Chronic Pain – NIH Director's Blog (original) (raw)
Pain Circuit Discovery in the Brain Suggests Promising Alternative to Opioid Painkillers
Posted on October 3rd, 2023 by Lawrence Tabak, D.D.S., Ph.D.
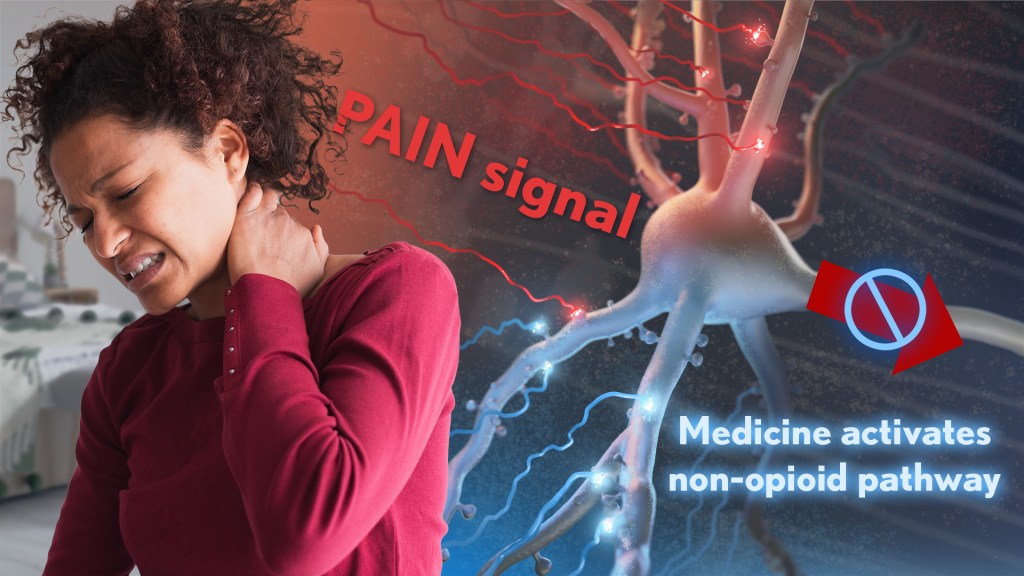
A pain signal (red) is sent to a neuron in the brain. Researchers have identified a novel anti-pain pathway (blue) that works differently from opioids. Medicines activating this pathway could deliver pain relief long-term with limited risk for withdrawal symptoms or addiction. Credit Donny Bliss/NIH
Chronic pain is an often-debilitating health condition and serious public health concern, affecting more than 50 million Americans.1 The opioid and overdose crisis, which stems from inadequate pain treatment, continues to have a devastating impact on families and communities across the country. To combat both challenges, we urgently need new ways to treat acute and chronic pain effectively without the many downsides of opioids.
While there are already multiple classes of non-opioid pain medications and other approaches to manage pain, unfortunately none have proved as effective as opioids when it comes to pain relief. So, I’m encouraged to see that an NIH-funded team now has preclinical evidence of a promising alternative target for pain-relieving medicines in the brain.2
Rather than activating opioid receptors, the new approach targets receptors for a nerve messenger known as acetylcholine in a portion of the brain involved in pain control. Based on findings from animal models, it appears that treatments targeting acetylcholine could offer pain relief even in people who have reduced responsiveness to opioids. Their findings suggest that the treatment approach has the potential to remain effective in combatting pain long-term and with limited risk for withdrawal symptoms or addiction.
The researchers, led by Daniel McGehee, University of Chicago, focused their attention on non-opioid pathways in the ventrolateral periaqueductal gray (vlPAG), a brain area involved in pain control. They had previously shown that activating acetylcholine receptors, which are part of the vlPAG’s underlying circuitry, could relieve pain.3 However, they found that when the body is experiencing pain, it unexpectedly suppresses acetylcholine rather than releasing more.
To understand how and why this is happening, McGehee and Shivang Sullere, now a postdoctoral fellow at Harvard Medical School, conducted studies in mice to understand how acetylcholine is released under various pain states. They found that mice treated with a drug that stimulates an acetylcholine receptor known as alpha-7 (⍺7) initially led to more activity in the nervous system. But this activity quickly gave way to a prolonged inactive or quiet state that delivered pain relief. Interestingly, this unexpected inhibitory effect lasted for several hours.
Additional studies in mice that had developed a tolerance to opioids showed the same long-lasting pain relief. This encouraging finding was expected since opioids use a pathway separate from acetylcholine. In more good news, the animals didn’t show any signs of dependence or addiction either. For instance, in the absence of pain, they didn’t prefer spending time in environments where they’d receive the ⍺7-targeted drug.
Imaging studies measuring brain activity revealed greater activity in cells expressing ⍺7 with higher levels of pain. When that activity was blocked, pain levels dropped. The finding suggests to the researchers it may be possible to monitor pain levels through brain imaging. It’s also possible the acetylcholine circuitry in the brain may play a role in the process whereby acute or temporary pain becomes chronic.
Finding treatments to modify acetylcholine levels or target acetylcholine receptors may therefore offer a means to treat pain and prevent it from becoming chronic. Encouragingly, drugs acting on these receptors already have been tested for use in people for treating other health conditions. It will now be important to learn whether these existing therapeutics or others like them may act as highly effective, non-addictive painkillers, with important implications for alleviating chronic pain.
References:
[1] NIH HEAL Initiative Research Plan. NIH HEAL Initiative.
[2] Sullere S et al. A cholinergic circuit that relieves pain despite opioid tolerance. Neuron. DOI: 10.1016/j.neuron.2023.08.017 (2023).
[3] Umana IC et al. Nicotinic modulation of descending pain control circuitry. Pain. PMID: 28817416; PMCID: PMC5873975 (2017).
Links:
The Helping to End Addiction Long-term® (HEAL) Initiative (NIH)
Pain (National Institute of Neurological Disorders and Stroke/NIH)
Opioids (National Institute on Drug Abuse/NIH)
Daniel McGehee (University of Chicago, Illinois)
NIH Support: National Institute of Neurological Disorders and Stroke, National Institute on Drug Abuse
Tags: acetylcholine, addiction, alpha-7, brain, Chronic Pain, neuron, opioid, opioid painkillers, opioid tolerance, overdose, pain, pain management
Visit the New NIH Virtual Tour
Posted on July 3rd, 2023 by Lawrence Tabak, D.D.S., Ph.D.
Happy Fourth of July! Before everyone heads out to celebrate the holiday with their family and friends, I want to share this brief video with you. It’s an introduction to the brand-new NIH Virtual Tour that’s now available on our website. When time permits, I encourage everyone to take the full tour of our Bethesda, MD, main campus and explore this great institution of science, technological innovation, and, above all, hope.
Among the virtual tour’s many features is an interactive, aerial map of the 32 buildings on our Bethesda campus. By clicking on a highlighted building, you can explore an impressive multimedia gallery of photos, video clips, and other resources. The tour will allow you to learn more about NIH and the ways in which we help people live longer and healthier lives.
You also can learn more about NIH’s 27 Institutes and Centers, including the NIH Clinical Center and 20 other in-depth tour stops—from research labs to patient rooms—and hear directly from some of our impressive researchers, leaders, and patients. For example, you can learn about chronic pain research from a lab in the NIH Clinical Center or see the largest zebrafish facility in the world, housed in Building 6.
What I like most about the virtual tour is that it captures what makes NIH so special—the many amazing people who collaborate every day to discover ways to solve seemingly intractable research problems. I admire their commitment to follow the science wherever it may lead.
In fact, from its humble beginnings in a one-room laboratory in 1887, NIH has become the world’s largest funder of medical research, whether that’s mobilizing to combat a deadly pandemic or strategizing to help people with a rare disorder find answers.
