biotechnology – NIH Director's Blog (original) (raw)
New Tool Predicts Response to Immunotherapy in Lung Cancer Patients
Posted on April 4th, 2023 by Douglas M. Sheeley, Sc.D., NIH Common Fund

Credit: XVIVO Scientific Animation, Wethersfield, CT
With just a blood sample from a patient, a promising technology has the potential to accurately diagnose non-small cell lung cancer (NSCLC), the most-common form of the disease, more than 90 percent of the time. The same technology can even predict from the same blood sample whether a patient will respond well to a targeted immunotherapy treatment.
This work is a good example of research supported by the NIH Common Fund. Many Common Fund programs support development of new tools that catalyze research across the full spectrum of biomedical science without focusing on a single disease or organ system.
The emerging NSCLC prediction technology was developed as part of our Extracellular RNA Communication Program. The program develops technologies to understand RNA circulating in the body, known as extracellular RNA (exRNA). These molecules can be easily accessed in bodily fluids such as blood, urine, and saliva, and they have enormous potential as biomarkers to better understand cancer and other diseases.
When the body’s immune system detects a developing tumor, it activates various immune cells that work together to kill the suspicious cells. But many tumors have found a way to evade the immune system by producing a protein called PD-L1.
Displayed on the surface of a cancer cell, PD-L1 can bind to a protein found on immune cells with the similar designation of PD-1. The binding of the two proteins keeps immune cells from killing tumor cells. One type of immunotherapy interferes with this binding process and can restore the natural ability of the immune system to kill the tumor cells.
However, tumors differ from person to person, and this form of cancer immunotherapy doesn’t work for everyone. People with higher levels of PD-L1 in their tumors generally have better response rates to immunotherapy, and that’s why oncologists test for the protein before attempting the treatment.
Because cancer cells within a tumor can vary greatly, a single biopsy taken at a single site in the tumor may miss cells with PD-L1. In fact, current prediction technologies using tissue biopsies correctly predict just 20 – 40 percent of NSCLC patients who will respond well to immunotherapy. This means some people receive immunotherapy who shouldn’t, while others don’t get it who might benefit.
To improve these predictions, a research team led by Eduardo Reátegui, The Ohio State University, Columbus, engineered a new technology to measure exRNA and proteins found within and on the surface of extracellular vesicles (EVs) [1]. EVs are tiny molecular containers released by cells. They carry RNA and proteins (including PD-L1) throughout the body and are known to play a role in communication between cells.
As the illustration above shows, EVs can be shed from tumors and then circulate in the bloodstream. That means their characteristics and internal cargo, including exRNA, can provide insight into the features of a tumor. But collecting EVs, breaking them open, and pooling their contents for assessment means that molecules occurring in small quantities (like PD-L1) can get lost in the mix. It also exposes delicate exRNA molecules to potential breakdown outside the protective EV.
The new technology solves these problems. It sorts and isolates individual EVs and measures both PD-1 and PD-L1 proteins, as well as exRNA that contains their genetic codes. This provides a more comprehensive picture of PD-L1 production within the tumor compared to a single biopsy sample. But also, measuring surface proteins and the contents of individual EVs makes this technique exquisitely sensitive.
By measuring proteins and the exRNA cargo from individual EVs, Reátegui and team found that the technology correctly predicted whether a patient had NSCLC 93.2 percent of the time. It also predicted immunotherapy response with an accuracy of 72.2 percent, far exceeding the current gold standard method.
The researchers are working on scaling up the technology, which would increase precision and allow for more simultaneous measurements. They are also working with the James Comprehensive Cancer Center at The Ohio State University to expand their testing. That includes validating the technology using banked clinical samples of blood and other bodily fluids from large groups of cancer patients. With continued development, this new technology could improve NSCLC treatment while, critically, lowering its cost.
The real power of the technology, though, lies in its flexibility. Its components can be swapped out to recognize any number of marker molecules for other diseases and conditions. That includes other cancers, neurodegenerative diseases, traumatic brain injury, viral diseases, and cardiovascular diseases. This broad applicability is an example of how Common Fund investments catalyze advances across the research spectrum that will help many people now and in the future.
Reference:
[1] An immunogold single extracellular vesicular RNA and protein (AuSERP) biochip to predict responses to immunotherapy in non-small cell lung cancer patients. Nguyen LTH, Zhang J, Rima XY, Wang X, Kwak KJ, Okimoto T, Amann J, Yoon MJ, Shukuya T, Chiang CL, Walters N, Ma Y, Belcher D, Li H, Palmer AF, Carbone DP, Lee LJ, Reátegui E. J Extracell Vesicles. 11(9):e12258. doi: 10.1002/jev2.12258.
Links:
Video: Unlocking the Mysteries of Extracellular RNA Communication (Common Fund)
Extracellular RNA Communication Program (ERCC) (Common Fund)
Upcoming Meeting: ERCC19 Research Meeting (May 1-2, 2023)
Eduardo Reátegui Group for Bioengineering Research (The Ohio State University College of Engineering, Columbus)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 27th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
Posted In: Generic
Tags: Aspirin in Reducing Events in the Elderly, biotechnology, cancer, cancer immunotherapy, Common Fund, exRNA, extracellular RNA, extracellular vesicles, immunotherapy, non-small cell lung cancer, NSCLC, PD-1, PD-1 inhibitors, PD-L1, RNA
On-the-Spot Gene Readouts Offer Clues to How Cells Work
Posted on March 11th, 2021 by Dr. Francis Collins

Credit: MIT and Harvard Medical School, Cambridge, MA
Just as two companies can merge to expand their capabilities, two technologies can become more powerful when integrated into one. That’s why researchers recently merged two breakthrough technologies into one super powerful new method called ExSeq. The two-in-one technology enables researchers for the first time to study an intact tissue sample and track genetic activity on the spot within a cell’s tiniest recesses, or microenvironments—areas that have been largely out of reach until now.
