chromosomes – NIH Director's Blog (original) (raw)
Study Offers New Clues to Why Most People with Autoimmune Diseases Are Women
Posted on February 15th, 2024 by Dr. Monica M. Bertagnolli
Xist molecules shut down one of two female X chromosomes to avoid toxic protein levels, but they may also play a role in triggering autoimmune diseases. Credit: Donny Bliss/NIH
As many as 50 million Americans have one of more than 100 known autoimmune diseases, making it the third most prevalent disease category, surpassed only by cancer and heart disease.1,2 This category of disease has also long held a mystery: Why are most people with a chronic autoimmune condition—as many as four out of every five—women? This sex-biased trend includes autoimmune diseases such as rheumatoid arthritis, multiple sclerosis, scleroderma, lupus, Sjögren’s syndrome, and many others.
Now, exciting findings from a study supported in part by NIH provide a clue to why this may be the case, with potentially important implications for the early detection, treatment, and prevention of autoimmune diseases. The new evidence, reported in the journal Cell, suggests that more women develop autoimmune diseases than men due in part to the most fundamental difference between the biological sexes: that females have two X chromosomes, while males have an X and a Y. More specifically, it has to do with molecules called Xist (pronounced “exist”), which are encoded on the X chromosome and transcribed into long non-coding stretches of RNA, only when there are two X chromosomes.
Those long Xist molecules wind themselves around sections of just one of a female’s two X chromosomes, shutting down the extra X chromosome in a process known as X-chromosome inactivation. It’s an essential process to ensure those cells won’t produce too many proteins encoded on X chromosomes, which would be a deadly mistake. It’s also something that males, with a single X chromosome and much smaller Y chromosome carrying almost no working genes, don’t have to worry about.
The new findings come from a team at Stanford University School of Medicine, Stanford, CA, led by Howard Chang and Diana Dou. What they suggest is that while Xist molecules play an essential role in X-chromosome inactivation, they also have a more nefarious ability to encourage the formation of odd clumps of RNA, DNA, and proteins that can in turn trigger strong autoimmune responses.
In earlier research, the team identified about 80 different proteins that bind to Xist either directly or indirectly. After taking a close look at the list, the researchers realized that many of the proteins had been shown to play some role in autoimmune conditions. This raised an intriguing question: Could the reason women develop autoimmune diseases so much more often than men be explained by those Xist-containing clumps?
To test the idea, the researchers first decided to study it in male mice. They made two different strains of male mice produce Xist to see if it would increase their risk for autoimmunity in ways they could measure. And it did. The researchers found that once Xist was activated in male mice that were genetically prone to autoimmunity, they became more susceptible to developing a lupus-like condition. It didn’t happen in every individual, which suggests, not surprisingly, that the development of autoimmune disease requires additional triggers as well.
In addition, in a different mouse strain that was resistant to developing autoimmunity, the addition of Xist in males wasn’t enough to cause autoimmunity, the researchers found. That also makes sense in that, while women are much more prone to developing autoimmune disease, most people don’t. Xist complexes likely lead to autoimmunity only when certain genetic and other factors are met.
The researchers also examined blood samples from 100 people with autoimmune conditions and found they had antibodies to many of their own Xist complexes. Some of those antibodies also appeared specific to a certain autoimmune disorder, suggesting that they might be useful for tests that could detect autoimmunity or particular autoimmune conditions even before symptoms arise.
There are still many questions to explore in future research, including why men sometimes do get autoimmune conditions, and what other key triggers drive the development of autoimmunity. But this fundamentally important discovery points to potentially new ways to think about the causes for the autoimmune conditions that affect so many people in communities here and around the world.
References:
[1] The American Autoimmune Related Diseases Association. Autoimmune Facts.
[2] Dou DR, et al. Xist ribonucleoproteins promote female sex-biased autoimmunity. Cell. DOI: 10.1016/j.cell.2023.12.037. (2024).
