dementia – NIH Director's Blog (original) (raw)
Study of Protective Gene Variant Provides Insight into Delaying Onset of Alzheimer’s Dementia
Posted on July 18th, 2024 by Dr. Monica M. Bertagnolli

Credit: Donny Bliss/NIH
Alzheimer’s disease is currently the seventh leading cause of death in the U.S. While your likelihood of developing Alzheimer’s-related cognitive impairment increases with age, risk for this disease and age of its onset depend on many factors, including the genes you carry. An intriguing new study suggests that having just one copy of a protective gene variant may be enough to delay cognitive impairment from this devastating disease in individuals who are otherwise genetically predisposed to developing early-onset Alzheimer’s dementia.
The findings, from a study supported in part by NIH and reported in The New England Journal of Medicine, offer important insights into the genetic factors and underlying pathways involved in Alzheimer’s dementia.1 While much more study is needed, the findings have potential implications for treatments that could one day work like this gene variant does to delay or perhaps even prevent Alzheimer’s dementia.
This research comes from an international team including Yakeel Quiroz, Massachusetts General Hospital, Boston; Joseph Arboleda-Velasquez, Mass Eye and Ear, Boston; and Francisco Lopera, University of Antioquia, Colombia. For the last 40 years, Lopera has been studying a Colombian family of about 6,000 blood relatives, 1,200 of whom carry a mutation known as Paisa (or Presenilin-1 E280A) that predisposes them to developing early-onset Alzheimer’s dementia. Those who carry a single copy of this gene variant typically show signs of cognitive decline in their early 40s, progressing to dementia by age 50. They frequently die from dementia-related complications in their 60s.
In 2019, the researchers reported on an extraordinary individual who was an exception to this prognosis.2 Even though she carried the Paisa mutation, she didn’t develop any notable cognitive decline until her late 70s—30 years later than expected. The researchers traced her protection against dementia to two copies of a rare variant of the APOE gene dubbed Christchurch. Further study of her brain after death also found lower levels of inflammation and tau protein, which forms damaging tangles inside neurons in the Alzheimer’s brain.
Christchurch is a rare variant, and it’s far more common for people to carry one copy of the protective variant versus two. Would a single copy of the Christchurch variant offer some protection against Alzheimer’s dementia, too? To find out in the new study, the researchers analyzed data from 27 members of this family carrying a single copy of the Christchurch variant among 1,077 carriers of the Paisa mutation.
The researchers compared Christchurch carriers to those without the protective variant and found the variant did delay the age of onset of Alzheimer’s-related cognitive decline and dementia. The median age at the onset of mild cognitive impairment was 52 in family members with the Christchurch variant, compared to approximately age 47 in a matched group without the variant. Similarly, the median age at the onset of dementia was 54, compared to the median age of 50 in noncarriers.
To learn more, the researchers imaged the brains of two of the individuals who had one copy of Christchurch. The brain scans showed lower levels of tau and more normal metabolic activity in brain areas that are known to play a role in Alzheimer’s. Interestingly, their brains still showed accumulations of amyloid proteins, which form plaques that are another hallmark of Alzheimer’s. The team also analyzed autopsy samples from four deceased individuals with one copy of the Christchurch variant and found that blood vessels in their brains appeared healthier, which may help to explain the protective effects of Christchurch. The findings suggest a significant role for blood vessel health in protecting the brain from cognitive decline, as well as a role for disease of the brain blood vessels in contributing to cognitive decline and dementia.
The researchers note this study is limited to a relatively small number of people with both the Paisa and Christchurch variants in one group of related individuals. Further studies involving larger and more diverse samples are needed to learn more about this protective gene variant and its effects on the brain in the general population. The hope is these findings may one day yield new approaches to delaying the onset of Alzheimer’s or slowing its progression in millions more people around the world at risk of developing this devastating disease.
References:
[1] Quiroz YT, et al. APOE3 Christchurch Heterozygosity and Autosomal Dominant Alzheimer’s Disease. The New England Journal of Medicine. DOI: 10.1056/NEJMoa2308583 (2024).
[2] Arboleda-Velasquez JF, et al. Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Nature Medicine. DOI: 10.1038/s41591-019-0611-3 (2019).
