brain – NIH Director's Blog (original) (raw)
Energy-Producing Enzyme Fuels the Brain with Promise for Treating Parkinson’s Disease
Posted on September 12th, 2024 by Dr. Monica M. Bertagnolli

Credit: Donny Bliss/NIH
In Parkinson’s disease, neurons in parts of the brain gradually weaken and die, leading people to experience worsening problems with movement and other symptoms. While the causes of this disease aren’t fully known, studies have suggested the Parkinson’s brain lacks fuel to power dopamine-producing neurons that are essential for movement. When too many of those neurons are lost, Parkinson’s disease symptoms appear. But what if there was a way to boost energy levels in the brain and stop the neurodegenerative process in its tracks?
While the findings are preliminary, an NIH-supported study reported in Science Advances takes an encouraging step toward this goal. The key element, according to the new work, is an energy-producing enzyme known as phosphoglycerate kinase (PGK1). In fact, these latest preclinical findings in models of the disease suggest that boosting this enzyme in the brain even slightly may be enough to restore energy and afford some protection against Parkinson’s disease.
The team, led by Timothy Ryan and Alexandros Kokotos, Weill Cornell Medicine, New York City, was inspired by recent discoveries suggesting an unexpectedly important role for PGK1 in protecting the normal function of neurons. They knew PGK1 plays an essential role in the pathway through which cells use glucose to generate and store energy in the form of adenosine 5′-triphosphate (ATP) molecules. The surprise came when studies showed the drug terazosin, which is used to treat high blood pressure and enlarged prostate, has an unexpected side effect: it enhances PGK1 activity, although perhaps weakly.
Could the boost in PGK1 activity be enough to fuel and protect dopamine-producing neurons? Studies in Parkinson’s models including mice, rats, flies, and human cells treated with terazosin suggested that it could. A retrospective study in people taking terazosin for an enlarged prostate also showed that those taking the drug were less likely to develop Parkinson’s.
As promising as that sounded, it was hard to imagine that such a seemingly small increase in PGK1 activity was enough to explain the findings. To investigate further in the new study, the researchers ran more sensitive studies to see just how much of a difference PGK1 can make when it comes to energy production in neurons. Their new studies show that even a small increase in PGK1 activity keeps neurons firing, even when glucose levels are low.
The researchers report that the increases in PGK1 they saw were enough to protect neurons carrying mutations in genes with known links to familial forms of Parkinson’s disease. They found that effects of a PGK1 boost also depend on another protein, called DJ-1, which has also been implicated in Parkinson’s disease. When the researchers experimentally increased PGK1 levels in mouse models of the disease, it strongly protected their dopamine neurons.
For the approximately one million Americans with Parkinson’s disease today, current treatments help to relieve symptoms but don’t stop the disease from progressing. These new findings raise the possibility that terazosin or drugs that enhance PGK1 activity even more may fuel the brain, helping to protect essential dopamine-producing neurons to treat or even prevent Parkinson’s disease, as well as other neurodegenerative conditions where PGK1 may play a role.
Reference:
Kokotos AC, et al. Phosphoglycerate kinase is a central leverage point in Parkinson’s Disease driven neuronal metabolic deficits. Science Advances. DOI: 10.1126/sciadv.adn6016 (2024).
NIH Support: National Institute of Neurological Disorders and Stroke, National Institute of General Medical Sciences
Study Identifies Previously Unknown Pain Control Pathway Underlying Placebo Effect
Posted on August 29th, 2024 by Dr. Monica M. Bertagnolli

Credit: Donny Bliss/NIH
When someone receives an inactive sugar pill for their pain, the expectation of benefit often leads them to experience some level of pain relief. Researchers have long known that this placebo effect is a very real phenomenon. However, the brain mechanisms underlying the placebo effect for pain have been difficult for researchers to understand.
Now, findings from an intriguing NIH-supported study in mice published in Nature offer insight into how this powerful demonstration of the mind-body connection works in the brain. Furthermore, the researchers identified a previously unknown neural pathway for pain control and suggest that specifically activating this pathway in the brain by other means could one day offer a promising alternative for treating pain more safely and effectively than with current methods, including opioids.
The findings come from a team led by Grégory Scherrer, University of North Carolina School of Medicine, Chapel Hill; with colleagues from Stanford University, Stanford, CA; the Howard Hughes Medical Institute, Ashburn, VA; and the Allen Institute for Brain Science, Seattle. The researchers knew from earlier human brain imaging studies that the placebo effect activates certain brain areas, including the anterior cingulate cortex, which is involved in emotion, attention, and mood. By conducting a series of more detailed studies in mice, the team sought to learn more about the specific brain activities involved.
To do this, the researchers set up an experiment in which mice were put in an apparatus with two distinct chambers, each with comfortably warm floors (about 86 degrees Fahrenheit). After a period of acclimatization, the floor of one of the chambers was made unpleasantly hot (about 118 degrees Fahrenheit—similar to hot pavement in the summertime). The mice learned that they could avoid their discomfort by spending time in the chamber with the comfortably warm floor. The researchers then equalized the floor temperature in both chambers to 118 degrees. Despite identical hot conditions in both chambers, the mice still exhibited fewer behaviors associated with discomfort, such as paw licking, in the chamber that was previously comfortable, indicating that the animals showed signs of a classic placebo effect.