ExSeq, which is described in a paper in the journal Science [1], will unleash many new experimental applications. Beyond enabling more precise analysis of the basic building blocks of life, these applications include analyzing tumor biopsies more comprehensively and even unlocking mysteries of how the brain works. The latter use is on display in this colorful cross-section of a mouse’s hippocampus, a region of the brain involved in the memory of facts and events.
Here you can see in precise and unprecedented detail the areas where genes are activated (magenta) in the brain’s neurons (green). In this particular example, the genes are working within subregions of the hippocampus called the CA1 and dentate gyrus regions (white, bottom and top left).
ExSeq is a joint effort from NIH grantees Ed Boyden, Massachusetts Institute of Technology (MIT), Cambridge, and George Church, Harvard Medical School, Boston. The new method combines a technology called tissue expansion with an _in sit_u sequencing approach.
Tissue expansion swells the contents of tissue sections up to 100 times their normal size but retains their same physical structure [2]. It’s sort of like increasing the font size and line spacing on a hard-to-read document. It makes cellular details that were outside the resolution range of the light microscope suddenly accessible.
With the information inside cells now easier to see, the next step involves a technique called FISSEQ (fluorescent in situ sequencing), which generates readouts of thousands of mRNA molecules in cells [3]. FISSEQ works by detecting individual RNA molecules where they are inside cells and amplifying them into “nanoballs,” or rolled-up copies of themselves. Each nanoball can be read using standard sequencing methods and a fluorescence microscope.
Using the combined ExSeq approach, the team can analyze precisely where gene activity changes within tiny cellular microenvironments. Or, it can compile a more-comprehensive readout of gene activity within cells by analyzing as many gene readouts as detectable. When used in the hippocampus, this untargeted, “agnostic” approach led to some surprises—revealing unusual forms of RNA and, by association, genes for proteins not previously linked with communication between neurons.
Like many technology developments, the scientists envision that ExSeq can be used in many ways, including for more precise analysis of tumor biopsies. To illustrate this point, the researchers analyzed breast cancer metastases, which are cells from breast tumors that have spread to other areas in the body. Metastases contain many different cell types, including cancer cells and immune cells.
Using ExSeq, Boyden and Church learned that these distinct cell types can behave differently depending on where they are inside a tumor. They discovered, for example, that immune B cells near tumor cells expressed certain inflammatory genes at a higher level than immune B cells that were further away. Precise information about a tumor’s composition and activity may lead to development of more targeted approaches to attack it.
Many discoveries come on the heels of transformative new technologies. ExSeq shines a much brighter light on the world of the very small. And that should help us better understand how different parts of cells work together, as well as how cells work with each other in the brain, in cancer, and throughout the body.
References:
[1] Expansion sequencing: Spatially precise in situ transcriptomics in intact biological systems. Alon S, Goodwin DR, Sinha A, Wassie AT, et al. Science. 2021 Jan 29;37:eaax2656.
[2] Expansion microscopy. Chen F, Tillberg PW, Boyden ES. Science. 2015;347:543-548.
[3]. Highly multiplexed subcellular RNA sequencing in situ. Lee JH, Daugharthy ER, Scheiman J, Kalhor R, et al. Science. 2014;343:1360-1363.
Links:
Ribonucleic Acid (RNA) (National Human Genome Research Institute/NIH)
Synthetic Neurobiology Group (Massachusetts Institute of Technology, Cambridge)
George Church (Harvard Medical School, Boston)
NIH Support: National Human Genome Research Institute; National Cancer Institute; National Institute of Biomedical Imaging and Bioengineering; National Institute of Mental Health; National Institute of Neurological Disorders and Stroke
Posted In: Snapshots of Life
Tags: B cells, biotechnology, brain, brain imaging, breast cancer, cancer imaging, expansion sequencing, ExSeq, FISSEQ, fluorescence in situ sequencing of RNA, gene activity, gene expression, gene readout, genes, genomics, hippocampus, imaging, inflammatory genes, metastases, microscopy, mRNA, nanoballs, neurons, technology, tissue expansion, tissue microenvironment
Meet the Researcher Leading NIH’s COVID-19 Vaccine Development Efforts
Posted on July 9th, 2020 by Dr. Francis Collins

A safe, effective vaccine is the ultimate tool needed to end the coronavirus disease 2019 (COVID-19) pandemic. Biomedical researchers are making progress every day towards such a vaccine, whether it’s devising innovative technologies or figuring out ways to speed human testing. In fact, just this week, NIH’s National Institute of Allergy and Infectious Diseases (NIAID) established a new clinical trials network that will enroll tens of thousands of volunteers in large-scale clinical trials testing a variety of investigational COVID-19 vaccines.
