the entirety of his professional career at the Clinical Center, working as a scientist in the Department of Transfusion Medicine since the 1960s.
Those who view this permanent exhibit will learn about how Harvey’s dedicated research has transformed the safety of the U.S. blood supply. Before the 1970s, nearly a third of patients who received multiple, lifesaving blood transfusions contracted hepatitis. Today, the risk of contracting hepatitis from a blood transfusion is essentially zero, thanks largely to Harvey’s research advances, including his work to identify the hepatitis C virus, which earned him the 2020 Nobel Prize in Physiology or Medicine. A cure for hepatitis C became available in 2014, and former NIH Director Dr. Francis Collins, who was at the event, has been working with President Biden to ensure greater access to these medications as part of an effort to eliminate hepatitis C in this country. This important work would not have been possible without Harvey’s foundational discoveries. Harvey is one of six Nobelists who did the entirety of their award-winning research at NIH as federal scientists, and the only NIH Nobel laureate to be recognized for clinical research.
This exhibit in the busy halls of the Clinical Center is a good reminder to the many who pass by of why we do what we do: It can take long hours and many years, but we can make a significant impact in clinical care when we try to understand the root causes of problems. Please stop by when you’re there to learn more about Harvey’s remarkable career.

For HIV, Treatment is Prevention
Posted on January 22nd, 2019 by Dr. Francis Collins
For almost four decades, researchers have worked tirelessly to find a cure for the human immunodeficiency virus (HIV), which causes AIDS. There’s still more work to do, but a recent commentary published in JAMA [1] by Anthony Fauci, director of NIH’s National Institute of Allergy and Infectious Diseases, and his colleagues serves as a reminder of just how far we’ve come. Today, thanks to scientific advances, especially the development of effective antiretroviral therapy (ART), most people living with HIV can live full and productive lives. These developments have started to change how our society views HIV infection.
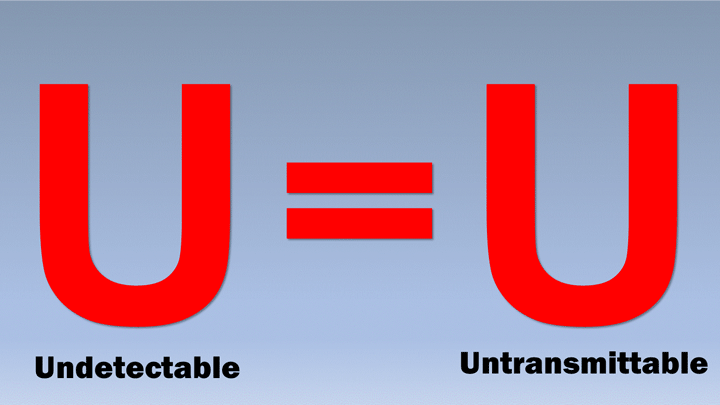
In their commentary, the NIH scientists describe the painstaking research that has now firmly established that people who take ART daily as prescribed, and who achieve and maintain an undetectable viral load (the amount of HIV in the blood), cannot sexually transmit the virus to others. To put it simply: Undetectable = Untransmittable (U=U).
The U=U message was introduced in 2016 by the Prevention Access Campaign, an international health equity initiative that aims to help end the HIV epidemic and HIV-related social stigma. The major breakthrough in combination ART regimens, which successfully reduced viral loads for many HIV patients, came over 20 years ago. But their importance for HIV prevention wasn’t immediately apparent.
There’d been some hints of U=U, but it was the results of the NIH-funded HIV Prevention Trials Network (HPTN) 052, published in The New England Journal of Medicine [2] in 2011, that offered the first rigorous clinical evidence. Among heterosexual couples in the randomized clinical trial, no HIV transmissions to an uninfected partner were observed when ART consistently, durably suppressed the virus in the partner living with HIV.
The data provided convincing evidence that ART not only treats HIV but also prevents the sexual transmission of HIV infection. The public health implications of what’s sometimes referred to as “treatment as prevention” were obvious and exciting. In fact, the discovery made Science’s 2011 list of top 10 Breakthroughs of the Year .
Three subsequent studies, known as PARTNER 1 and 2 and Opposites Attract, confirmed and extended the findings of the HPTN 052 study. All three showed that people with HIV taking ART, who had undetectable HIV levels in their blood, had essentially no risk of passing the virus on to their HIV-negative partners.