Not only does NIH conduct groundbreaking research in its own labs and clinics, it also supports much of the medical research conducted at universities and institutions in your states and local communities. Whether in Bethesda or beyond the Beltway, this national research effort will continue to yield the needed understanding to turn discovery into better health, helping more people to flourish and lead fully productive lives, now and in the generations to come.
That’s certainly something we can all celebrate this holiday, the 247th birthday of our great nation that I’m so honored to serve. Have a great, but safe, Fourth of July, and I’ll see you back here soon to share another blog post and another story of NIH-supported research progress.
Links:
Virtual Tour (NIH)
Visitor Information (NIH)
The Office of NIH History & Stetten Museum (NIH)
NIH HEAL Initiative Meets People Where They Are
Posted on April 18th, 2023 by Rebecca Baker, Ph.D., NIH Helping to End Addiction Long-term® (HEAL) Initiative

Credit: NIH HEAL Initiative
The opioid crisis continues to devastate communities across America. Dangerous synthetic opioids, like fentanyl, have flooded the illicit drug supply with terrible consequences. Tragically, based on our most-recent data, about 108,000 people in the U.S. die per year from overdoses of opioids or stimulants [1]. Although this complex public health challenge started from our inability to treat pain effectively, chronic pain remains a life-altering problem for 50 million Americans.
To match the size and complexity of the crisis, in 2018 NIH developed the NIH Helping to End Addiction Long-term® (HEAL) Initiative, an aggressive effort involving nearly all of its 27 institutes and centers. Through more than 1,000 research projects, including basic science, clinical testing of new and repurposed drugs, research with communities, and health equity research, HEAL is dedicated to building a new future built on hope.
In this future:
- A predictive tool used during a health visit personalizes treatment for back pain. The tool estimates the probability that a person will benefit from physical therapy, psychotherapy, or surgery.
- Visits to community health clinics and emergency departments serve as routine opportunities to prevent and treat opioid addiction.
- Qualified school staff and pediatricians screen all children for behavioral and other mental health conditions that increase risk for harmful developmental outcomes, including opioid misuse.
- Infants born exposed to opioids during a mother’s pregnancy receive high-quality care—setting them up for a healthy future.
Five years after getting started (and interrupted by a global pandemic), HEAL research is making progress toward achieving this vision. I’ll highlight three ways in which scientific solutions are meeting people where they are today.
A Window of Opportunity for Treatment in the Justice System
Sadly, jails and prisons are “ground zero” for the nation’s opioid crisis. Eighty-five percent of people who are incarcerated have a substance use disorder or a history of substance use. Our vision at HEAL is that every person in jail, prison, or a court-supervised program receives medical care, which includes effective opioid use disorder treatment.
Some research results already are in supporting this approach: A recent HEAL study learned that individuals who had received addiction treatment while in one Massachusetts jail were about 30 percent less likely to be arrested, arraigned, or incarcerated again compared with those incarcerated during the same time period in a neighboring jail that did not offer treatment [2]. Research from the HEAL-supported Justice Community Opioid Innovation Network also is exploring public perceptions about opioid addiction. One such survey showed that most U.S. adults see opioid use disorder as a treatable medical condition rather than as a criminal matter [3]. That’s hopeful news for the future.
A Personalized Treatment Plan for Chronic Back Pain
Half of American adults live with chronic back pain, a major contributor to opioid use. The HEAL-supported Back Pain Consortium (BACPAC) is creating a whole-system model for comprehensive testing of everything that contributes to chronic low back pain, from anxiety to tissue damage. It also includes comprehensive testing of promising pain-management approaches, including psychotherapy, antidepressants, or surgery.
Refining this whole-system model, which is nearing completion, includes finding computer-friendly ways to describe the relationship between the different elements of pain and treatment. That might include developing mathematical equations that describe the physical movements and connections of the vertebrae, discs, and tendons.
Or it might include an artificial intelligence technique called machine learning, in which a computer looks for patterns in existing data, such as electronic health records or medical images. In keeping with HEAL’s all-hands-on-deck approach, BACPAC also conducts clinical trials to test new (or repurposed) treatments and develop new technologies focused on back pain, like a “wearable muscle” to help support the back.
Harnessing Innovation from the Private Sector
The HEAL research portfolio spans basic science to health services research. That allows us to put many shots on goal that will need to be commercialized to help people. Through its research support of small businesses, HEAL funding offers a make-or-break opportunity to advance a great idea to the marketplace, providing a bridge to venture capital or other larger funding sources needed for commercialization.
This bridge also allows HEAL to invest directly in the heart of innovation. Currently, HEAL funds nearly 100 such companies across 20 states. While this is a relatively small portion of all HEAL research, it is science that will make a difference in our communities, and these researchers are passionate about what they do to build a better future.
A couple of current examples of this research passion include: delivery of controlled amounts of non-opioid pain medications after surgery using a naturally absorbable film or a bone glue; immersive virtual reality to help people with opioid use disorder visualize the consequences of certain personal choices; and mobile apps that support recovery, taking medications, or sensing an overdose.
In 2023, HEAL is making headway toward its mission to accelerate development of safe, non-addictive, and effective strategies to prevent and treat pain, opioid misuse, and overdose. We have 314 clinical trials underway and 41 submissions to the Food and Drug Administration to begin clinical testing of investigational new drugs or devices: That number has doubled in the last year. More than 100 projects alone are addressing back pain, and more than 200 projects are studying medications for opioid use disorder.
The nation’s opioid crisis is profoundly difficult and multifaceted—and it won’t be solved with any single approach. Our research is laser-focused on its vision of ending addiction long-term, including improving pain management and expanding access to underused, but highly effective, addiction medications. Every day, we imagine a better future for people with physical and emotional pain and communities that are hurting. Hundreds of researchers and community members across the country are working to achieve a future where people and communities have the tools they need to thrive.
References:
[1] Provisional drug overdose death counts. Ahmad FB, Cisewski JA, Rossen LM, Sutton P. National Center for Health Statistics. 2023.
[2] Recidivism and mortality after in-jail buprenorphine treatment for opioid use disorder. Evans EA, Wilson D, Friedmann PD. Drug Alcohol Depend. 2022 Feb 1;231:109254.
[3] Social stigma toward persons with opioid use disorder: Results from a nationally representative survey of U.S. adults. Taylor BG, Lamuda PA, Flanagan E, Watts E, Pollack H, Schneider J. Subst Use Misuse. 2021;56(12):1752-1764.
Links:
SAMHSA’s National Helpline (Substance Abuse and Mental Health Services Administration, Rockville, MD)
NIH Helping to End Addiction Long-term® (HEAL) Initiative
Video: The NIH HEAL Initiative–HEAL Is Hope
Justice Community Opioid Innovation Network (HEAL)
Back Pain Consortium Research Program (HEAL)
NIH HEAL Initiative 2023 Annual Report (HEAL)
Small Business Programs (HEAL)
Rebecca Baker (HEAL)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 28th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
Posted In: Generic
Tags: AI, artificial intelligence, Back Pain, Back Pain Consortium, BACPAC, child health, Chronic Pain, clinical trial, drug repurposing, drugs, FDA, HEAL, health equity, Helping to End Addiction Long-term, jails, Justice Community Opioid Innovation Network, machine learning, opioid addiction, opioid crisis, pain, prisons, psychotherapy, small business, substance use disorders, wearable muscle
A Whole Person Approach to Lifting the Burden of Chronic Pain Among Service Members and Veterans
Posted on March 28th, 2023 by Helene M. Langevin, M.D., National Center for Complementary and Integrative Health

Credit: U.S. Army; Getty Images; U.S. Department of Veterans Affairs; Sgt. Jennifer Spradlin, U.S. Army
Chronic pain and its companion crisis of opioid misuse have taken a terrible toll on Americans. But the impact has been even greater on U.S. service members and veterans, who often deal with the compounded factors of service-related injuries and traumatic stress.