The researchers used a variety of sophisticated imaging techniques to visualize the activity of individual neurons in mice to better understand their behaviors. They found the placebo effect links the anterior cingulate cortex in the front of the brain through the pontine nucleus, a part of the brain with clusters of cells in the brainstem that has not previously been associated with pain or pain relief, to the cerebellum in the back of the brain. This research indicates that the pontine nucleus could be a promising target for novel pain-relieving therapies.
The experiments also revealed an unexpected abundance of opioid receptors in the pontine nucleus, lending further support for the role of this brain area in responding to the body’s natural opioids that modulate pain.
While the experience of pain is exceedingly complex, and this research is in mice, the researchers expect that these findings will have relevance to people. The next step is to explore the role of activity in this newly discovered pain pathway in humans’ experience of the placebo effect. The hope is that with continued study it may one day be possible to target this brain area using small molecules or neural stimulation as a potentially more effective and safer means to ease pain compared to current methods.
Reference:
Chen C, et al. Neural circuit basis of placebo pain relief. Nature. DOI: 10.1038/s41586-024-07816-z (2024).
NIH Support: National Institute of Neurological Disorders and Stroke, National Institute on Drug Abuse
Mapping Psilocybin’s Brain Effects to Explore Potential for Treating Mental Health Disorders
Posted on August 15th, 2024 by Dr. Monica M. Bertagnolli

Credit: Donny Bliss/NIH
Psilocybin is a natural ingredient found in “magic mushrooms.” A single dose of this psychedelic can distort a person’s perception of time and space, as well as their sense of self, for hours. It can also trigger strong emotions, ranging from euphoria to fear. While psilocybin comes with health risks and isn’t recommended for recreational use, there’s growing evidence that—under the right conditions—its effects on the brain might be harnessed in the future to help treat substance use disorders or mental illnesses.
To explore this potential, it will be important to understand how psilocybin exerts its effects on the human brain. Now, a study in Nature supported in part by NIH has taken a step in this direction, using functional brain mapping in healthy adults before, during, and after taking psilocybin to visualize its impact. While earlier studies in animals suggested that psilocybin makes key brain areas more adaptable or “plastic,” this new research aims to clarify changes in the function of larger brain networks and their connection to the experiences people have with this psychedelic drug.
In the study, the team led by Joshua Siegel, Nico Dosenbach and Ginger Nicol, Washington University School of Medicine in St. Louis, recruited seven healthy adults to take, in separate sessions, a high dose of psilocybin and the stimulant methylphenidate, the generic form of Ritalin, under controlled conditions. Because taking psilocybin comes with a risk for having disturbing or negative experiences, a pair of trained experts stayed with each participant throughout the sessions to provide guidance and support. They also helped participants prepare for and process the experience afterwards.
To visually capture the impact of psilocybin on the brain, the researchers had each participant undergo an average of 18 functional MRI brain scan visits. Four of the study’s participants also returned six to 12 months later to take an additional psilocybin dose. Comparisons of the brain images revealed profound and widespread, but temporary, changes to the brain’s functional networks. While an individual’s functional brain network is typically as distinctive as a fingerprint, psilocybin made the participants’ brain networks look so similar in the scans that the researchers couldn’t tell them apart. In addition, by following the brain scans with specialized questionnaires given to elicit details about participants’ subjective experiences with psilocybin, the researchers were able to generate precise data on each person’s unique impressions and associated changes in their brain networks.
For all the participants, psilocybin desynchronized the brain’s default mode network, an interconnected set of brain areas that are most active when people are daydreaming or otherwise not engaged in any focused, goal-directed mental activity. By comparison, the default mode network remained stable after study participants took methylphenidate. Once the effects of psilocybin wore off, brain function returned almost to its original state. However, the researchers did note small but potentially important differences in each participant’s brain scans after taking psilocybin that remained for weeks.
The researchers suggest that the short-term changes in the default mode network likely explain psilocybin’s psychedelic effects, including the way it changes the way a person thinks about themselves in relation to other people and the world. They also suggest that the more subtle, longer-term effects they observed might indicate that the brain is more flexible in the weeks following a dose of psilocybin in ways that could allow for a healthier state. This may help explain preliminary research showing that psilocybin may have benefits for treating substance use disorders, as well as depression, anxiety, and other mental health conditions.
Though these findings are encouraging, they should not be seen as a reason to try psilocybin without clinician supervision or use it to self-medicate. The drug is not proven or approved as a treatment for any condition, and its unsupervised use comes with serious risks. The researchers hope that with much more clinical study of how and why this drug affects individuals in the powerful ways that it does, this kind of research may one day lead to a greater understanding of the human brain and promising new interventions that improve mental health.
Reference:
Siegel JS, et al. Psilocybin desynchronizes the human brain. Nature. DOI: 10.1038/s41586-024-07624-5 (2024).