Among the vaccines moving rapidly through the development pipeline is one developed by NIAID’s Dale and Betty Bumpers Vaccine Research Center (VRC), in partnership with Moderna, Inc., Cambridge, MA. So, I couldn’t think of a better person to give us a quick overview of the COVID-19 vaccine research landscape than NIH’s Dr. John Mascola, who is Director of the VRC. Our recent conversation took place via videoconference, with John linking in from his home in Rockville, MD, and me from my place in nearby Chevy Chase. Here’s a condensed transcript of our chat:
Collins: Vaccines have been around since Edward Jenner and smallpox in the late 1700s. But how does a vaccine actually work to protect someone from infection?
Mascola: The immune system works by seeing something that’s foreign and then responding to it. Vaccines depend on the fact that if the immune system has seen a foreign protein or entity once, the second time the immune response will be much brisker. So, with these principles in mind, we vaccinate using part of a viral protein that the immune system will recognize as foreign. The response to this viral protein, or antigen, calls in specialized T and B cells, the so-called memory cells, and they remember the encounter. When you get exposed to the real thing, the immune system is already prepared. Its response is so rapid that you clear the virus before you get sick.
Collins: What are the steps involved in developing a vaccine?
Mascola: One can’t make a vaccine, generally speaking, without knowing something about the virus. We need to understand its surface proteins. We need to understand how the immune system sees the virus. Once that knowledge exists, we can make a candidate vaccine in the laboratory pretty quickly. We then transfer the vaccine to a manufacturing facility, called a pilot plant, that makes clinical grade material for testing. When enough testable material is available, we do a first-in-human study, often at our vaccine clinic at the NIH Clinical Center.
If those tests look promising, the next big step is finding a pharmaceutical partner to make the vaccine at large scale, seek regulatory approval, and distribute it commercially. That usually takes a while. So, from start to finish, the process often takes five or more years.
Collins: With this global crisis, we obviously don’t have five years to wait. Tell us about what the VRC started to do as soon as you learned about the outbreak in Wuhan, China.
Mascola: Sure. It’s a fascinating story. We had been talking with NIAID Director Dr. Anthony Fauci and our colleagues about how to prepare for the next pandemic. Pretty high on our list were coronaviruses, having already worked on past outbreaks of SARS and MERS [other respiratory diseases caused by coronaviruses]. So, we studied coronaviruses and focused on the unique spike protein crowning their surfaces. We designed a vaccine that presented the spike protein to the immune system.
Collins: Knowing that the spike protein was likely your antigen, what was your approach to designing the vaccine?
Mascola: Our approach was a nucleic acid-based vaccine. I’m referring to vaccines that are based on genetic material, either DNA or RNA. It’s this type of vaccine that can be moved most rapidly into the clinic for initial testing.
When we learned of the outbreak in Wuhan, we simply accessed the nucleic acid sequence of SARS-CoV-2, the novel coronavirus that causes COVID-19. Most of the sequence was on a server from Chinese investigators. We looked at the spike sequence and built that into an RNA vaccine. This is called in silico vaccine design. Because of our experience with the original SARS back in the 2000s, we knew its sequence and we knew this approach worked. We simply modified the vaccine design to the sequence of the spike protein of SARS-CoV-2. Literally within days, we started making the vaccine in the lab.
At the same time, we worked with a biotechnology company called Moderna that creates personalized cancer vaccines. From the time the sequence was made available in early January to the start of the first in-human study, it was about 65 days.
Collins: Wow! Has there ever been a vaccine developed in 65 days?
Mascola: I don’t think so. There are a lot of firsts with COVID, and vaccine development is one of them.
Collins: For the volunteers who enrolled in the phase 1 study, what was actually in the syringe?
Mascola: The syringe included messenger RNA (mRNA), the encoded instructions for making a specific protein, in this case the spike protein. The mRNA is formulated in a lipid nanoparticle shell. The reason is mRNA is less stable than DNA, and it doesn’t like to hang around in a test tube where enzymes can break it down. But if one formulates it just right into a nanoparticle, the mRNA is protected. Furthermore, that protective particle allows one to inject it into muscle and facilitates the uptake of the mRNA into the muscle cells. The cells translate the mRNA into spike proteins, and the immune system sees them and mounts a response.
Collins: Do muscle cells know how to take that protein and put it on their cell surfaces, where the immune system can see it?
Mascola: They do if the mRNA is engineered just the right way. We’ve been doing this with DNA for a long time. With mRNA, the advantage is that it just has to get into the cell [not into the nucleus of the cell as it does for DNA]. But it took about a decade of work to figure out how to do nucleotide silencing, which allows the cell to see the mRNA, not destroy it, and actually treat it as a normal piece of mRNA to translate into protein. Once that was figured out, it becomes pretty easy to make any specific vaccine.
Collins: That’s really an amazing part of the science. While it seems like this all happened in a blink of an eye, 65 days, it was built on years of basic science work to understand how cells treat mRNA. What’s the status of the vaccine right now?
Mascola: Early data from the phase 1 study are very encouraging. There’s a manuscript in preparation that should be out shortly showing that the vaccine was safe. It induced a very robust immune response to that spike protein. In particular, we looked for neutralizing antibodies, which are the ones that attach to the spike, blocking the virus from binding to a cell. There’s a general principle in vaccine development: if the immune system generates neutralizing antibodies, that’s a very good sign.
Collins: You’d be the first to say that you’re not done yet. Even though those are good signs, that doesn’t prove that this vaccine will work. What else do you need to know?