For example, among soldiers in a leading U.S. Army unit, 44 percent had chronic pain and 15 percent used opioids after a combat deployment. That compares to 26 percent and 4 percent, respectively, in the general population [1,2].
This disproportionate burden of chronic pain among veterans [3] and service members led NIH’s National Center for Complementary and Integrative Health (NCCIH) to act. We forged a collaboration in 2017 across NIH, U.S. Department of Defense (DOD), and U.S. Department of Veteran’s Affairs (VA) to establish the Pain Management Collaboratory (PMC).
The PMC’s research focusing on the implementation and evaluation of nondrug approaches for the management of pain is urgently needed in the military and across our entire country. Nondrug approaches require a shift in thinking. Rather than focusing solely on blocking pain temporarily using analgesics, nondrug approaches work with the mind and body to promote the resolution of chronic pain and the long-term restoration of health through techniques and practices such as manual therapy, yoga, and mindfulness-based interventions.
Addressing chronic pain in ways that don’t only rely on drugs means addressing underlying issues, such as joints and connective tissue that lack adequate movement or training our brains to “turn down the volume” on pain signals. Using mind and body practices to reduce pain can help promote health in other ways. Possible “fringe benefits” include better sleep, more energy for physical activity, a better mindset for making good nutritional choices, and/or improved mood.
Indeed, there is a growing body of research on the benefits of nondrug approaches to address chronic pain. What is so powerful about PMC is it puts this knowledge to work by embedding research within military health care settings.
The PMC supports a shared resource center and 11 large-scale pragmatic clinical trials. Within this real-world health care setting, the clinical trials have enrolled more than 8,200 participants across 42 veteran and military health systems. These studies offer both strength in numbers and insights into what happens when learnings from controlled clinical trials collide with the realities of health care delivery and the complexities of daily life. [4]
Central to the PMC partnership is whole person health. Too often, we see health through the prism of separate parts—for example, a person’s cardiovascular, digestive, and mental health problems are viewed as co-occurring rather than as interrelated conditions. A whole person framework—a central focus of NCCIH’s current Strategic Plan—brings the parts back together and recognizes that health exists across multiple interconnected body systems and domains: biological, behavioral, social, and environmental.
The VA’s implementation of a whole health model [5] and their unique closed-loop health care system offers an opportunity to deliver care, conduct research, and illustrate what happens when people receive coordinated care that treats the whole person. In fact, VA’s leadership in this area was the impetus for a recent report by the National Academies of Sciences, Engineering, and Medicine. The report underscored the importance of implementing whole person health care in all settings and for every American.
There are many opportunities ahead for this interagency collaboration. It will help to achieve an important shift, from treating problems one at a time to promoting overall military readiness, resilience, and well-being for U.S. service members and veterans.
Congress appropriated $5 million to NCCIH in fiscal year 2023 to enhance pain research with a special emphasis on military populations. These additional resources will allow NCCIH to support more complex studies in understanding how multiple therapeutic approaches that impact multiple body systems can impact chronic pain.
Meanwhile, programs like the DOD’s Consortium for Health and Military Performance (CHAMP) will continue to translate these lessons learned into accessible pain management information that service members can use in promoting and maintaining their health.
While the PMC’s research program specifically targets the military community, this growing body of knowledge will benefit us all. Understanding how to better manage chronic pain and offering more treatment options for those who want to avoid the risks of opioids will help us all build resilience and restore health of the whole person.
References:
[1] Chronic pain and opioid use in US soldiers after combat deployment. Toblin RL, Quartana PJ, Riviere LA, Walper KC, Hoge CW. JAMA Intern. Med. 2014 Aug;174(8):1400-1401.
[2] Pain and opioids in the military: We must do better. Jonas WB, Schoomaker EB. JAMA Intern. Med. 2014 Aug;174(8):1402-1403
[3] Severe pain in veterans: The effect of age and sex, and comparisons with the general population. Nahin RL. J Pain. 2017 Mar; 18(3):247-254.
[4] Justice and equity in pragmatic clinical trials: Considerations for pain research within integrated health systems. Ali J, Davis AF, Burgess DJ, Rhon DI, Vining R, Young-McCaughan S, Green S, Kerns RD. Learn Health Sys. 2021 Oct 19;6(2): e10291
[5] The APPROACH trial: Assessing pain, patient-reported outcomes, and complementary and integrative health. Zeliadt S, Coggeshall S, Thomas E, Gelman H, Taylor S. Clin. Trials. 2020 Aug;17(4):351-359.
Links:
National Center for Complementary and Integrative Health (NIH)
NCCIH Strategic Plan FY 2021-2025: Mapping a Pathway to Research on Whole Person Health (NIH)
Pain Management Collaboratory (Yale University, New Haven, CT)
Whole Health (U.S Department of Veteran’s Affairs, Washington, D.C.)
Consortium for Health and Military Performance (Department of Defense, Bethesda, MD)
Achieving Whole Health: A New Approach for Veterans and the Nation. (National Academies of Sciences, Engineering, and Medicine, Washington, D.C.)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes, Centers, and Offices to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 26th in the series of NIH guest posts that will run until a new permanent NIH director is in place.
Posted In: Generic
Tags: APPROACH trial, CHAMP, Chronic Pain, clinical trial, Consortium for Health and Military Performance, DOD, National Academy of Sciences Engineering and Medicine, NCCIH, opioid addiction, opioid misuse, pain, pain management, Pain Management Collaboratory, PMC, pragmatic trials, service members, Veteran's Health Administration, whole health
Biomedical Research Leads Science’s 2021 Breakthroughs
Posted on January 4th, 2022 by Lawrence Tabak, D.D.S., Ph.D.

Hi everyone, I’m Larry Tabak. I’ve served as NIH’s Principal Deputy Director for over 11 years, and I will be the acting NIH director until a new permanent director is named. In my new role, my day-to-day responsibilities will certainly increase, but I promise to carve out time to blog about some of the latest research progress on COVID-19 and any other areas of science that catch my eye.
I’ve also invited the directors of NIH’s Institutes and Centers (ICs) to join me in the blogosphere and write about some of the cool science in their research portfolios. I will publish a couple of posts to start, then turn the blog over to our first IC director. From there, I envision alternating between posts from me and from various IC directors. That way, we’ll cover a broad array of NIH science and the tremendous opportunities now being pursued in biomedical research.
Since I’m up first, let’s start where the NIH Director’s Blog usually begins each year: by taking a look back at _Science_’s Breakthroughs of 2021. The breakthroughs were formally announced in December near the height of the holiday bustle. In case you missed the announcement, the biomedical sciences accounted for six of the journal _Science_’s 10 breakthroughs. Here, I’ll focus on four biomedical breakthroughs, the ones that NIH has played some role in advancing, starting with _Science_’s editorial and People’s Choice top-prize winner:
Breakthrough of the Year: AI-Powered Predictions of Protein Structure
The biochemist Christian Anfinsen, who had a distinguished career at NIH, shared the 1972 Nobel Prize in Chemistry, for work suggesting that the biochemical interactions among the amino acid building blocks of proteins were responsible for pulling them into the final shapes that are essential to their functions. In his Nobel acceptance speech, Anfinsen also made a bold prediction: one day it would be possible to determine the three-dimensional structure of any protein based on its amino acid sequence alone. Now, with advances in applying artificial intelligence to solve biological problems—Anfinsen’s bold prediction has been realized.