NIH Support: National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Neurological Disorders and Stroke
Tags: anxiety, brain, default mode network, depression, imaging, mental health disorders, neuroscience, psilocybin, psychedelic, substance use disorders
Epigenetic Editor Silences Toxic Proteins in the Mouse Brain, Offering Promising Path to Treat Deadly Prion Diseases
Posted on July 25th, 2024 by Dr. Monica M. Bertagnolli

Credit: Donny Bliss/NIH
Prion diseases are fatal neurodegenerative disorders caused by a malfunction of the prion protein in the brain. Exposure to a misfolded version of the protein triggers normal proteins of the same type in the brain to misfold, forming clumps that produce infectious disease and fatal brain damage over time. There are currently no treatments, preventive vaccines, or cures for prion diseases, which can be acquired, like mad cow disease, or inherited, like fatal familial insomnia. But an encouraging new study in mice suggests a potentially promising path for developing a treatment for people with these deadly conditions.
Findings from an NIH-supported study reported in Scienceshow that the key to this approach is a molecular tool capable of silencing prion protein throughout the brain using epigenetic editing.1 Unlike gene editing approaches, which change the sequence of genes, epigenetic editing can turn gene expression off with the addition of a chemical tag that prevents genes from being translated into proteins. Such a strategy may be able to deliver modifying tools to the brain or other parts of the body to silence specific toxic or disease-causing genes, including the one encoding the prion protein, without the risks associated with altering DNA sequences.
Earlier findings in mouse studies have shown that reducing prion protein levels can halt disease progression. However, attempting this with gene editing approaches has been a challenge. In the new study, researchers led by Jonathan Weissman at the Whitehead Institute and Sonia Vallabh at the Broad Institute, both in Cambridge, MA, pursued an epigenetic approach to this problem as a potentially more feasible and safer option than gene editing. To do it, they first had to develop an epigenetic silencer that was compact enough for delivery into cells using an adeno-associated virus (AAV) vector, which is the preferred way to get therapeutic payloads into the central nervous system, including the brain.
They call their programmable epigenetic silencer “CHARM,” short for Coupled Histone tail for Autoinhibition Release of Methyltransferase. To target a gene with the needed specificity, CHARM uses a guide protein to direct the tool to a target site in DNA. The tool also recruits enzymes that are naturally present in cells to deliver a silencing methyl group. The researchers engineered their tool to include parts that successfully switch the methyltransferase enzyme on to do its work of silencing the prion gene in just the right spot.
To get the molecular tools into the brain, the researchers built on a previous NIH-supported advance made by a team including study co-author Benjamin Deverman, also at the Broad Institute, that improves the delivery of therapeutic molecular tools such as CHARM into the brain. As reported in Science, the researchers showed they could shuttle an AAV across the blood-brain barrier using a receptor that normally brings iron into the brain.2
The latest study shows that, when delivered to the mouse brain using this new AAV, CHARM efficiently silences the prion gene in most neurons without altering the underlying DNA sequence. As a result, prion protein levels dropped by more than 80%. That’s important given that earlier studies have shown that reducing prion protein by as little as 20% can improve symptoms. The researchers also engineered their CHARM editors such that they turn themselves off after silencing the target gene to limit the possibility of unwanted effects.
This study was supported in part by the NIH Common Fund as part of the NIH Somatic Cell Genome Editing (SCGE) Program. The researchers report they are now fine-tuning their tool to make it more effective, safer, and easier to manufacture in quantities that are necessary for future testing in clinical trials enrolling people with degenerative and otherwise fatal prion diseases. It’s likely to be a long road, but if all goes well, this impressive work could one day enable effective treatments for people with prion diseases. This approach may also hold promise for treating other neurodegenerative conditions that involve the accumulation of toxic protein aggregates in the brain.
References:
[1] Neumann EN, et al. Brainwide silencing of prion protein by AAV-mediated delivery of an engineered compact epigenetic editor. Science. DOI: 10.1126/science.ado7082 (2024).
[2] Huang Q, et al. An AAV capsid reprogrammed to bind human transferrin receptor mediates brain-wide gene delivery. Science. DOI: 10.1126/science.adm8386 (2024).
NIH Support: Common Fund Somatic Cell Genome Editing Program, National Institute of Neurological Disorders and Stroke, National Human Genome Research Institute, National Institute of Biomedical Imaging and Bioengineering
Study of Protective Gene Variant Provides Insight into Delaying Onset of Alzheimer’s Dementia
Posted on July 18th, 2024 by Dr. Monica M. Bertagnolli

Credit: Donny Bliss/NIH
Alzheimer’s disease is currently the seventh leading cause of death in the U.S. While your likelihood of developing Alzheimer’s-related cognitive impairment increases with age, risk for this disease and age of its onset depend on many factors, including the genes you carry. An intriguing new study suggests that having just one copy of a protective gene variant may be enough to delay cognitive impairment from this devastating disease in individuals who are otherwise genetically predisposed to developing early-onset Alzheimer’s dementia.