NIH Support: National Institute on Aging, National Institute of Neurological Disorders and Stroke
Posted In: Health, News, Science
Tags: aging, Alzheimer’s disease, brain, cognitive decline, dementia, DNA, gene variants, genetics, neurological disease
Taking a Deep Dive into the Alzheimer’s Brain in Search of Understanding and New Targets
Posted on October 10th, 2023 by Lawrence Tabak, D.D.S., Ph.D.

Researchers characterized gene activity at the single-cell level in more than 2 million cells from brain tissue. The findings detailed the molecular drivers of Alzheimer’s disease and which cell types in the brain are most likely to be affected. Credit: Donny Bliss/NIH
People living with Alzheimer’s disease experience a gradual erosion of memory and thinking skills until they can no longer carry out daily activities. Hallmarks of the disease include the buildup of plaques that collect between neurons, accumulations of tau protein inside neurons and weakening of neural connections. However, there’s still much to learn about what precisely happens in the Alzheimer’s brain and how the disorder’s devastating march might be slowed or even stopped. Alzheimer’s affects more than six million people in the United States and is the seventh leading cause of death among adults in the U.S., according to the National Institute on Aging.
NIH-supported researchers recently published a trove of data in the journal Cell detailing the molecular drivers of Alzheimer’s disease and which cell types in the brain are most likely to be affected.1,2,3,4 The scientists, led by Li-Huei Tsai and Manolis Kellis, both at the Massachusetts Institute of Technology, Cambridge, MA, characterized gene activity at the single-cell level in more than two million cells from postmortem brain tissue. They also assessed DNA damage and surveyed epigenetic changes in cells, which refers to chemical modifications to DNA that alter gene expression in the cell. The findings could help researchers pinpoint new targets for Alzheimer’s disease treatments.
In the first of four studies, the researchers examined 54 brain cell types in 427 brain samples from a cohort of people with varying levels of cognitive impairment that has been followed since 1994.1 The MIT team generated an atlas of gene activity patterns within the brain’s prefrontal cortex, an important area for memory retrieval.
Their analyses in brain samples taken from people with Alzheimer’s dementia showed altered activity in genes involved in various functions. Additional findings showed that people with normal cognitive abilities with evidence of plaques in their brains had more neurons that inhibit or dampen activity in the prefrontal cortex compared to those with Alzheimer’s dementia. The finding suggests that the workings of inhibitory neurons may play an unexpectedly important role in maintaining cognitive resilience despite age-related changes, including the buildup of plaques. It’s one among many discoveries that now warrant further study.
In another report, the researchers compared brain tissues from 48 people without Alzheimer’s to 44 people with early- or late-stage Alzheimer’s.2 They developed a map of the various elements that regulate function within cells in the prefrontal cortex. By cross-referencing epigenomic and gene activity data, the researchers showed changes in many genes with known links to Alzheimer’s disease development and risk.
Their single-cell analysis also showed that these changes most often occur in microglia, which are immune cells that remove cellular waste products from the brain. At the same time, every cell type they studied showed a breakdown over time in the cells’ normal epigenomic patterning, a process that may cause a cell to behave differently as it loses essential aspects of its original identity and function.
In a third report, the researchers looked even deeper into gene activity within the brain’s waste-clearing microglia.3 Based on the activity of hundreds of genes, they were able to define a dozen distinct microglia “states.” They also showed that more microglia enter an inflammatory state in the Alzheimer’s brain compared to a healthy human brain. Fewer microglia in the Alzheimer’s brain were in a healthy, balanced state as well. The findings suggest that treatments that target microglia to reduce inflammation and promote balance may hold promise for treating Alzheimer’s disease.
The fourth and final report zeroed in on DNA damage, inspired in part by earlier findings suggesting greater damage within neurons even before Alzheimer’s symptoms appear.4 In fact, breaks in DNA occur as part of the normal process of forming new memories. But those breaks in the healthy brain are quickly repaired as the brain makes new connections.
The researchers studied postmortem brain tissue samples and found that, over time in the Alzheimer’s brain, the damage exceeds the brain’s ability to repair it. As a result, attempts to put the DNA back together leads to a patchwork of mistakes, including rearrangements in the DNA and fusions as separate genes are merged. Such changes appear to arise especially in genes that control neural connections, which may contribute to the signs and symptoms of Alzheimer’s.