Mascola: The only real way to learn if a vaccine works is to test it in people. We break clinical studies into phases 1, 2, and 3. Phase 1 has already been done to evaluate safety. Phase 2 is a larger evaluation of safety and immune response. That’s ongoing and has enrolled 500 or 600 people, which is good. The plan for the phase 3 study will be to start in July. Again, that’s incredibly fast, considering that we didn’t even know this virus existed until January.
Collins: How many people do you need to study in a phase 3 trial?
Mascola: We’re thinking 20,000 or 30,000.
Collins: And half get the vaccine and half get a placebo?
Mascola: Sometimes it can be done differently, but the classic approach is half placebo, half vaccine.
Collins: We’ve been talking about the VRC-Moderna nucleic acid vaccine. But there are others that are coming along pretty quickly. What other strategies are being employed, and what are their timetables?
Mascola: There are many dozens of vaccines under development. The response has been extraordinary by academic groups, biotech companies, pharmaceutical companies, and NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership. I don’t think I’ve ever seen so much activity in a vaccine space moving ahead at such a rapid clip.
As far as being ready for advanced clinical trials, there are a just handful and they involve different types of vaccines. At least three nucleic acid vaccines are in clinical trials. There are also two vaccines that use proteins, which is a more classic approach.
In addition, there are several vaccines based on a viral vector. To make these, one puts the genes for the spike protein inside an adenovirus, which is an innocuous cold virus, and injects it into muscle. In regard to phase 3 trials, there are maybe three or four vaccines that could be formally in such tests by the fall.
Collins: How is it possible to do this so much more rapidly than in the past, without imposing risks?
Mascola: It’s a really important question, Francis. A number of things are being done in parallel, and that wouldn’t usually be the case. We can get a vaccine into a first-in-human study much more quickly because of time-saving technologies.
But the real important point is that for the phase 3 trial, there are no timesavers. One must enroll 30,000 people and watch them over months in a very rigorous, placebo-controlled environment. The NIH has stood up what’s called a Data Safety Monitoring Board for all the trials. That’s an independent group of investigators that will review all vaccine trial data periodically. They can see what the data are showing: Should the trial be stopped early because the vaccine is working? Is there a safety signal that raises concern?
While the phase 3 trial is going on, the U.S. government also will be funding large-scale manufacture of the vaccine. Traditionally, you would do the vaccine trial, wait until it’s all done, and analyze the data. If it worked, you’d build a vaccine plant to make enough material, which takes two or three years, and then go to the Food and Drug Administration (FDA) for regulatory approval.
Everything here is being done in parallel. So, if the vaccine works, it’s already in supply. And we have been engaging the FDA to get real-time feedback. That does save a lot of time.
Collins: Is it possible that we’ll manufacture a whole lot of doses that may have to be thrown out if the vaccine doesn’t work?
Mascola: It certainly is possible. One would like to think that for coronaviruses, vaccines are likely to work, in part because the natural immune response clears them. People get quite sick, but eventually the immune system clears the virus. So, if we can prime it with a vaccine, there is reason to believe vaccines should work.
Collins: If the vaccine does work, will this be for lifelong prevention of COVID-19? Or will this be like the flu, where the virus keeps changing and new versions of the vaccine are needed every year?
Mascola: From what we know about coronaviruses, we think it’s likely COVID-19 is not like the flu. Coronaviruses do have some mutation rate, but the data suggest it’s not as rapid as influenza. If we’re fortunate, the vaccine won’t need to be changed. Still, there’s the matter of whether the immunity lasts for a year, five years, or 10 years. That we don’t know without more data.
Collins: Do we know for sure that somebody who has had COVID-19 can’t get it again a few months later?
Mascola: We don’t know yet. To get the answer, we must do natural history studies, where we follow people who’ve been infected and see if their risk of getting the infection is much lower. Although classically in virology, if your immune system shows neutralizing antibodies to a virus, it’s very likely you have some level of immunity.
What’s a bit tricky is there are people who get very mild symptoms of COVID-19. Does that mean their immune system only saw a little bit of the viral antigen and didn’t respond very robustly? We’re not sure that everyone who gets an infection is equally protected. That’s going to require a natural history study, which will take about a year of follow-up to get the answers.
Collins: Let’s go back to trials that need to happen this summer. You talked about 20,000 to 30,000 people needing to volunteer just for one vaccine. Whom do you want to volunteer?
Mascola: The idea with a phase 3 trial is to have a broad spectrum of participation. To conduct a trial of 30,000 people is an enormous logistical operation, but it has been done for the rotavirus and HPV vaccines. When you get to phase 3, you don’t want to enroll just healthy adults. You want to enroll people who are representative of the diverse population that you want to protect.
Collins: Do you want to enrich for high-risk populations? They’re the ones for whom we hope the vaccine will provide greatest benefit: for example, older people with chronic illnesses, African Americans, and Hispanics.
Mascola: Absolutely. We want to make sure that we can feel comfortable to recommend the vaccine to at-risk populations.
Collins: Some people have floated another possibility. They ask why do we need expensive, long-term clinical trials with tens of thousands of people? Couldn’t we do a human challenge trial in which we give the vaccine to some healthy, young volunteers, wait a couple of weeks, and then intentionally expose them to SARS-CoV-2. If they don’t get sick, we’re done. Are challenge studies a good idea for COVID-19?