The findings, from a study supported in part by NIH and reported in The New England Journal of Medicine, offer important insights into the genetic factors and underlying pathways involved in Alzheimer’s dementia.1 While much more study is needed, the findings have potential implications for treatments that could one day work like this gene variant does to delay or perhaps even prevent Alzheimer’s dementia.
This research comes from an international team including Yakeel Quiroz, Massachusetts General Hospital, Boston; Joseph Arboleda-Velasquez, Mass Eye and Ear, Boston; and Francisco Lopera, University of Antioquia, Colombia. For the last 40 years, Lopera has been studying a Colombian family of about 6,000 blood relatives, 1,200 of whom carry a mutation known as Paisa (or Presenilin-1 E280A) that predisposes them to developing early-onset Alzheimer’s dementia. Those who carry a single copy of this gene variant typically show signs of cognitive decline in their early 40s, progressing to dementia by age 50. They frequently die from dementia-related complications in their 60s.
In 2019, the researchers reported on an extraordinary individual who was an exception to this prognosis.2 Even though she carried the Paisa mutation, she didn’t develop any notable cognitive decline until her late 70s—30 years later than expected. The researchers traced her protection against dementia to two copies of a rare variant of the APOE gene dubbed Christchurch. Further study of her brain after death also found lower levels of inflammation and tau protein, which forms damaging tangles inside neurons in the Alzheimer’s brain.
Christchurch is a rare variant, and it’s far more common for people to carry one copy of the protective variant versus two. Would a single copy of the Christchurch variant offer some protection against Alzheimer’s dementia, too? To find out in the new study, the researchers analyzed data from 27 members of this family carrying a single copy of the Christchurch variant among 1,077 carriers of the Paisa mutation.
The researchers compared Christchurch carriers to those without the protective variant and found the variant did delay the age of onset of Alzheimer’s-related cognitive decline and dementia. The median age at the onset of mild cognitive impairment was 52 in family members with the Christchurch variant, compared to approximately age 47 in a matched group without the variant. Similarly, the median age at the onset of dementia was 54, compared to the median age of 50 in noncarriers.
To learn more, the researchers imaged the brains of two of the individuals who had one copy of Christchurch. The brain scans showed lower levels of tau and more normal metabolic activity in brain areas that are known to play a role in Alzheimer’s. Interestingly, their brains still showed accumulations of amyloid proteins, which form plaques that are another hallmark of Alzheimer’s. The team also analyzed autopsy samples from four deceased individuals with one copy of the Christchurch variant and found that blood vessels in their brains appeared healthier, which may help to explain the protective effects of Christchurch. The findings suggest a significant role for blood vessel health in protecting the brain from cognitive decline, as well as a role for disease of the brain blood vessels in contributing to cognitive decline and dementia.
The researchers note this study is limited to a relatively small number of people with both the Paisa and Christchurch variants in one group of related individuals. Further studies involving larger and more diverse samples are needed to learn more about this protective gene variant and its effects on the brain in the general population. The hope is these findings may one day yield new approaches to delaying the onset of Alzheimer’s or slowing its progression in millions more people around the world at risk of developing this devastating disease.
References:
[1] Quiroz YT, et al. APOE3 Christchurch Heterozygosity and Autosomal Dominant Alzheimer’s Disease. The New England Journal of Medicine. DOI: 10.1056/NEJMoa2308583 (2024).
[2] Arboleda-Velasquez JF, et al. Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nature Medicine. DOI: 10.1038/s41591-019-0611-3 (2019).
NIH Support: National Institute on Aging, National Institute of Neurological Disorders and Stroke
Posted In: Health, News, Science
Tags: aging, Alzheimer’s disease, brain, cognitive decline, dementia, DNA, gene variants, genetics, neurological disease
Insights into Molecular Basis of PTSD and Major Depression Could One Day Aid in Diagnosis and Treatment
Posted on June 13th, 2024 by Dr. Monica M. Bertagnolli

Credit: S. Thomas Carmichael/UCLA, Yuliia/Adobe Stock
We know stress can take a toll on our mental health. Yet, it’s unclear why some people develop stress-related mental health disorders and others don’t. The risk for developing a stress-related mental health disorder such as post-traumatic stress disorder (PTSD) or major depressive disorder (MDD) depends on a complex interplay between the genetic vulnerabilities we are born with and the impact of traumatic stress we experience over our lifetimes.
Given this complexity, it’s been difficult for researchers to pinpoint the underlying biological pathways in the body that ultimately produce changes associated with PTSD, major depression, or other mental health conditions. Now, a study reported in a special issue of Science on decoding the brain uses a comprehensive approach to examine multiple biological processes across brain regions, cell types, and blood to elucidate this complexity. It’s an unprecedented effort to understand in a more holistic way the essential biological networks involved in PTSD and MDD.