But getting there wasn’t easy. Every two years since 1994, research teams from around the world have gathered to compete against each other in developing computational methods for predicting protein structures from sequences alone. A score of 90 or above means that a predicted structure is extremely close to what’s known from more time-consuming work in the lab. In the early days, teams more often finished under 60.
In 2020, a London-based company called DeepMind made a leap with their entry called AlphaFold. Their deep learning approach—which took advantage of 170,000 proteins with known structures—most often scored above 90, meaning it could solve most protein structures about as well as more time-consuming and costly experimental protein-mapping techniques. (AlphaFold was one of _Science_’s runner-up breakthroughs last year.)
This year, the NIH-funded lab of David Baker and Minkyung Baek, University of Washington, Seattle, Institute for Protein Design, published that their artificial intelligence approach, dubbed RoseTTAFold, could accurately predict 3D protein structures from amino acid sequences with only a fraction of the computational processing power and time that AlphaFold required [1]. They immediately applied it to solve hundreds of new protein structures, including many poorly known human proteins with important implications for human health.
The DeepMind and RoseTTAFold scientists continue to solve more and more proteins [1,2], both alone and in complex with other proteins. The code is now freely available for use by researchers anywhere in the world. In one timely example, AlphaFold helped to predict the structural changes in spike proteins of SARS-CoV-2 variants Delta and Omicron [3]. This ability to predict protein structures, first envisioned all those years ago, now promises to speed fundamental new discoveries and the development of new ways to treat and prevent any number of diseases, making it this year’s Breakthrough of the Year.
Anti-Viral Pills for COVID-19
The development of the first vaccines to protect against COVID-19 topped _Science_’s 2020 breakthroughs. This year, we’ve also seen important progress in treating COVID-19, including the development of anti-viral pills.
First, there was the announcement in October of interim data from Merck, Kenilworth, NJ, and Ridgeback Biotherapeutics, Miami, FL, of a significant reduction in hospitalizations for those taking the anti-viral drug molnupiravir [4] (originally developed with an NIH grant to Emory University, Atlanta). Soon after came reports of a Pfizer anti-viral pill that might target SARS-CoV-2, the novel coronavirus that causes COVID-19, even more effectively. Trial results show that, when taken within three days of developing COVID-19 symptoms, the pill reduced the risk of hospitalization or death in adults at high risk of progressing to severe illness by 89 percent [5].
On December 22, the Food and Drug Administration (FDA) granted Emergency Use Authorization (EUA) for Pfizer’s Paxlovid to treat mild-to-moderate COVID-19 in people age 12 and up at high risk for progressing to severe illness, making it the first available pill to treat COVID-19 [6]. The following day, the FDA granted an EUA for Merck’s molnupiravir to treat mild-to-moderate COVID-19 in unvaccinated, high-risk adults for whom other treatment options aren’t accessible or recommended, based on a final analysis showing a 30 percent reduction in hospitalization or death [7].
Additional promising anti-viral pills for COVID-19 are currently in development. For example, a recent NIH-funded preclinical study suggests that a drug related to molnupiravir, known as 4’-fluorouridine, might serve as a broad spectrum anti-viral with potential to treat infections with SARS-CoV-2 as well as respiratory syncytial virus (RSV) [8].
Artificial Antibody Therapies
Before anti-viral pills came on the scene, there’d been progress in treating COVID-19, including the development of monoclonal antibody infusions. Three monoclonal antibodies now have received an EUA for treating mild-to-moderate COVID-19, though not all are effective against the Omicron variant [9]. This is also an area in which NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) public-private partnership has made big contributions.
Monoclonal antibodies are artificially produced versions of the most powerful antibodies found in animal or human immune systems, made in large quantities for therapeutic use in the lab. Until recently, this approach had primarily been put to work in the fight against conditions including cancer, asthma, and autoimmune diseases. That changed in 2021 with success using monoclonal antibodies against infections with SARS-CoV-2 as well as respiratory syncytial virus (RSV), human immunodeficiency virus (HIV), and other infectious diseases. This earned them a prominent spot among _Science_’s breakthroughs of 2021.
Monoclonal antibodies delivered via intravenous infusions continue to play an important role in saving lives during the pandemic. But, there’s still room for improvement, including new formulations highlighted on the blog last year that might be much easier to deliver.
CRISPR Fixes Genes Inside the Body
One of the most promising areas of research in recent years has been gene editing, including CRISPR/Cas9, for fixing misspellings in genes to treat or even cure many conditions. This year has certainly been no exception.
CRISPR is a highly precise gene-editing system that uses guide RNA molecules to direct a scissor-like Cas9 enzyme to just the right spot in the genome to cut out or correct disease-causing misspellings. Science highlights a small study reported in The New England Journal of Medicine by researchers at Intellia Therapeutics, Cambridge, MA, and Regeneron Pharmaceuticals, Tarrytown, NY, in which six people with hereditary transthyretin (TTR) amyloidosis, a condition in which TTR proteins build up and damage the heart and nerves, received an infusion of guide RNA and CRISPR RNA encased in tiny balls of fat [10]. The goal was for the liver to take them up, allowing Cas9 to cut and disable the TTR gene. Four weeks later, blood levels of TTR had dropped by at least half.
In another study not yet published, researchers at Editas Medicine, Cambridge, MA, injected a benign virus carrying a CRISPR gene-editing system into the eyes of six people with an inherited vision disorder called Leber congenital amaurosis 10. The goal was to remove extra DNA responsible for disrupting a critical gene expressed in the eye. A few months later, two of the six patients could sense more light, enabling one of them to navigate a dimly lit obstacle course [11]. This work builds on earlier gene transfer studies begun more than a decade ago at NIH’s National Eye Institute.
Last year, in a research collaboration that included former NIH Director Francis Collins’s lab at the National Human Genome Research Institute (NHGRI), we also saw encouraging early evidence in mice that another type of gene editing, called DNA base editing, might one day correct Hutchinson-Gilford Progeria Syndrome, a rare genetic condition that causes rapid premature aging. Preclinical work has even suggested that gene-editing tools might help deliver long-lasting pain relief. The technology keeps getting better, too. This isn’t the first time that gene-editing advances have landed on _Science_’s annual Breakthrough of the Year list, and it surely won’t be the last.
The year 2021 was a difficult one as the pandemic continued in the U.S. and across the globe, taking far too many lives far too soon. But through it all, science has been relentless in seeking and finding life-saving answers, from the rapid development of highly effective COVID-19 vaccines to the breakthroughs highlighted above.
As this list also attests, the search for answers has progressed impressively in other research areas during these difficult times. These groundbreaking discoveries are something in which we can all take pride—even as they encourage us to look forward to even bigger breakthroughs in 2022. Happy New Year!
References:
[1] Accurate prediction of protein structures and interactions using a three-track neural network. Baek M, DiMaio F, Anishchenko I, Dauparas J, Grishin NV, Adams PD, Read RJ, Baker D., et al. Science. 2021 Jul 15:eabj8754.
[2] Highly accurate protein structure prediction with AlphaFold. Jumper J, Evans R, Pritzel A, Green T, Senior AW, Kavukcuoglu K, Kohli P, Hassabis D. et al. Nature. 2021 Jul 15.
[3] Structural insights of SARS-CoV-2 spike protein from Delta and Omicron variants. Sadek A, Zaha D, Ahmed MS. preprint bioRxiv. 2021 Dec 9.
[5] Pfizer’s novel COVID-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 EPIC-HR Study. Pfizer. 5 November 52021.
[6] Coronavirus (COVID-19) Update: FDA authorizes first oral antiviral for treatment of COVID-19. Food and Drug Administration. 22 Dec 2021.