Mascola: Not right now. First, one has to make a challenge stock of the SARS-CoV-2 that’s not too pathogenic. We don’t want to make something in the lab that causes people to get severe pneumonia. Also, for challenge studies, it would be preferable to have a very effective small drug or antibody treatment on hand. If someone were to get sick, you could take care of the infection pretty readily with the treatments. We don’t have curative treatments, so the current thinking is we’re not there yet for COVID-19 challenge studies [1]. If you look at our accelerated timeline, formal vaccine trials still may be the fastest and safest way to get the answers.
Collins: I’m glad you’re doing it the other way, John. It’s going to take a lot of effort. You’re going to have to go somewhere where there is still ongoing spread, otherwise you won’t know if the vaccine works or not. That’s going to be tricky.
Mascola: Yes. How do we know where to test the vaccine? We are using predictive analytics, which is just a fancy way of saying that we are trying to predict where in the country there will be ongoing transmission. If we can get really good at it, we’ll have real-time data to say transmission is ongoing in a certain area. We can vaccinate in that community, while also possibly protecting people most at risk.
Collins: John, this conversation has been really informative. What’s your most optimistic view about when we might have a COVID-19 vaccine that’s safe and effective enough to distribute to the public?
Mascola: An optimistic scenario would be that we get an answer in the phase 3 trial towards the end of this year. We have scaled up the production in parallel, so the vaccine should be available in great supply. We still must allow for the FDA to review the data and be comfortable with licensing the vaccine. Then we must factor in a little time for distributing and recommending that people get the vaccine.
Collins: Well, it’s wonderful to have someone with your skills, experience, and vision taking such a leading role, along with your many colleagues at the Vaccine Research Center. People like Kizzmekia Corbett, Barney Graham, and all the others who are a part of this amazing team that you’ve put together, overseen by Dr. Fauci.
While there is still a ways to go, we can take pride in how far we have come since this virus emerged just about six months ago. In my 27 years at NIH, I’ve never seen anything quite like this. There’s been a willingness among people to set aside all kinds of other concerns. They’ve gathered around the same table, worked on vaccine design and implementation, and gotten out there in the real world to launch clinical trials.
John, thank you for what you are doing 24/7 to make this kind of progress possible. We’re all watching, hoping, and praying that this will turn out to be the answer that people desperately need after such a terribly difficult time so far in 2020. I believe 2021 will be a very different kind of experience, largely because of the vaccine science that we’ve been talking about today.
Mascola: Thank you so much, Francis. And thanks for recognizing all the people behind the scenes who are making this happen. They’re working really hard!
Reference:
[1] Accelerating Development of SARS-CoV-2 Vaccines—The Role for Controlled Human Infection Models. Deming ME, Michael, NL, Robb M, Cohen MS, Neuzil KM. N Engl J Med. 2020 July 1. [Epub ahead of print].
Links:
Coronavirus (COVID-19) (NIH)
John R. Mascola (National Institute of Allergy and Infectious Diseases/NIH)
Novel Vaccine Technologies for the 21st Century. Mascola JR, Fauci AS. Nat Rev Immunol. 2020 Feb;20(2):87-88.
Vaccine Research Center (NIAID/NIH)
Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)
Posted In: Generic
Tags: Accelerating COVID-19 Therapeutic Interventions and Vaccines, ACTIV, Anthony Fauci, Barney Graham, biotechnology, challenge trial, coronavirus, COVID-19, COVID-19 Prevention Trials Network, COVID-19 vaccine, COVPN, Data Safety Monitoring Board, Edward Jenner, FDA, first-in-human, John Mascola, Kizzmekia Corbett, Moderna, mRNA, mRNA vaccine, NIH Clinical Center, novel coronavirus, nucleic acid-based vaccine, pandemic, SARS-CoV-2, spike protein, Vaccine Research Center, vaccines
Combating Mosquitoes with an Engineered Fungus
Posted on June 11th, 2019 by Dr. Francis Collins

Caption: Anopheles coluzzii mosquito with transgenic fungus (green) emerging from its body after death. Credit: Brian Lovett, University of Maryland, College Park
Almost everywhere humans live on this planet, mosquitoes carry microbes that cause potentially deadly diseases, from West Nile virus to malaria. While chemical insecticides offer a line of defense, mosquito populations often grow resistant to them. So, it’s intriguing to learn that we may now have another ally in this important fight: a genetically engineered fungus!
Reporting in the journal Science, an international research team supported by NIH describes how this new approach might be used to combat malaria [1]. A fungus called Metarhizium pingshaense is a natural enemy of the mosquito, but, by itself, it kills mosquitoes too slowly to control transmission of malaria. To make this fungus an even more efficient mosquito killer, researchers engineered it to carry a gene encoding a toxin, derived from a spider, that is deadly to insects. Tests of the souped-up fungus in a unique contained facility designed to simulate a West African village found it safely and rapidly killed insecticide-resistant mosquitoes, reducing their numbers by more than 99 percent within 45 days.
Mosquitoes are the deadliest animals in the world. More than 3.2 billion people—about half of all humans—are at risk for malaria, and more than 400,000 die each year from the disease. Other mosquito-borne illnesses, including Zika and dengue viruses, sicken millions more each year. By combining existing insect control strategies with the latest technical innovation, it should be possible to lower those numbers.
In the latest study, Raymond St. Leger and Brian Lovett, University of Maryland, College Park, teamed with Abdoulaye Diabate and colleagues from Institut de Recherche en Sciences de la Santé/Cente Muraz, Burkina Faso, West Africa. The researchers employed a strategy that’s been in use around the world for more than 100 years to control agricultural pests.