While earlier studies looked at stress hormones, the immune system, and other molecular signatures of stress in blood samples, what had been largely missing from the picture of PTSD and MDD were links between those changes in the body and changes in the brain. To get a more complete picture, a multisite research team led by Nikolaos P. Daskalakis and Kerry Ressler of McLean Hospital, Belmont, MA, developed a vast molecular dataset including DNA variants, RNA, proteins, and chemical modifications to DNA. This “multi-omic” dataset was generated by the NIH-supported PTSD Brainomics Project of the PsychENCODE Consortium, and included postmortem data from 231 individuals with PTSD and/or MDD, as well as from individuals who didn’t have known mental health conditions.
In the study, the researchers looked at three essential brain regions: the medial prefrontal cortex (mPFC), the hippocampal dentate gyrus, and the central nucleus of the amygdala. They conducted single-cell RNA sequencing analysis of 118 dorsolateral prefrontal cortex (dlPFC) samples to look at cell-type-specific patterns and evaluated protein changes in the blood of more than 50,000 UK Biobank samples to look for biomarkers of stress-related disorders. After identifying key brain-based genes whose expression was altered in PTSD and/or MDD, the researchers compared them to genes linked to increased risk for these conditions.
Among many findings, the study results show an important role for the mPFC in both stress-related conditions, which is interesting, as the mPFC is essential for integrating signals from other brain areas and is known to play a role in cognitive processes, emotional regulation, motivation, and sociability. The findings also highlight important roles for molecular pathways known to play a role in immune function, the regulation of neurons and neural connections, and stress hormones. The single-cell RNA sequencing in the dlPFC also uncovered dysregulated stress-related signals in neurons and other brain cell types.
Furthermore, the findings reveal shared changes in gene activity between PTSD and MDD, as well as notable differences in the patterns of methyl marks on the DNA, suggesting changes in the way genes are switched on or off, and at the level of cell-type-specific gene activity. The researchers also found that history of childhood trauma and suicide were drivers of molecular changes in both disorders.
The data point to a short list of proteins that may be important in regulating key genetic pathways underlying these disorders. They also reveal links to gene networks related to aging, inflammation, stress, and more. Similarities in disease signals in the brain and blood suggest that blood-based tests might one day offer an additional avenue for assessing these disorders. Interestingly, there was little overlap between PTSD and MDD risk genes and those involved in the underlying molecular-level changes in the brains of people with one or both conditions. This shows that there’s a need for more research into how genetic risk factors are related to molecular-level disease processes.
There’s clearly much more to discover in the years ahead. But these insights already point to important roles for known stress-related pathways in fundamental brain changes underlying PTSD and MDD, while also revealing more novel pathways as potentially promising new treatment targets. With further study, the researchers hope these findings can also begin to answer vexing questions, such as why some people develop PTSD or major depression after stressful events and others don’t.
Reference:
Daskalakis NP, et al. Systems biology dissection of PTSD and MDD across brain regions, cell types, and blood. Science. DOI: 10.1126/science.adh3707 (2024).
This paper is part of a larger collection of studies from the PsychENCODE Consortium looking at the underlying mechanisms of neuropsychiatric diseases.
NIH Support: National Institute of Mental Health
Posted In: Health, News, Science
Tags: biomarkers, brain, depression, mental health, mental health disorders, molecular signature, multiomics, neuroscience, post-traumatic stress disorder, PTSD, stress
Study Suggests Computerized Brain Implant Could One Day Decode Internal Speech for Those Who Can No Longer Speak
Posted on June 6th, 2024 by Dr. Monica M. Bertagnolli

Credit: Tom Merton/KOTO/Adobe Stock
The ability to communicate using only your thoughts might sound like the stuff of science fiction. But for people who don’t have the ability to speak or move due to injury or disease, there’s great hope that this may one day be possible using brain-computer interfaces (BCIs) that can “read” relevant brain signals and translate them into written or spoken words. A research team has made a preliminary advance in this direction by showing for the first time that a computerized brain implant can decode internal speech with minimal training.
In the new NIH-supported study, researchers implanted such a device in a brain area known to be important for representing spoken words called the supramarginal gyrus in two people with tetraplegia, a condition marked by full body paralysis from the neck down due to cervical spinal cord injury. The researchers found that the device could decode several words the participants “spoke” only in their minds. While we are far from using such a device to decode whole sentences or even phrases, and the exact mechanisms of internal speech are still under study, the findings, reported in Nature Human Behavior, are notable because it had been unclear whether the brain signals involved in thinking words could be reproducibly translated.
The findings come from a team led by Richard Andersen at the California Institute of Technology, Pasadena, CA, and Sarah Wandelt, now at the Feinstein Institutes for Medical Research in Manhasset, NY, and the study was supported by the NIH _Brain Research Through Advancing Innovative Neurotechnologies_® (BRAIN) Initiative Research Opportunities in Humans program. Though earlier research had shown that brain implants could decode vocalized, attempted, and mimed speech, it had yet to be seen whether internal speech could be similarly decoded.
An earlier advance in decoding speech signals from the brain came in 2022, when the researchers reported they could accurately predict the words that a person with tetraplegia was thinking using a BCI. In the new study, they’ve shown that the device works in a second person with tetraplegia. The finding is an indication that the approach can work in different individuals and doesn’t depend on the brain characteristics of a particular person or the precise orientation of the implant in their brain.