[7] Coronavirus (COVID-19) Update: FDA authorizes additional oral antiviral for treatment of COVID-19 in certain adults. Food and Drug Administration. 23 Dec 2021.
[8] 4′-Fluorouridine is an oral antiviral that blocks respiratory syncytial virus and SARS-CoV-2 replication. Sourimant J, Lieber CM, Aggarwal M, Cox RM, Wolf JD, Yoon JJ, Toots M, Ye C, Sticher Z, Kolykhalov AA, Martinez-Sobrido L, Bluemling GR, Natchus MG, Painter GR, Plemper RK. Science. 2021 Dec 2.
[9] Anti-SARS-CoV-2 monoclonal antibodies. NIH COVID-19 Treatment Guidelines. 16 Dec 2021.
[10] CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. Gillmore JD, Gane E, Taubel J, Kao J, Fontana M, Maitland ML, Seitzer J, O’Connell D, Walsh KR, Wood K, Phillips J, Xu Y, Amaral A, Boyd AP, Cehelsky JE, McKee MD, Schiermeier A, Harari O, Murphy A, Kyratsous CA, Zambrowicz B, Soltys R, Gutstein DE, Leonard J, Sepp-Lorenzino L, Lebwohl D. N Engl J Med. 2021 Aug 5;385(6):493-502.
[11] Editas Medicine announces positive initial clinical data from ongoing phase 1/2 BRILLIANCE clinical trial of EDIT-101 For LCA10. Editas Medicine. 29 Sept 2021.
Links:
Structural Biology (National Institute of General Medical Sciences/NIH)
The Structures of Life (NIGMS)
COVID-19 Research (NIH)
2021 Science Breakthrough of the Year (American Association for the Advancement of Science, Washington, D.C)
Posted In: News
Tags: 4'-fluorouridine, Accelerating COVID-19 Therapeutic Interventions and Vaccines, ACTIV, AI, AlphaFold, amino acids, artificial antibodies, artificial intelligence, biochemistry, Christian Anfinsen, Chronic Pain, computational biology, coronavirus, COVID pill, COVID-19, CRISPR, CRISPR/Cas9, DeepMind, Delta variant, Editas Medicine, EUA, gene editing, hereditary transthyretin amyloidosis, Hutchinson-Gilford progeria syndrome, Intellia Therapeutics, Leber congenital amaurosis, Merck, molnupiravir, monoclonal antibodies, Omicron variant, pandemic, Pfizer, progeria, protein structure, rare disease, Regeneron, RoseTTAFold, SARS-CoV-2, Science Breakthrough of the Year, Science Breakthroughs of 2021, structural biology
U.S. Surgeon General on Emotional Well-Being and Fighting the Opioid Epidemic
Posted on June 10th, 2021 by Dr. Francis Collins

From September 2019 to September 2020, the Centers for Disease Control and Prevention reported nearly 90,000 overdose deaths in the United States. These latest data on the nation’s opioid crisis offer another stark reminder that help is desperately needed in communities across the land. NIH’s research efforts to address the opioid crisis have been stressed during the pandemic, but creative investigators have come up with workarounds like wider use of telemedicine to fill the gap.
Much of NIH’s work on the opioid crisis is supported by the Helping to End Addiction Long-term (HEAL) Initiative. Recently, the more-than 500 investigators supported by HEAL came together virtually for their second annual meeting to discuss the initiative’s latest research progress and challenges.
As part of the meeting, I had a conversation with Dr. Vivek Murthy, the U.S. Surgeon General. Dr. Murthy served as the 19th U.S. Surgeon General under the Obama Administration and was recently confirmed as the 21st Surgeon General under the Biden Administration. In his first term as America’s Doctor, in which I had the privilege of working with him, Dr. Murthy created initiatives to tackle our country’s most urgent public health issues, including addiction and the opioid crisis. He also issued the nation’s first Surgeon General’s Report on addiction, presenting the latest scientific data and issuing a call to action to recognize addiction as a chronic illness—and not a moral failing.
In 2016, Dr. Murthy sent a letter to 2.3 million healthcare professionals urging them to join a movement to tackle the opioid epidemic. This was the first time in the history of the office that a Surgeon General had issued a letter calling the medical profession to action on this issue. In 2017, Dr. Murthy focused his attention on chronic stress and isolation as prevalent problems with profound implications for health, productivity, and happiness.
Our conversation during the HEAL meeting took place via videoconference, with the Surgeon General connecting from Washington, D.C., and me linking in from my home in Maryland. Here’s a condensed transcript of our chat:
Collins: Welcome, Dr. Murthy. We’ve known each other for a few years, and I know that you’ve talked extensively about the national epidemic of loneliness. What have you learned about loneliness and how it affects our emotional wellbeing?
Murthy: Thanks, Francis. Loneliness and perceived social isolation are profound challenges for communities struggling with addiction, including opioid use disorders. I had no real background in these issues when I started as Surgeon General in 2014. I was educated by people I met all across the country, who in their own way would tell me their stories of isolation and loneliness. It’s a common stressor, especially for those who struggle with opioid use disorders. Stress can be a trigger for relapse. It’s also connected with overdose attempts and overdose deaths.
But loneliness is bigger than addiction. It is not just a bad feeling. Loneliness increases our risk of anxiety and depression, dementia, cardiac disease, and a host of other conditions. However you cut it, addressing social isolation and loneliness is an important public-health issue if we care about addiction, if we care about mental health—if we care about the physical wellbeing of people in our country.
Collins: Vivek, you made the diagnosis of an epidemic of American loneliness back before COVID-19 came along. With the emergence of COVID-19 a little more than a year ago, it caused us to isolate ourselves even more. Now that you’re back as Surgeon General and seeing the consequences of the worst pandemic in 103 years, is loneliness even worse now than before the pandemic?
Murthy: I think there are many people for whom that sense of isolation and loneliness has increased during the pandemic. But the pandemic has been a very heterogenous experience. There are some people who have found themselves more surrounded by their extended family or a close set of friends. That has been, in many ways, a luxury. For many people who are on the frontlines as essential workers, whose jobs don’t permit them to just pick up and leave and visit extended family, these have been very stressful and isolating times.
So, I am worried. And I’m particularly worried about young people—adolescents and young adults. They already had high rates of depression, anxiety, and suicide before the pandemic, and they’re now struggling with loneliness. I mention this because young people are so hyperconnected by technology, they seem to be on TikTok and Instagram all the time. They seem to be chatting with their friends constantly, texting all the time. How could they feel isolated or lonely?
But one of the things that has become increasingly clear is what matters when it comes to loneliness is the quality of your human connections, not the quantity. For many young people that I spoke to while traveling across the country, they would say that, yes, we’re connected to people all the time. But we don’t necessarily feel like we can always be ourselves in our social media environment. That’s where comparison culture is at its height. That’s where we feel like our lives are always falling short, whether it’s not having a fancy enough job, not having as many friends, or not having the right clothing or other accessories.
We talk a lot about resilience in our country. But how do we develop more resilient people? One of the keys is to recognize that social connections are an important source of resilience. They are our natural buffers for stress. When hard things happen in our lives, so many of us just instinctively will pick up the phone to call a friend. Or we’ll get into the car and go visit a member of our family or church. The truth is, if we want to build a society that’s healthier mentally and physically, that is more resilient, and that is also more happy and fulfilled, we have to think about how we build a society that is more centered around human connection and around relationships.
My hope is that one of the things we will reevaluate is building a people-centered society. That means designing workplaces that allow people to prioritize relationships. It means designing schools that equip our children with social and emotional learning tools to build healthy relationships from the earliest ages. It means thinking about public policy, not from just the standpoint of financial impact but in terms of how it impacts communities and how it can fracture communities.