The approach involves the fungal species Metarhizium, which kills a variety of insects. Earlier studies had shown that spores from a specific Metarhizium strain could make a big enough dent in a mosquito population to raise the possibility of using the fungus to reduce infective bites among humans [2]. But killing off the mosquitoes required very large quantities of fungal spores and usually took a couple of weeks.
Here’s where things turned innovative. To boost the fungus’s potency, St. Leger and colleagues used genetic engineering to add a toxin derived from the Australian Blue Mountains funnel-web spider. The toxin came with a major advantage: the U.S. Environmental Protection Agency (EPA) already has approved its use as a safe-and-effective insecticidal protein.
Besides giving the engineered fungus that ability to produce a spider toxin, the researchers added another clever element. They didn’t want the fungus to produce the toxin all the time—only after it comes in contact with a mosquito’s hemolymph, the insect equivalent of blood. So, they needed to insert a control switch, and the researchers knew just where to find the needed part.
Once inside a mosquito, the fungus naturally produces a structural protein called collagen that shields it from the insect’s immune system. A genetic switch that turns “on” when it detects an insect’s hemolymph controls that collagen production. To ensure that the spider toxin was produced at just the right time, the researchers hotwired their Metarhizium to begin producing it under the control of this same genetic switch.
The next step was to test this modified organism in a more natural, but controlled, environment. The researchers spent more than a year in Burkina Faso building a specialized facility called a MosquitoSphere. It’s similar to a very large greenhouse, but with mosquito netting instead of glass.
The MosquitoSphere has six separate compartments, four of which contain West African huts, along with native plants and breeding sites for mosquitoes. The researchers hung a black cotton sheet, previously soaked in sesame oil, on the wall of a hut in each of three chambers.
In one hut, the sesame oil contained genetically engineered fungal spores. In the second hut, the oil contained natural fungal spores. In the third hut, there were no spores at all. Then, they released 1,000 adult male and 500 adult female mosquitoes into each chamber and watched what happened over the next 45 days.
In the hut without spores, the mosquitoes established a stable population of almost 1,400. In the chamber with the natural spores, 450 mosquitoes survived. But, in the chamber with the engineered fungus, the researchers counted just 13 survivors—too few to sustain a viable population.
The researchers say they suspect the fungus would be relatively easy to contain in nature. It’s sticky and not easily airborne. The spores are also extremely sensitive to sunlight, making it difficult for them to travel far. Importantly, the fungus didn’t harm other beneficial insects, including honeybees.
Caution is warranted before considering the release of a genetically engineered organism into the wild. In the meantime, the genetically engineered fungus also will serve as a platform for continued technology development.
The system can be readily adapted to target mosquitoes or other insects , perhaps using different natural toxins if insects might grow resistant to Metarhizium just as they have to traditional insecticides. Interestingly, the researchers note that the engineered fungi appear to make mosquitoes sensitive to chemical insecticides again, suggesting that the two types of insect-killers might be used successfully in combination.
References:
[1] Transgenic Metarhizium rapidly kills mosquitoes in a malaria-endemic region of Burkina Faso. Lovett B, Bilgo E, Millogo SA, Ouattarra AK, Sare I, Gnambani EJ, Dabire RK, Diabate A, St Leger RJ. Science. 2019 May 31;364(6443):894-897.
[2] An entomopathogenic fungus for control of adult African malaria mosquitoes. Scholte EJ, Ng’habi K, Kihonda J, Takken W, Paaijmans K, Abdulla S, Killeen GF, Knols BG. Science. 2005 Jun 10;308(5728):1641-2.
Links:
Transgenic Fungus Rapidly Killed Malaria Mosquitoes in West African Study (University of Maryland News Release)
Malaria (National Institute of Allergy and Infectious Diseases/NIH)
Funnel-Web Spiders (Australian Museum, Sydney)
Video: 2016 Grand Challenges Spotlight Talk: Abdoulaye Diabaté (YouTube)
Raymond St. Leger (University of Maryland, College Park)
NIH Support: National Institute of Allergy and Infectious Diseases
Posted In: News
Tags: Australian Blue Mountains funnel-web spider, bioengineering, biotechnology, Burkina Faso, dengue virus, entomology, fungus, genetic engineering, global health, insect pathology, insecticide, insects, malaria, Metarhizium pingshaense, mosquito, mosquito-borne illnesses, MosquitoSphere, spider, toxin, transgenic fungus, West Africa, West Nile, Zika
How to Make Biopharmaceuticals Quickly in Small Batches
Posted on October 8th, 2018 by Dr. Francis Collins

Caption: InSCyT system. Image shows (1) production module, (2) purification module, and (3) formulation module.
Credit: Felice Frankel Daniloff, Massachusetts Institute of Technology, Cambridge
Today, vaccines and other protein-based biologic drugs are typically made in large, dedicated manufacturing facilities. But that doesn’t always fit the need, and it could one day change. A team of researchers has engineered a miniaturized biopharmaceutical “factory” that could fit on a dining room table and produce hundreds to thousands of doses of a needed treatment in about three days.
As published recently in the journal Nature Biotechnology, this on-demand manufacturing system is called Integrated Scalable Cyto-Technology (InSCyT). It is fully automated and can be readily reconfigured to produce virtually any approved or experimental vaccine, hormone, replacement enzyme, antibody, or other biopharmaceutical. With further improvements and testing, InSCyT promises to give researchers and health care providers easy access to specialty biologics needed to treat rare diseases, as well as treatments for combating infectious disease outbreaks in remote towns or villages around the globe.