The researchers say they now plan to apply artificial intelligence and other analytic tools to learn even more about Alzheimer’s disease from this trove of data. To speed progress even more, they’ve made all the data freely available online to the research community, where it promises to yield many more fundamentally important discoveries about the precise underpinnings of Alzheimer’s disease in the brain and new ways to intervene in Alzheimer’s dementia.
References:
[1] Mathys H, et al. Single-cell atlas reveals correlates of high cognitive function, dementia, and resilience to Alzheimer’s disease pathology. Cell. DOI: 10.1016/j.cell.2023.08.039. (2023).
[2] Xiong X, et al. Epigenomic dissection of Alzheimer’s disease pinpoints causal variants and reveals epigenome erosion. Cell. DOI: 10.1016/j.cell.2023.08.040. (2023).
[3] Sun N, et al. Human microglial state dynamics in Alzheimer’s disease progression. Cell. DOIi: 10.1016/j.cell.2023.08.037. (2023).
[4] Dileep V, et al. Neuronal DNA double-strand breaks lead to genome structural variations and 3D genome disruption in neurodegeneration. Cell. 2023 DOI: 10.1016/j.cell.2023.08.038. (2023).
NIH Support: National Institute on Aging, National Institute of Neurological Disorders and Stroke, National Institute of Mental Health, National Institute of General Medical Sciences
Changes in Human Microbiome Precede Alzheimer’s Cognitive Declines
Posted on June 27th, 2023 by Lawrence Tabak, D.D.S., Ph.D.
Caption: The human gut teems with bacteria and other microbes. They contribute to our health but also influence our susceptibility to certain diseases, including Alzheimer’s disease. Credit: Donny Bliss, NIH
In people with Alzheimer’s disease, the underlying changes in the brain associated with dementia typically begin many years—or even decades—before a diagnosis. While pinpointing the exact causes of Alzheimer’s remains a major research challenge, they likely involve a combination of genetic, environmental, and lifestyle factors. Now an NIH-funded study elucidates the role of another likely culprit that you may not have considered: the human gut microbiome, the trillions of diverse bacteria and other microbes that live primarily in our intestines [1].
Earlier studies had showed that the gut microbiomes of people with symptomatic Alzheimer’s disease differ from those of healthy people with normal cognition [2]. What this new work advances is that these differences arise early on in people who will develop Alzheimer’s, even before any obvious symptoms appear.
The science still has a ways to go before we’ll know if specific dietary changes can alter the gut microbiome and modify its influence on the brain in the right ways. But what’s exciting about this finding is it raises the possibility that doctors one day could test a patient’s stool sample to determine if what’s present from their gut microbiome correlates with greater early risk for Alzheimer’s dementia. Such a test would help doctors detect Alzheimer’s earlier and intervene sooner to slow or ideally even halt its advance.
The new findings, reported in the journal Science Translational Medicine, come from a research team led by Gautam Dantas and Beau Ances, Washington University School of Medicine, St. Louis. Ances is a clinician who treats and studies people with Alzheimer’s; Dantas is a basic researcher and expert on the gut microbiome.
The pair struck up a conversation one day about the possible connection between the gut microbiome and Alzheimer’s. While they knew about the earlier studies suggesting a link, they were surprised that nobody had looked at the gut microbiomes of people in the earliest, so-called preclinical, stages of the disease. That’s when dementia isn’t detectable, but the brain has formed amyloid-beta plaques, which are associated with Alzheimer’s.
To take a look, they enrolled 164 healthy volunteers, age 68 to 94, who performed normally on standard tests of cognition. They also collected stool samples from each volunteer and thoroughly analyzed them all the microbes from their gut microbiome. Study participants also kept food diaries and underwent extensive testing, including two types of brain scans, to look for signs of amyloid-beta plaques and tau protein accumulation that precede the onset of Alzheimer’s symptoms.
Among the volunteers, about a third (49 individuals) unfortunately had signs of early Alzheimer’s disease. And, as it turned out, their microbiomes showed differences, too.
The researchers found that those with preclinical Alzheimer’s disease had markedly different assemblages of gut bacteria. Their microbiomes differed in many of the bacterial species present. Those species-level differences also point to differences in the way their microbiomes would be expected to function at a metabolic level. These microbiome changes were observed even though the individuals didn’t seem to have any apparent differences in their diets.