In the study, the researchers trained their device to recognize brain patterns associated with certain internal “spoken” words including six actual words (battlefield, cowboy, python, spoon, swimming, and telephone) and two nonsense words (nifzig and bindip). During three sessions, the researchers flashed words on a screen and asked each participant to think about “saying” the words without speaking or moving. The BCI then used measurements of brain activity during the sessions and a computer model to predict the words being “spoken” internally.
The researchers found that in this task the device could decode the words with an average accuracy of 79% with the first participant and 23% with the second participant. They noted that the second participant had fewer unique patterns of brain activity associated with the different words, which may explain the lower results. Nevertheless, the findings show that the brain region in question generally contains signals for internal speech, although there is likely also variation among people in how thoughts of particular words are represented in patterns of brain activity. Furthermore, the device’s ability to decode nonsense words suggests that the words are represented in this part of the brain phonetically and not necessarily based on their meanings.
While there is much more to learn about how to decode internal speech more reliably across individuals, the findings offer proof-of-concept for a high-performance internal speech BCI. The new research adds to a growing portfolio of rapidly advancing technologies supported by the BRAIN Initiative that could one day routinely restore the ability to communicate for those who can no longer speak or even move, including people with brain injuries, paralysis, or diseases such as amyotrophic lateral sclerosis (ALS).
Reference:
Wandelt SK, et al. Representation of internal speech by single neurons in human supramarginal gyrus. Nature Human Behaviour. DOI: 10.1038/s41562-024-01867-y (2024).
NIH Support: NIH BRAIN Initiative
Posted In: Health, News, Science
Tags: amyotrophic lateral sclerosis, brain, BRAIN Initiative, brain-computer interfaces, internal speech decoder, neuroscience, paralysis, speech, tetraplegia, thoughts
Most Detailed 3D Reconstruction of Human Brain Tissue Ever Produced Yields Surprising Insights
Posted on May 30th, 2024 by Dr. Monica M. Bertagnolli
Researchers have developed a detailed 3D reconstruction of a cubic millimeter of brain tissue. Credit: Images in video from Google Research & Lichtman Lab, Harvard University. Renderings by D. Berger, Harvard. Video compiled by Donny Bliss/NIH
The NIH _Brain Research Through Advancing Innovative Neurotechnologies_® (BRAIN) Initiative has expanded scientists’ understanding of the human brain in recent years, offering fascinating insights into the ways that individual cells and complex neural circuits interact dynamically to enable us to think, feel, and act. But neuroscientists still have much more to learn about how our brains are put together at the most fundamental, subcellular level.
As a step in that direction, in a new study supported in part by the NIH BRAIN Initiative and reported in the journal Science, researchers have created the most detailed nanoscale resolution map ever produced of a cubic millimeter of brain tissue, about the size of half a grain of rice.
Despite its small size, this fragment of healthy brain contained about 57,000 cells of various types, 230 millimeters of blood vessels, 150 million neural connections, or synapses, and the protective myelin that insulates neurons. To capture it all in vivid detail, the researchers relied on electron microscopy to amass an impressive 1,400 terabytes of imaging data. For perspective, one terabyte of data is enough to store 100,000 photos on your smartphone.
While there are many more details yet to analyze given the sheer quantity of data, this impressively detailed subcellular map has already revealed multiple brain structures that have never been seen before. This includes a class of triangular neurons in deep brain layers being described for the first time. The map also revealed axons, the long extensions of nerve cells that carry electrical impulses, with as many as 50 synapses and other unusual structures, including axons arranged into extensive spiraling patterns that now warrant further study.
The findings come from a team led by Jeff W. Lichtman, Harvard University, Cambridge, MA, and Viren Jain, Google Research, Mountain View, CA. They recognized that fully understanding the human brain requires knowledge of its most basic construction. While the imaging technologies needed to produce this kind of map were available, there were other barriers, including a limited availability of healthy and high-quality human brain tissue samples for study.
Most biopsies of the brain are done to examine or take out abnormal growths of cells or tissues, making them unsuitable for understanding the normal makeup of the brain. In this case, the researchers were able to obtain a tiny sample from the brain tissue removed and destined for disposal during the normal course of surgery for a patient with epilepsy. The researchers first stained the preserved sample to make the cells easier to trace individually before slicing it into 5,000 thin layers for microscopic imaging.
To put those slices back together into a complete 3D reconstruction, the researchers relied on artificial intelligence (AI) models. Because the dataset is too large for any one group to fully analyze, they’ve made it all freely available to the research community in an online resource. They’ve also provided tools for its further analysis and proofreading.
While there is plenty still left to uncover, the findings offer proof-of-principle that it’s possible to visualize the brain at this very detailed level. This is crucial groundwork for new research now supported by the BRAIN Initiative Connectivity Across Scales (BRAIN CONNECTS) program. BRAIN CONNECTS will develop and scale up tools to produce an equally detailed map of a complete mouse brain, which is about 1,000 times larger than the human brain fragment. The researchers now hope their 3D map and others like it will be put to work to understand both normal and disordered brain function more fully.