Of course, the success of U=U depends on people with HIV having the needed access to health care and taking their medications as prescribed every day of their lives [3]. ART works by preventing the virus from making more copies of itself. It’s important to note that achieving an undetectable viral load with treatment can take time—up to 6 months. Viral load testing should be performed on a regular basis to ensure that the virus remains at undetectable levels. If treatment is stopped, the virus typically rebounds within a matter of weeks. So, strict adherence to ART over the long term is absolutely essential.
Practically speaking, though, ART alone won’t be enough to end the spread of HIV, and other methods of HIV prevention are still needed. In fact, we’re now at a critical juncture in HIV research as work continues on preventive vaccines that could one day bring about a durable end to the pandemic.
But for now, there are more than 35 million people worldwide who are HIV positive [4]. With currently available interventions, experts have predicted that about 50 million people around the world will become HIV positive from 2015 to 2035 [5]. Work is proceeding actively on the vaccine, and also on ways to totally eradicate the virus from infected individuals (a “cure”), but that is proving to be extremely challenging.
Meanwhile, with continued advances, including improved accessibility to testing, adherence to existing medications, and use of pre-exposure prophylaxis (PrEP) in high risk individuals, the goal is to reduce greatly the number of new cases of HIV/AIDS.
References:
[1] HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. Eisinger RW, Dieffenbach CW, Fauci AS. JAMA. 2019 Jan 10.
[2] Prevention of HIV-1 infection with early antiretroviral therapy. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR; HPTN 052 Study Team. N Engl J Med. 2011 Aug 11;365(6):493-505.
[3] HIV Treatment (U.S. Department of Health and Human Services)
[4] HIV/AIDS (World Health Organization)
[5] Effectiveness of UNAIDS targets and HIV vaccination across 127 countries. Medlock J, Pandey A, Parpia AS, Tang A, Skrip LA, Galvani AP. Proc Natl Acad Sci U S A. 2017 Apr 11;114(15):4017-4022.
Links:
HIV/AIDS (National Institute of Allergy and Infectious Diseases/NIH)
Treatment as HIV Prevention (NIAID)
Anthony S. Fauci (NIAID)
HIV Prevention Trials Network (Durham, NC)
Posted In: News, Uncategorized
Tags: AIDS, antiretroviral therapy, art, HIV, HIV epidemic, HIV prevention, HIV Prevention Trials Network, HIV treatment, HIV vaccine, HIV/AIDS, human immunodeficiency virus, pandemic, Partnership for Public Service, prevention, Prevention Access Campaign, Science Breakthrough of the Year, social stigma, U=U, Undetectable=Untransmittable, viral load
Gene Editing: Gold Nanoparticle Delivery Shows Promise
Posted on October 10th, 2017 by Dr. Francis Collins
 About a month ago, I had the pleasure of welcoming the Juip (pronounced “Yipe”) family from Michigan to NIH. Although you’d never guess it from this photo, two of the Juip’s five children—9-year-old Claire and 11-year-old Jake (both to my left)—have a rare genetic disease called Friedreich’s ataxia (FA). This inherited condition causes progressive damage to their nervous systems and their hearts. No treatment currently exists for kids like Claire and Jake, yet this remarkable family has turned this serious health challenge into an opportunity to raise awareness about the need for biomedical research.
About a month ago, I had the pleasure of welcoming the Juip (pronounced “Yipe”) family from Michigan to NIH. Although you’d never guess it from this photo, two of the Juip’s five children—9-year-old Claire and 11-year-old Jake (both to my left)—have a rare genetic disease called Friedreich’s ataxia (FA). This inherited condition causes progressive damage to their nervous systems and their hearts. No treatment currently exists for kids like Claire and Jake, yet this remarkable family has turned this serious health challenge into an opportunity to raise awareness about the need for biomedical research.
One thing that helps keep the Juips optimistic is the therapeutic potential of CRISPR/Cas9, an innovative gene editing system that may someday make it possible to correct the genetic mutations responsible for FA and many other conditions. So, I’m sure the Juips were among those encouraged by the recent news that NIH-funded researchers have developed a highly versatile approach to CRISPR/Cas9-based therapies. Instead of relying on viruses to carry the gene-editing system into cells, the new approach uses tiny particles of gold as the delivery system!
Posted In: Health, Science, technology, Uncategorized
Tags: CRISPR, CRISPR-Gold, CRISPR/Cas9, DMD, Duchenne muscular dystrophy, dystrophin, FA, Friedreich’s ataxia, gene editing, Juip, rare diseases, stem cells