The team also found that the microbiome changes correlated with amyloid-beta and tau levels in the brain. But they did not find any relationship to degenerative changes in the brain, which tend to happen later in people with Alzheimer’s.
The team is now conducting a five-year study that will follow volunteers to get a better handle on whether the differences observed in the gut microbiome are a cause or a consequence of the brain changes seen in Alzheimer’s. If it’s a cause, this discovery would raise the tantalizing possibility that specially formulated probiotics or fecal transplants that promote the growth of “good” bacteria over “bad” bacteria in the gut might slow the development of Alzheimer’s and its most devastating symptoms. It’s an exciting area of research and definitely one worth following in the years ahead.
References:
[1] Gut microbiome composition may be an indicator of preclinical Alzheimer’s disease. Ferreiro AL, Choi J, Ryou J, Newcomer EP, Thompson R, Bollinger RM, Hall-Moore C, Ndao IM, Sax L, Benzinger TLS, Stark SL, Holtzman DM, Fagan AM, Schindler SE, Cruchaga C, Butt OH, Morris JC, Tarr PI, Ances BM, Dantas G. Sci Transl Med. 2023 Jun 14;15(700):eabo2984. doi: 10.1126/scitranslmed.abo2984. Epub 2023 Jun 14. PMID: 37315112.
[2] Gut microbiome alterations in Alzheimer’s disease. Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE. Sci Rep. 2017 Oct 19;7(1):13537. doi: 10.1038/s41598-017-13601-y. PMID: 29051531; PMCID: PMC5648830.
Links:
Alzheimer’s Disease and Related Dementias (National Institute on Aging/NIH)
Video: How Alzheimer’s Changes the Brain (NIA)
Dantas Lab (Washington University School of Medicine. St. Louis)
Ances Bioimaging Laboratory (Washington University School of Medicine, St. Louis)
NIH Support: National Institute on Aging; National Institute of Diabetes and Digestive and Kidney Diseases
Posted In: News
Tags: aging, Alzheimer’s disease, amyloid plaques, beta amyloid, brain, dementia, diet, fecal transplant, gut, gut bacteria, gut microbiome, microbiome, probiotics, tau
Case Study Unlocks Clues to Rare Resilience to Alzheimer’s Disease
Posted on May 30th, 2023 by Lawrence Tabak, D.D.S., Ph.D.
Caption: Newly discovered Reelin-COLBOS gene variation may delay or prevent Alzheimer’s disease. Credit: Donny Bliss, NIH
Biomedical breakthroughs most often involve slow and steady research in studies involving large numbers of people. But sometimes careful study of even just one truly remarkable person can lead the way to fascinating discoveries with far-reaching implications.
An NIH-funded case study published recently in the journal Nature Medicine falls into this far-reaching category [1]. The report highlights the world’s second person known to have an extreme resilience to a rare genetic form of early onset Alzheimer’s disease. These latest findings in a single man follow a 2019 report of a woman with similar resilience to developing symptoms of Alzheimer’s despite having the same strong genetic predisposition for the disease [2].
The new findings raise important new ideas about the series of steps that may lead to Alzheimer’s and its dementia. They’re also pointing the way to key parts of the brain for cognitive resilience—and potentially new treatment targets—that may one day help to delay or even stop progression of Alzheimer’s.
The man in question is a member of a well-studied extended family from the country of Colombia. This group of related individuals, or kindred, is the largest in the world with a genetic variant called the “Paisa” mutation (or Presenilin-1 E280A). This Paisa variant follows an autosomal dominant pattern of inheritance, meaning that those with a single altered copy of the rare variant passed down from one parent usually develop mild cognitive impairment around the age of 44. They typically advance to full-blown dementia around the age of 50 and rarely live past the age of 60. This contrasts with the most common form of Alzheimer’s, which usually begins after age 65.
The new findings come from a team led by Yakeel Quiroz, Massachusetts General Hospital, Boston; Joseph Arboleda-Velasquez, Massachusetts Eye and Ear, Boston; Diego Sepulveda-Falla, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; and Francisco Lopera, University of Antioquia, Medellín, Colombia. Lopera first identified this family more than 30 years ago and has been studying them ever since.