We have an opportunity to do that now, but it won’t happen by default. We have to think through this very proactively, and it starts with our own lives. What does it mean for each of us to live a truly people-centered life? What decisions would we make differently about work, about how we spend time, about where we put our attention and energy?
Collins: Those are profound and very personal words that I think we can all relate to. Let me ask you about another vulnerable population that we care deeply about. There are 50 million Americans who are living with chronic pain, invisible to many, especially during the pandemic, for whom being even more isolated has been particularly rough—and who are perhaps in a circumstance where getting access to medical care has been challenging. As Surgeon General, are you also looking closely at the folks with chronic pain?
Murthy: You’re right, the populations that were more vulnerable pre-pandemic have really struggled during this pandemic—whether that’s getting medications for treatment, needed counseling services, or taking part in social support groups, which are an essential part of the overall treatment approach and staying in recovery. It’s a reminder of how urgent it is for us, number one, to improve access to healthcare in our country. We’ve made huge strides in this area, but millions are still out of reach of the healthcare system.
A potential silver lining of this pandemic is telemedicine, which has extraordinary potential to improve and extend access to services for people living with substance use disorders. In 2016, I remember visiting a small Alaskan fishing village that you can only get to by boat or plane. In that tiny village of 150 people, I walked into the small cabin where they had first-aid supplies and provided some basic medical care. There I saw a small monitor mounted on the wall and a chair. They told me that the monitor is where people, if they’re dealing with a substance use disorder, come and sit to get counseling services from people in the lower 48 states. I was so struck by that. To know that telemedicine could reach this remote Alaskan village was really extraordinary.
I think the pandemic has accelerated our adoption of telemedicine by perhaps five years or more. But we must sustain this momentum not only with investment in broadband infrastructure, but with other things that seem mundane, like the reimbursement structure around telemedicine. I talk to clinicians now who say they are seeing some private insurers go back on reimbursement for telemedicine because the pandemic is starting to get better. But the lesson learned is not that telemedicine should go away; it’s that we should be integrating it even more deeply into the practice of medicine.
The future of care, I believe, is bringing care closer to where people are, integrating it into their workflow, bringing it to their homes and their neighborhoods. I saw this so clearly for many of the patients I cared for who fell into that category of being in vulnerable populations. They were working two, three jobs, trying to take care of their children at the same time. Having a conversation with them about how they could find time to go to the gym was almost a laughable matter because they were literally dealing with issues of survival and putting food on the table for their kids. As a society we have to do more to understand the lives of people who fall into those categories and provide services that bring what they need to them, as opposed to expecting them to come to us.
If we continue in a purely fee-for-service-based environment where people must go multiple places to get their care, we will not ultimately get care to the vulnerable populations that have struggled the most and that are hoping that we will do better this time around. I think we can. I think we must. And I think COVID may just be, in part, the impetus to move forward in a different way that we need.
Collins: Let’s talk a minute about the specifics of the opioid crisis. If we’re going to move this crisis in the right direction, are there particular areas that you would say we really need more rigorous data in order to convince the medical care system—both the practitioners and the people deciding about reimbursement—that these are things we must do?
Murthy: There are a few areas that come to mind, and I’ve jotted them down. It is so important for us to do research with vulnerable populations, recognizing they often get left out. It’s essential that we conduct studies specifically for these populations so that we can better target interventions to them.
The second area is prevention programs. People want to prevent illnesses. I have not met anybody anywhere in the United States who has said, “I’d rather get diabetes first and treat it versus prevent it in the first place.” As silly as that might sound, it is the exact opposite of how we finance health interventions in our country. We put the lion’s share of our dollars in treatment. We do very little in prevention.
The third piece is the barriers faced by primary-care clinicians, who we want to be at the heart of providing a lot of these treatment services. I’ll tell you, just from my conversations with primary-care docs around the country, they worry about not having enough for their patients in the way of social work and social support services in their offices.
Finally, it has become extraordinarily clear to me that social support is one of the critical elements of treatment for substance use disorders. That it is what helps keep people in recovery. I think about the fact that many people I met who struggle with opioid use disorders had family members who were wondering how they could be helpful. They weren’t sure. They said, “Should I just keep badgering my relative to go to treatment? Should I take a tough love approach? What should I do to be helpful?”
This actually is one of the most pressing issues: social support is most often going to come from family, from friends, and from other community members. So, being able to guide them in an evidence-based way about what measures, what forms actually can be helpful to people struggling with opioid use disorders could also be immensely helpful to a group that is looking to provide assistance and support, but often is struggling to figure out how best to do that.
Collins: Vivek, you were focused as Surgeon General in the Obama Administration on the importance of changing how America thinks about addiction—that it is not a moral failing but a chronic illness that has to be treated with compassion, urgency, skill, and medical intervention. Are we getting anywhere with making that case?
Murthy: Sometimes people shy away from addressing the stigma around addiction because it feels too hard to address. But it is one of the most important issues to address. If people are still feeling judged for their disorders, they are not going to feel comfortable coming forward and getting treatment. And others will hesitate to step up and provide support.
I will always remember the young couple I met in Oklahoma who had lost their son to an opioid overdose. They told me that previously in their life whenever they had a struggle—a job loss or other health issue in the family—neighbors would come over, they would drop off food, they would visit and sit with them in their living room and hold their hands to see if they were okay. When their son died after opioid use disorder, it was silent. Nobody came over. It’s a very common story of how people feel ashamed, they feel uncomfortable, they don’t know quite what to say. So they stay away, which is the worst thing possible during these times of great pain and distress.
I do think we have made progress in the last few years. There are more people stepping forward to tell their stories. There are more people and practitioners who are embracing the importance of talking to their patients about substance use disorders and getting involved in treating them. But the truth is, we still have many people in the country who feel ashamed of what they’re dealing with. We still have many family members who feel that this is a source of shame to have a loved one struggling with a substance use disorder.
To me, this is much bigger than substance use disorders. This is a broader cultural issue of how we think about strength and vulnerability. We have defined strength in modern society as the loudest voice in the room or the person with the most physical prowess, the person who’s aggressive in negotiations, and the person who’s famous.
But I don’t think that’s what strength really is. Strength is so often displayed in moments of vulnerability when people have the courage to open up and be themselves. Strength is defined by the people who have the courage to display love, patience, and compassion, especially when it’s difficult. That’s what real strength is.
One of my hopes is that, as a society, we can ultimately redefine strength. As we think about our children and what we want them to be, we cannot aspire for them to be the loudest voice in the room. We can aspire for them to be the most-thoughtful, the most-welcoming, the most-inviting, the most-compassionate voice in the room.
If we truly want to be a society that’s grounded in love, compassion, and kindness, if we truly recognize those as the sources of strength and healing, we have to value those in our workplaces. They have to be reflected in our promotion systems. We have to value them in the classroom. Ultimately, we’ve got to build our lives around them.
That is a broader lesson that I took from all of the conversations I’ve had with people who struggle with opioid use disorders. What I took was, yes, we need medication and assisted treatment; yes, we need counseling services; yes, we need social services and wraparound services and recovery services. But the engine that will drive our healing is fundamentally the love and compassion that come from human relationships.
We all have the ability to heal because we all have the ability to be kind and to love one another. That’s the lesson that it took me more than two decades to learn in medicine. More important than any prescription that I could write is the compassion that I could extend to patients simply by listening, by showing up, by being present in their lives. We all have that ability, regardless of what degrees follow our name.
Collins: Vivek, this has been a wonderful conversation. We are fortunate to have you as our Surgeon General at this time, when we need lots of love and compassion.
Murthy: Thank you so much, Francis.