Posted In: News
Tags: Bill and Melinda Gates Foundation, biological therapies, biologics, biopharmaceuticals, biotechnology, drug manufacturing, drug production, granulocyte colony-stimulating factor, human growth hormone, InSCyT, Integrated Scalable Cyto-Technology, interferon-alpha 2b, rare diseases, rotavirus, vaccines, yeast
DNA Barcodes Make for Better Single-Cell Analysis
Posted on April 16th, 2018 by Dr. Francis Collins
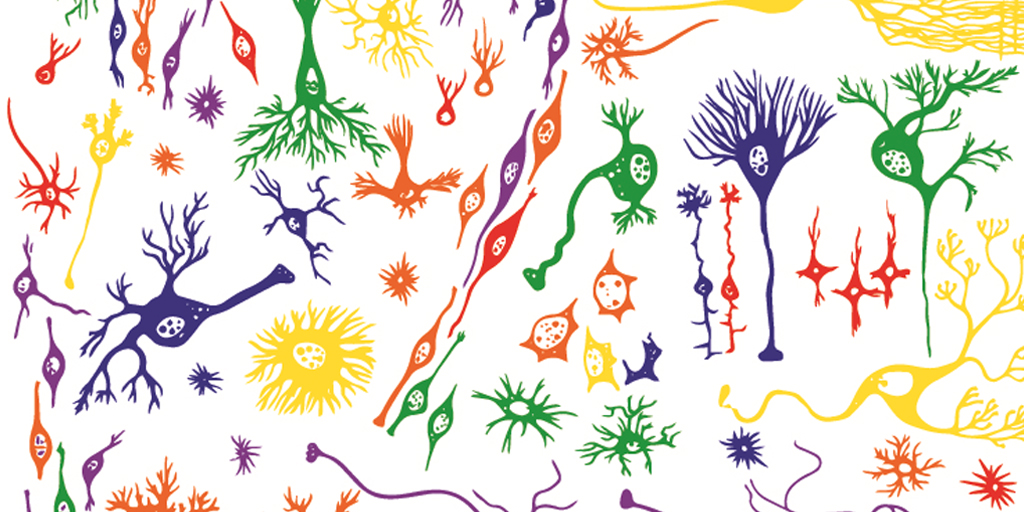
Caption: Single-cell analysis helps to reveal subtle, but important, differences among human cells, including many types of brain cells.
Credit: Shutterstock, modified by Ryan M. Mulqueen
Imagine how long it would take to analyze the 37 trillion or so cells that make up the human body if you had to do it by hand, one by one! Still, single-cell analysis is crucial to gaining a comprehensive understanding of our biology. The cell is the unit of life for all organisms, and all cells are certainly not the same. Think about it: even though each cell contains the same DNA, some make up your skin while others build your bones; some of your cells might be super healthy while others could be headed down the road to cancer or Alzheimer’s disease.
So, it’s no surprise that many NIH-funded researchers are hard at work in the rapidly emerging field known as single-cell analysis. In fact, one team recently reported impressive progress in improving the speed and efficiency of a method to analyze certain epigenetic features of individual cells [1]. Epigenetics refers to a multitude of chemical and protein “marks” on a cell’s DNA—patterns that vary among cells and help to determine which genes are switched on or off. That plays a major role in defining cellular identity as a skin cell, liver cell, or pancreatic cancer cell.
The team’s rather simple but ingenious approach relies on attaching a unique combination of two DNA barcodes to each cell prior to analyzing epigenetic marks all across the genome, making it possible for researchers to pool hundreds of cells without losing track of each of them individually. Using this approach, the researchers could profile thousands of individual cells simultaneously for less than 50 cents per cell, a 50- to 100-fold drop in price. The new approach promises to yield important insights into the role of epigenetic factors in our health, from the way neurons in our brains function to whether or not a cancer responds to treatment.
Posted In: Health, News, Science, technology
Tags: barcodes, biotechnology, chromatin, combinatorial indexing, DNA, DNA barcode, DNA methylation, DNA sequencing, epigenetics, neurons, single cell analysis
Sequencing Human Genome with Pocket-Sized “Nanopore” Device
Posted on February 6th, 2018 by Dr. Francis Collins

Caption: MinION sequencing device plugged into a laptop/Oxford Nanopore Technologies
It’s hard to believe, but it’s been almost 15 years since we successfully completed the Human Genome Project, ahead of schedule and under budget. I was proud to stand with my international colleagues in a celebration at the Library of Congress on April 14, 2003 (which happens to be my birthday), to announce that we had stitched together the very first reference sequence of the human genome at a total cost of about $400 million. As remarkable as that achievement was, it was just the beginning of our ongoing effort to understand the human genome, and to use that understanding to improve human health.
That first reference human genome was sequenced using automated machines that were the size of small phone booths. Since then, breathtaking progress has been made in developing innovative technologies that have made DNA sequencing far easier, faster, and more affordable. Now, a report in Nature Biotechnology highlights the latest advance: the sequencing and assembly of a human genome using a pocket-sized device [1]. It was generated using several “nanopore” devices that can be purchased online with a “starter kit” for just $1,000. In fact, this new genome sequence—completed in a matter of weeks—includes some notoriously hard-to-sequence stretches of DNA, filling several key gaps in our original reference genome.