Reference:
[1] Shapson-Coe A, et al. A petavoxel fragment of human cerebral cortex reconstructed at nanoscale resolution. Science. DOI: 10.1126/science.adk4858 (2024).
NIH Support: NIH BRAIN Initiative, National Institute of Mental Health
Posted In: News, Science, technology
Tags: 3d reconstruction, brain, BRAIN Initiative, brain tissue, data, imaging, neural circuitry, neuroscience, research tools
Researchers Map Neural Connections Key to Wakefulness in the Human Brain
Posted on May 23rd, 2024 by Dr. Monica M. Bertagnolli

Credit: Donny Bliss/NIH
Human consciousness requires a person to be both awake and aware. While neuroscientists have learned a great deal from research about the underlying brain networks that sustain awareness, surprisingly little has been known about the networks that keep us awake.
Now, an NIH-supported team of researchers has mapped the connectivity of a neural network they suggest is essential for wakefulness, or arousal, in the human brain. According to the researchers, this advance, reported in Science Translational Medicine, is essential for understanding human consciousness. It may also lead to new ways of understanding what happens in the brain when people lose consciousness, with potentially important implications for treating those who have entered a coma or vegetative state.
The team—led by Brian Edlow, Massachusetts General Hospital and Harvard Medical School, Boston, and Hannah Kinney, Boston Children’s Hospital and Harvard Medical School—set out to map the brain network that sustains wakefulness in a manner similar to earlier research that identified the default mode network, which influences awareness. Default networks in the brain are most active when people are at rest rather than focused on a goal-oriented task.
To map what they call the default ascending arousal network, the researchers knew they needed to capture connections deep within human brain areas previously implicated in wakefulness in animal studies. They wanted to use high resolution to uncover fine structural details. Because it isn’t currently possible to capture this in the brain of a living person within a reasonable scan time, the researchers looked to the brains of three organ donors who died without any neurological problems.
The researchers stained different types of brain cells in key areas of the brainstem, hypothalamus, thalamus, and basal forebrain, and took images of the donor brains using a sophisticated form of magnetic resonance imaging (MRI). Their data allowed them to map underlying structures and individual neural connections deep in the brain.
To learn more about how this wakefulness network functions, they next looked to a wealth of functional MRI data from 84 healthy study participants in the NIH-supported Human Connectome Project. Those data revealed functional connections between the arousal network and the previously identified default mode network that is active when people are awake but not attending to their surroundings. Further study revealed a “connectivity hub” between these networks in an area of the midbrain known as the dopaminergic ventral tegmental area, which helps in understanding how arousal and awareness are integrated in human consciousness.
These findings suggest that stimulating this key arousal hub for human consciousness may hold promise for helping people recover from a coma. In fact, the researchers have already launched a clinical trial to see whether stimulating the hub in people in a coma after traumatic brain injury could restore consciousness.
This new guide to brain areas that are essential to wakefulness may ultimately aid understanding of many conditions in which people have altered consciousness, including coma, seizures, and sudden infant death syndrome (SIDS), according to the researchers. And, to enable others to continue studying and uncovering other aspects of human consciousness, the team has made its MRI data, methods, and atlas freely available.
Reference:
[1] Edlow BL, et al. Multimodal MRI reveals brainstem connections that sustain wakefulness in human consciousness. Science Translational Medicine. DOI: 10.1126/scitranslmed.adj4303 (2024).
NIH Support: National Institute of Neurological Disorders and Stroke, National Institute for Biomedical Imaging and Bioengineering, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute on Deafness and Other Communication Disorders, National Institute on Aging, National Institute of Mental Health, NIH BRAIN Initiative
Study Suggests Treatments that Unleash Immune Cells in the Brain Could Help Combat Alzheimer’s
Posted on April 25th, 2024 by Dr. Monica M. Bertagnolli
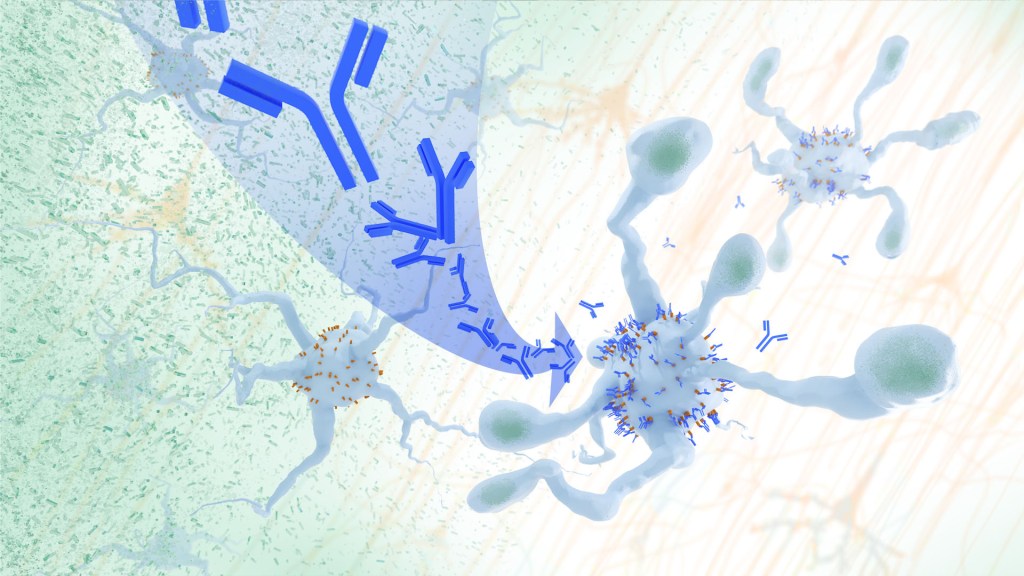
In a study, an antibody treatment blocked interaction between APOE proteins and LILRB4 receptors in the brain, enabling microglia immune cells to clear amyloid plaques, a feature of Alzheimer’s. Credit: Donny Bliss/NIH
In Alzheimer’s disease, a buildup of sticky amyloid proteins in the brain clump together to form plaques, causing damage that gradually leads to worsening dementia symptoms. A promising way to change the course of this disease is with treatments that clear away damaging amyloid plaques or stop them from forming in the first place. In fact, the Food and Drug Administration recently approved the first drug for early Alzheimer’s that moderately slows cognitive decline by reducing amyloid plaques.1 Still, more progress is needed to combat this devastating disease that as many as 6.7 million Americans were living with in 2023.