Links:
Opioids (National Institute on Drug Abuse/NIH)
Opioid Overdose Crisis (NIDA)
Vice Admiral Vivek H. Murthy (U.S. Department of Health and Human Services, Washington, D.C.)
Helping to End Addiction Long-term (HEAL) Initiative (NIH)
Video: Emotional Well Being and the Power of Connections to Fight the Opioid Epidemic (HEAL/NIH)
Posted In: Generic
Tags: addiction, adolescents, anxiety, Chronic Pain, COVID-19, depression, HEAL, HEAL Initiative, Helping to End Addiction Long-term, Instagram, loneliness, mental health, opioid crisis, opioid use disorder, opioids, overdose, pandemic, personal relationships, prevention, resilience, social media, social stigma, social support, stigma, stress, substance use disorders, support systems, Surgeon General Murthy, teen depression, telemedicine, TikTok, U. S. Surgeon General, Vivek Murthy, well-being, young adults
Could CRISPR Gene-Editing Technology Be an Answer to Chronic Pain?
Posted on April 1st, 2021 by Dr. Francis Collins

Credit: iStock/Firstsignal
Gene editing has shown great promise as a non-heritable way to treat a wide range of conditions, including many genetic diseases and more recently, even COVID-19. But could a version of the CRISPR gene-editing tool also help deliver long-lasting pain relief without the risk of addiction associated with prescription opioid drugs?
In work recently published in the journal Science Translational Medicine, researchers demonstrated in mice that a modified version of the CRISPR system can be used to “turn off” a gene in critical neurons to block the transmission of pain signals [1]. While much more study is needed and the approach is still far from being tested in people, the findings suggest that this new CRISPR-based strategy could form the basis for a whole new way to manage chronic pain.
This novel approach to treating chronic pain occurred to Ana Moreno, the study’s first author, when she was a Ph.D. student in the NIH-supported lab of Prashant Mali, University of California, San Diego. Mali had been studying a wide range of novel gene- and cell-based therapeutics. While reading up on both, Moreno landed on a paper about a mutation in a gene that encodes a pain-enhancing protein in spinal neurons called NaV1.7.
Moreno read that kids born with a loss-of-function mutation in this gene have a rare condition known as congenital insensitivity to pain (CIP). They literally don’t sense and respond to pain. Although these children often fail to recognize serious injuries because of the absence of pain to alert them, they have no other noticeable physical effects of the condition.
For Moreno, something clicked. What if it were possible to engineer a new kind of treatment—one designed to turn this gene down or fully off and stop people from feeling chronic pain?
Moreno also had an idea about how to do it. She’d been working on repressing or “turning off” genes using a version of CRISPR known as “dead” Cas9 [2]. In CRISPR systems designed to edit DNA, the Cas9 enzyme is often likened to a pair of scissors. Its job is to cut DNA in just the right spot with the help of an RNA guide. However, CRISPR-dead Cas9 no longer has any ability to cut DNA. It simply sticks to its gene target and blocks its expression. Another advantage is that the system won’t lead to any permanent DNA changes, since any treatment based on CRISPR-dead Cas9 might be safely reversed.
After establishing that the technique worked in cells, Moreno and colleagues moved to studies of laboratory mice. They injected viral vectors carrying the CRISPR treatment into mice with different types of chronic pain, including inflammatory and chemotherapy-induced pain.
Moreno and colleagues determined that all the mice showed evidence of durable pain relief. Remarkably, the treatment also lasted for three months or more and, importantly, without any signs of side effects. The researchers are also exploring another approach to do the same thing using a different set of editing tools called zinc finger nucleases (ZFNs).
The researchers say that one of these approaches might one day work for people with a large number of chronic pain conditions that involve transmission of the pain signal through NaV1.7. That includes diabetic polyneuropathy, sciatica, and osteoarthritis. It also could provide relief for patients undergoing chemotherapy, along with those suffering from many other conditions. Moreno and Mali have co-founded the spinoff company Navega Therapeutics, San Diego, CA, to work on the preclinical steps necessary to help move their approach closer to the clinic.
Chronic pain is a devastating public health problem. While opioids are effective for acute pain, they can do more harm than good for many chronic pain conditions, and they are responsible for a nationwide crisis of addiction and drug overdose deaths [3]. We cannot solve any of these problems without finding new ways to treat chronic pain. As we look to the future, it’s hopeful that innovative new therapeutics such as this gene-editing system could one day help to bring much needed relief.
References:
[1] Long-lasting analgesia via targeted in situ repression of NaV1.7 in mice. Moreno AM, Alemán F, Catroli GF, Hunt M, Hu M, Dailamy A, Pla A, Woller SA, Palmer N, Parekh U, McDonald D, Roberts AJ, Goodwill V, Dryden I, Hevner RF, Delay L, Gonçalves Dos Santos G, Yaksh TL, Mali P. Sci Transl Med. 2021 Mar 10;13(584):eaay9056.
[2] Nuclease dead Cas9 is a programmable roadblock for DNA replication. Whinn KS, Kaur G, Lewis JS, Schauer GD, Mueller SH, Jergic S, Maynard H, Gan ZY, Naganbabu M, Bruchez MP, O’Donnell ME, Dixon NE, van Oijen AM, Ghodke H. Sci Rep. 2019 Sep 16;9(1):13292.
[3] Drug Overdose Deaths. Centers for Disease Control and Prevention.
Links:
Congenital insensitivity to pain (National Center for Advancing Translational Sciences/NIH)
Opioids (National Institute on Drug Abuse/NIH)
Mali Lab (University of California, San Diego)
Navega Therapeutics (San Diego, CA)
NIH Support: National Human Genome Research Institute; National Cancer Institute; National Institute of General Medical Sciences; National Institute of Neurological Disorders and Stroke
Posted In: News
Tags: chemotherapy-induced pain, Chronic Pain, CIP, congenital insensitivity to pain, CRISPR, CRISPR/Cas9, dead CAS9, diabetic polyneuropathy, gene editing, inflammatory pain, mice, NaV1.7, Navega Therapeutics, nuclease dead Cas9, osteoarthritis, pain, pain management, sciatica, spinal neurons, ZFN, zinc finger nucleases
The Amazing Brain: Deep Brain Stimulation
Posted on August 1st, 2019 by Dr. Francis Collins

Credit: Andrew Janson, Butson Lab, University of Utah
August is here, and many folks have plans to enjoy a well-deserved vacation this month. I thought you might enjoy taking a closer look during August at the wonder and beauty of the brain here on my blog, even while giving your own brains a rest from some of the usual work and deadlines.
Some of the best imagery—and best science—comes from the NIH-led Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative, a pioneering project aimed at revolutionizing our understanding of the human brain. Recently, the BRAIN Initiative held a “Show Us Your Brain Contest!”, which invited researchers involved in the effort to submit their coolest images. So, throughout this month, I’ve decided to showcase a few of these award-winning visuals.
Let’s start with the first-place winner in the still-image category. What you see above is an artistic rendering of deep brain stimulation (DBS), an approach now under clinical investigation to treat cognitive impairment that can arise after a traumatic brain injury and other conditions.
The vertical lines represent wire leads with a single electrode that has been inserted deep within the brain to reach a region involved in cognition, the central thalamus. The leads are connected to a pacemaker-like device that has been implanted in a patient’s chest (not shown). When prompted by the pacemaker, the leads’ electrode emits electrical impulses that stimulate a network of neuronal fibers (blue-white streaks) involved in arousal, which is an essential component of human consciousness. The hope is that DBS will improve attention and reduce fatigue in people with serious brain injuries that are not treatable by other means.