NIH Support: National Institute of Arthritis and Musculoskeletal and Skin Diseases
Tags: autoimmune disease, autoimmune disorders, chromosomes, lupus, multiple sclerosis, rheumatoid arthritis, scleroderma, Sjogren's syndrome, women's health, Xist
Building a 3D Map of the Genome
Posted on October 4th, 2018 by Dr. Francis Collins
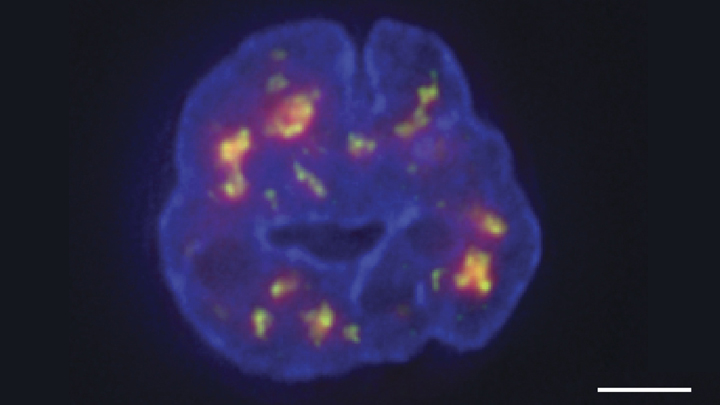
Credit: Chen et al., 2018
Researchers have learned a lot in recent years about how six-plus feet of human DNA gets carefully packed into a tiny cell nucleus that measures less than .00024 of an inch. Under those cramped conditions, we’ve been learning more and more about how DNA twists, turns, and spatially orients its thousands of genes within the nucleus and what this positioning might mean for health and disease.
Thanks to a new technique developed by an NIH-funded research team, there is now an even more refined view [1]. The image above features the nucleus (blue) of a human leukemia cell. The diffuse orange-red clouds highlight chemically labeled DNA found in close proximity to the tiny nuclear speckles (green). You’ll need to look real carefully to see the nuclear speckles, but these structural landmarks in the nucleus have long been thought to serve as storage sites for important cellular machinery.
Posted In: Snapshots of Life
Tags: 3D genome map, cell biology, cell nucleus, chromatin, chromosomes, DNA, genome, genome organization, nuclear speckles, Santiago Ramon y Cajal, transcription, TSA-Seq, tyramide
Cool Videos: A Biological Fireworks Display
Posted on June 29th, 2017 by Dr. Francis Collins
Let’s kick off the Fourth of July weekend with some biological fireworks! While we’ve added a few pyrotechnic sound effects just for fun, what you see in this video is the product of some serious research. Using a specialized microscope equipped with a time-lapse camera to image fluorescence-tagged proteins in real-time, an NIH-funded team has captured a critical step in the process of cell division, or mitosis: how filaments called microtubules (red) form new branches (green) and fan out to form mitotic spindles.
In this particular experimental system, the team led by Sabine Petry at Princeton University, Princeton, NJ, studies the dynamics of microtubules in a cell-free extract of cytoplasm taken from the egg of an African clawed frog (Xenopus laevis). Petry’s ultimate goal is to learn how to build mitotic spindles, molecule by molecule, in the lab. Such an achievement would mark a major step forward in understanding the complicated mechanics of cell division, which, when disrupted, can cause cancer and many other health problems.
Posted In: Health, Science, Video
Tags: biological fireworks, branching microtubule nucleation, branching microtubules, cell biology, cell division, chromosomes, fluorescence microscopy, frog, frog eggs, gamma-TuRC, microtubules, mitotic spindle, NIH Director’s 2016 New Innovator Award, Princeton’s 2017 Art of Science, Ran, TPX2, xenopus, Xenopus laevis
“OMG” Microscope Lives Up To Its Name
Posted on March 21st, 2013 by Dr. Francis Collins
Courtesy of Indiana University
The scientists at the IU School of Medicine-Bloomington nicknamed their new microscope the “OMG” for good reason—the images it produces are showstoppers. The DeltaVision OMX imaging system (its official title) is a $1.2 million dollar microscope that can peek inside a cell and image fluorescent proteins in unprecedented detail.
Jane Stout, a researcher in the NIH-funded lab, used the OMG to create this spectacular image that won her first place in the high- and super-resolution microscopy category of the 2012 GE Healthcare Life Sciences Cell Imaging Competition.
What you’re looking at is a cell in the midst of dividing into two identical copies—a process called mitosis. Here, the chromosomes (in blue) are aligned at the cell’s equator. Microtubules (red) from opposite poles of the cell attach to the chromosomes using the kinetochores (green) and pull them to opposite ends of the cell, which then splits in half. But sometimes cells do not divide properly—a common problem in cancer. Understanding the mechanics of cell division could help us correct this process when it goes wrong.
Jane Stout’s prize: her mitosis image will light up a billboard in Times Square in New York City in April. That is a wonderful celebration of science!
NIH support: the National Institute of General Medical Sciences