Recent findings from a study in mice, supported in part by NIH and reported in Science Translational Medicine, offer another potential way to clear amyloid plaques in the brain. The key component of this strategy is using the brain’s built-in cleanup crew for amyloid plaques and other waste products: immune cells known as microglia that naturally help to limit the progression of Alzheimer’s. The findings suggest it may be possible to develop immunotherapies—treatments that use the body’s immune system to fight disease—to activate microglia in the brains of people with Alzheimer’s and clear amyloid plaques more effectively.2
In their report, the research team—including Marco Colonna, Washington University School of Medicine in St. Louis, and Jinchao Hou, now at Children’s Hospital of Zhejiang University School of Medicine in Zhejiang Province, China—wrote that microglia in the brain surround plaques to create a barrier that controls their spread. Microglia can also destroy amyloid plaques directly. But how microglia work in the brain depends on a fine-tuned balance of signals that activate or inhibit them. In people with Alzheimer’s, microglia don’t do their job well enough.
The researchers suspected this might have something to do with a protein called apolipoprotein E (APOE). This protein normally helps carry cholesterol and other fats in the bloodstream. But the gene encoding the protein is known for its role in influencing a person’s risk for developing Alzheimer’s, and in the Alzheimer’s brain, the protein is a key component of amyloid plaques. The protein can also inactivate microglia by binding to a receptor called LILRB4 found on the immune cells’ surfaces.
Earlier studies in mouse models of Alzheimer’s showed that the LILRB4 receptor is expressed at high levels in microglia when amyloid plaques build up. This suggested that treatments targeting this receptor on microglia might hold promise for treating Alzheimer’s. In the new study, the research team looked for evidence that an increase in LILRB4 receptors on microglia plays an important role in the brains of people with Alzheimer’s.
To do this, the researchers first studied brain tissue samples from people who died with this disease and discovered unusually high amounts of the LILRB4 receptor on the surfaces of microglia, similar to what had been seen in the mouse models. This could help explain why microglia struggle to control amyloid plaques in the Alzheimer’s brain.
Next, the researchers conducted studies of mouse brains with accumulating amyloid plaques that express the LILRB4 receptor to see if an antibody targeting the receptor could lower amyloid levels by boosting activity of immune microglia. Their findings suggest that the antibody treatment blocked the interaction between APOE proteins and LILRB4 receptors and enabled microglia to clear amyloid plaques. Intriguingly, the team’s additional studies found that this clearing process also changed the animals’ behavior, making them less likely to take risks. That’s important because people with Alzheimer’s may engage in risky behaviors as they lack memories of earlier experiences that they could use to make decisions.
There’s plenty more to learn. For instance, the researchers don’t know yet whether this approach will affect the tau protein, which forms damaging tangles inside neurons in the Alzheimer’s brain. They also want to investigate whether this strategy of clearing amyloid plaques might come with other health risks.
But overall, these findings add to evidence that immunotherapies of this kind could be a promising way to treat Alzheimer’s. This strategy may also have implications for treating other neurodegenerative conditions characterized by toxic debris in the brain, such as Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and Huntington’s disease. The hope is that this kind of research will ultimately lead to more effective treatments for Alzheimer’s and other conditions affecting the brain.
References:
[1] FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval. U.S. Food and Drug Administration (2023).
[2] Hou J, et al. Antibody-mediated targeting of human microglial leukocyte Ig-like receptor B4 attenuates amyloid pathology in a mouse model. Science Translational Medicine. DOI: 10.1126/scitranslmed.adj9052 (2024).
NIH Support: National Institute of General Medical Sciences, National Institute on Aging