Andrew Janson, who is a graduate student in Christopher Butson’s NIH-supported lab at the Scientific Computing and Imaging Institute, University of Utah, Salt Lake City, composed this image using a software program called Blender. It’s an open-source, 3D computer graphics program often used to create animated films or video games, but not typically used in biomedical research. That didn’t stop Janson.
With the consent of a woman preparing to undergo experimental DBS treatment for a serious brain injury suffered years before in a car accident, Janson used Blender to transform her clinical brain scans into a 3D representation of her brain and the neurostimulation process. Then, he used a virtual “camera” within Blender to capture the 2D rendering you see here. Janson plans to use such imagery, along with other patient-specific modeling and bioelectric fields simulations, to develop a virtual brain stimulation surgery to predict the activation of specific fiber pathways, depending upon lead location and stimulation settings.
DBS has been used for many years to relieve motor symptoms of certain movement disorders, including Parkinson’s disease and essential tremor. More recent experimental applications include this one for traumatic brain injury, and others for depression, addiction, Alzheimer’s disease, and chronic pain. As the BRAIN Initiative continues to map out the brain’s complex workings in unprecedented detail, it will be exciting to see how such information can lead to even more effective applications of to DBS to help people living with a wide range of neurological conditions.
Links:
Deep Brain Stimulation for Movement Disorders (National Institute of Neurological Disorders and Stroke/NIH)
Video: Deep Brain Stimulation (University of Utah, Salt Lake City)
Deep Brain Stimulation for the Treatment of Parkinson’s Disease and Other Movement Disorders (NINDS/NIH)
Butson Lab (University of Utah)
Show Us Your Brain! (BRAIN Initiative/NIH)
Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
NIH Support: National Institute of Neurological Disorders and Stroke
Posted In: Snapshots of Life
Tags: addiction, Alzheimer’s disease, Blender, brain, BRAIN Initiative, brain injury, central thalamus, Chronic Pain, cognitive impairment, DBS, deep brain stimulation, depression, essential tremor, movement disorder, movement disorders, Parkinson's disease, summer series 2019, thalamus, Traumatic Brain Injury
Discovering a Source of Laughter in the Brain
Posted on February 12th, 2019 by Dr. Francis Collins
Illustration showing how an electrode was inserted into the cingulum bundle. Courtesy of American Society for Clinical Investigation
If laughter really is the best medicine, wouldn’t it be great if we could learn more about what goes on in the brain when we laugh? Neuroscientists recently made some major progress on this front by pinpointing a part of the brain that, when stimulated, never fails to induce smiles and laughter.
In their study conducted in three patients undergoing electrical stimulation brain mapping as part of epilepsy treatment, the NIH-funded team found that stimulation of a specific tract of neural fibers, called the cingulum bundle, triggered laughter, smiles, and a sense of calm. Not only do the findings shed new light on the biology of laughter, researchers hope they may also lead to new strategies for treating a range of conditions, including anxiety, depression, and chronic pain.
In people with epilepsy whose seizures are poorly controlled with medication, surgery to remove seizure-inducing brain tissue sometimes helps. People awaiting such surgeries must first undergo a procedure known as intracranial electroencephalography (iEEG). This involves temporarily placing 10 to 20 arrays of tiny electrodes in the brain for up to several weeks, in order to pinpoint the source of a patient’s seizures in the brain. With the patient’s permission, those electrodes can also enable physician-researchers to stimulate various regions of the patient’s brain to map their functions and make potentially new and unexpected discoveries.
In the new study, published in The Journal of Clinical Investigation, Jon T. Willie, Kelly Bijanki, and their colleagues at Emory University School of Medicine, Atlanta, looked at a 23-year-old undergoing iEEG for 8 weeks in preparation for surgery to treat her uncontrolled epilepsy [1]. One of the electrodes implanted in her brain was located within the cingulum bundle and, when that area was stimulated for research purposes, the woman experienced an uncontrollable urge to laugh. Not only was the woman given to smiles and giggles, she also reported feeling relaxed and calm.
As a further and more objective test of her mood, the researchers asked the woman to interpret the expression of faces on a computer screen as happy, sad, or neutral. Electrical stimulation to the cingulum bundle led her to see those faces as happier, a sign of a generally more positive mood. A full evaluation of her mental state also showed she was fully aware and alert.
To confirm the findings, the researchers looked to two other patients, a 40-year-old man and a 28-year-old woman, both undergoing iEEG in the course of epilepsy treatment. In those two volunteers, stimulation of the cingulum bundle also triggered laughter and reduced anxiety with otherwise normal cognition.
Willie notes that the cingulum bundle links many brain areas together. He likens it to a super highway with lots of on and off ramps. He suspects the spot they’ve uncovered lies at a key intersection, providing access to various brain networks regulating mood, emotion, and social interaction.
Previous research has shown that stimulation of other parts of the brain can also prompt patients to laugh. However, what makes stimulation of the cingulum bundle a particularly promising approach is that it not only triggers laughter, but also reduces anxiety.
The new findings suggest that stimulation of the cingulum bundle may be useful for calming patients’ anxieties during neurosurgeries in which they must remain awake. In fact, Willie’s team did so during their 23-year-old woman’s subsequent epilepsy surgery. Each time she became distressed, the stimulation provided immediate relief. Also, if traditional deep brain stimulation or less invasive means of brain stimulation can be developed and found to be safe for long-term use, they may offer new ways to treat depression, anxiety disorders, and/or chronic pain.
Meanwhile, Willie’s team is hard at work using similar approaches to map brain areas involved in other aspects of mood, including fear, sadness, and anxiety. Together with the multidisciplinary work being mounted by the NIH-led BRAIN Initiative, these kinds of studies promise to reveal functionalities of the human brain that have previously been out of reach, with profound consequences for neuroscience and human medicine.
Reference:
[1] Cingulum stimulation enhances positive affect and anxiolysis to facilitate awake craniotomy. Bijanki KR, Manns JR, Inman CS, Choi KS, Harati S, Pedersen NP, Drane DL, Waters AC, Fasano RE, Mayberg HS, Willie JT. J Clin Invest. 2018 Dec 27.
Links:
Video: Patient’s Response (Bijanki et al. The Journal of Clinical Investigation)
Epilepsy Information Page (National Institute of Neurological Disease and Stroke/NIH)
Jon T. Willie (Emory University, Atlanta, GA)
NIH Support: National Institute of Neurological Disease and Stroke; National Center for Advancing Translational Sciences
Posted In: News
Tags: anxiety, awake neurosurgery, brain, BRAIN Initiative, brain mapping surgery, brain surgery, calm, Chronic Pain, cingulum bundle, depression, epilepsy, iEEG, intracranial electroencephalography, laughter, neurosurgery, pain, seizures, smiles
Researchers Elucidate Role of Stress Gene in Chronic Pain
Posted on September 4th, 2018 by Dr. Francis Collins

Credit: Getty Images/simonkr
For most people, pain eventually fades away as an injury heals. But for others, the pain persists beyond the initial healing and becomes chronic, hanging on for weeks, months, or even years. Now, we may have uncovered an answer to help explain why: subtle differences in a gene that controls how the body responds to stress.
In a recent study of more than 1,600 people injured in traffic accidents, researchers discovered that individuals with a certain variant in a stress-controlling gene, called FKBP5, were more likely to develop chronic pain than those with other variants [1]. These findings may point to new non-addictive strategies for preventing or controlling chronic pain, and underscore the importance of NIH-funded research for tackling our nation’s opioid overuse crisis.
Posted In: News
Tags: Acute Pain, car accident, Chronic Pain, cortisol, FKBP5, gene variants, genomics, HEAL, HEAL Initiative, mi-320a, MicroRNA, non-addictive medications, opiates, opioid addiction, opioid crisis, opioids, pain, pain management, Project CRASH, stress, trauma