Posted In: Health, Science, technology
Tags: biotechnology, Biowulf, DNA, DNA sequencing, Ebola virus, genome assembly, hand-held sequencing device, human genome, Human Genome Project, International Space Station, MinION, nanopore sequencing, Oxford Nanopore Technologies, precision medicine, repetitive DNA, telomeres, Zika virus
DNA-Encoded Movie Points Way to ‘Molecular Recorder’
Posted on July 18th, 2017 by Dr. Francis Collins

Credit: Seth Shipman, Harvard Medical School, Boston
There’s a reason why our cells store all of their genetic information as DNA. This remarkable molecule is unsurpassed for storing lots of data in an exceedingly small space. In fact, some have speculated that, if encoded in DNA, all of the data ever generated by humans could fit in a room about the size of a two-car garage and, if that room happens to be climate controlled, the data would remain intact for hundreds of thousands of years! [1]
Scientists have already explored whether synthetic DNA molecules on a chip might prove useful for archiving vast amounts of digital information. Now, an NIH-funded team of researchers is taking DNA’s information storage capabilities in another intriguing direction. They’ve devised their own code to record information not on a DNA chip, but in the DNA of living cells. Already, the team has used bacterial cells to store the data needed to outline the shape of a human hand, as well the data necessary to reproduce five frames from a famous vintage film of a horse galloping (see above).
But the researchers’ ultimate goal isn’t to make drawings or movies. They envision one day using DNA as a type of “molecular recorder” that will continuously monitor events taking place within a cell, providing potentially unprecedented looks at how cells function in both health and disease.
Posted In: Health, Science, Video
Tags: biosensor, biotechnology, Cas1, Cas2, CRISPR, CRISPR-Cas, DNA, DNA movie, DNA storage, E. coli, film, gene editing, genomics, Human and Animal Locomotion, imaging, information storage, molecular recorder, movie, spacers
DNA Barcodes Could Streamline Search for New Drugs to Combat Cancer
Posted on March 8th, 2016 by Dr. Francis Collins
 A little more than a decade ago, researchers began adapting a familiar commercial concept to genomics: the barcode. Instead of the black, printed stripes of the Universal Product Codes (UPCs) that we see on everything from package deliveries to clothing tags, they used short, unique snippets of DNA to label cells. These biological “barcodes” enable scientists to distinguish one cell type from another, in much the same way that a supermarket scanner recognizes different brands of cereal.
A little more than a decade ago, researchers began adapting a familiar commercial concept to genomics: the barcode. Instead of the black, printed stripes of the Universal Product Codes (UPCs) that we see on everything from package deliveries to clothing tags, they used short, unique snippets of DNA to label cells. These biological “barcodes” enable scientists to distinguish one cell type from another, in much the same way that a supermarket scanner recognizes different brands of cereal.
DNA barcoding has already empowered single-cell analysis, including for nerve cells in the brain. Now, in a new NIH-supported study, DNA barcoding helps in the development of a new method that could greatly streamline an increasingly complex and labor-intensive process: screening for drugs to combat cancer.
Tags: barcoded cancer cell lines, biotechnology, BRD-7880, cancer, cancer cell lines, cancer drugs, DNA barcode, drug discovery, drug screening, genomics, mechanism of action, oncology, Precision Medicine Initiative, PRISM, Profiling Relative Inhibition Simultaneously in Mixtures, single cell analysis
Happy New Year … and a Look Back at a Memorable 2015
Posted on January 5th, 2016 by Dr. Francis Collins
 A new year has arrived, and it’s going to be an amazing one for biomedical research. But before diving into our first “new science” post of 2016, let’s take a quick look back at 2015 and some of its remarkable accomplishments. A great place to reflect on “the year that was” is the journal Science’s annual Top 10 list of advances in all of scientific research worldwide. Four of 2015’s Top 10 featured developments directly benefited from NIH support—including Science’s “Breakthrough of the Year,” the CRISPR/Cas9 gene-editing technique. Here’s a little more on the NIH-assisted breakthroughs:
A new year has arrived, and it’s going to be an amazing one for biomedical research. But before diving into our first “new science” post of 2016, let’s take a quick look back at 2015 and some of its remarkable accomplishments. A great place to reflect on “the year that was” is the journal Science’s annual Top 10 list of advances in all of scientific research worldwide. Four of 2015’s Top 10 featured developments directly benefited from NIH support—including Science’s “Breakthrough of the Year,” the CRISPR/Cas9 gene-editing technique. Here’s a little more on the NIH-assisted breakthroughs:
CRISPR Makes the Cut: I’ve highlighted CRISPR/Cas9 in several posts. This gene-editing system consists of a short segment of RNA that is attached to an enzyme. The RNA is preprogrammed to find a distinct short sequence of DNA and deliver the enzyme, which acts like a scalpel to slice the sequence out of the genome. It’s fast and pretty precise. Although CRISPR/Cas9 isn’t brand-new—it’s been under development as a gene-editing tool for a few years—Science considered 2015 to be “the year that it broke away from the pack.”
Tags: baker's yeast, biotechnology, brain, CRISPR, CRISPR/Cas9, drug development, Ebola, Ebola vaccine, gene drive, global health, hydrocodone, immune system, immunology, infectious diseases, lymphatic system, neuroscience, opiates, opioid, PMI, Precision Medicine Initiative, rVSV-ZEBOV, Science Breakthrough of the Year, Science's Top 10 Science Advances, thebaine, Top 25 Science Stories of 2015, yeast, ZMapp