In the new case report, the researchers identified a Colombian man who’d been married with two children and retired from his job as a mechanic in his early 60s. Despite carrying the Paisa mutation, his first cognitive assessment at age 67 showed he was cognitively intact, having limited difficulties with verbal learning skills or language. It wasn’t until he turned 70 that he was diagnosed with mild cognitive impairment—more than 20 years later than the expected age for this family—showing some decline in short-term memory and verbal fluency.
At age 73, he enrolled in the Colombia-Boston biomarker research study (COLBOS). This study is a collaborative project between the University of Antioquia and Massachusetts General Hospital involving approximately 6,000 individuals from the Paisa kindred. About 1,500 of those in the study carry the mutation that sets them up for early Alzheimer’s. As a member of the COLBOS study, the man underwent thorough neuroimaging tests to look for amyloid plaques and tau tangles, both of which are hallmarks of Alzheimer’s.
While this man died at age 74 with Alzheimer’s, the big question is: how did he stave off dementia for so long despite his poor genetic odds? The COLBOS study earlier identified a woman with a similar resilience to Alzheimer’s, which they traced to two copies of a rare, protective genetic variant called Christchurch. This variant affects a gene called apolipoprotein E (APOE3), which is well known for its influence on Alzheimer’s risk. However, the man didn’t carry this same protective variant.
The researchers still thought they’d find an answer in his genome and kept looking. While they found several variants of possible interest, they zeroed in on a single gene variant that they’ve named Reelin-COLBOS. What helped them to narrow it down to this variant is the man also had a sister with the Paisa mutation who only progressed to advanced dementia at age 72. It turned out, in addition to the Paisa variant, the siblings also shared an altered copy of the newly discovered Reelin-COLBOS variant.
This Reelin-COLBOS gene is known to encode a protein that controls signals to chemically modify tau proteins, which form tangles that build up over time in the Alzheimer’s brain and have been linked to memory loss. Reelin is also functionally related to APOE, the gene that was altered in the woman with extreme Alzheimer’s protection. Reelin and APOE both interact with common protein receptors in neurons. Together, the findings add to evidence that signaling pathways influencing tau play an important role in Alzheimer’s pathology and protection.
The neuroimaging exams conducted when the man was age 73 have offered further intriguing clues. They showed that his brain had extensive amyloid plaques. He also had tau tangles in some parts of his brain. But one brain region, called the entorhinal cortex, was notable for having a very minimal amount of those hallmark tau tangles.
The entorhinal cortex is a hub for memory, navigation, and the perception of time. Its degeneration also leads to cognitive impairment and dementia. Studies of the newly identified Reelin-COLBOS variant in Alzheimer’s mouse models also help to confirm that the variant offers its protection by diminishing the pathological modifications of tau.
Overall, the findings in this one individual and his sister highlight the Reelin pathway and brain region as promising targets for future study and development of Alzheimer’s treatments. Quiroz and her colleagues report that they are actively exploring treatment approaches inspired by the Christchurch and Reelin-COLBOS discoveries.
Of course, there’s surely more to discover from continued study of these few individuals and others like them. Other as yet undescribed genetic and environmental factors are likely at play. But the current findings certainly offer some encouraging news for those at risk for Alzheimer’s disease—and a reminder of how much can be learned from careful study of remarkable individuals.
References:
[1] Resilience to autosomal dominant Alzheimer’s disease in a Reelin-COLBOS heterozygous man. Lopera F, Marino C, Chandrahas AS, O’Hare M, Reiman EM, Sepulveda-Falla D, Arboleda-Velasquez JF, Quiroz YT, et al. Nat Med. 2023 May;29(5):1243-1252.
[2] Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Arboleda-Velasquez JF, Lopera F, O’Hare M, Delgado-Tirado S, Tariot PN, Johnson KA, Reiman EM, Quiroz YT et al. Nat Med. 2019 Nov;25(11):1680-1683.
Links:
Alzheimer’s Disease & Related Dementias (National Institute on Aging/NIH)
“NIH Support Spurs Alzheimer’s Research in Colombia,” Global Health Matters, January/February 2014, Fogarty International Center/NIS
“COLBOS Study Reveals Mysteries of Alzheimer’s Disease,” NIH Record, August 19, 2022.
Yakeel Quiroz (Massachusetts General Hospital, Harvard Medical School, Boston)
Joseph Arboleda-Velasquez (Massachusetts Eye and Ear, Harvard Medical School, Boston)
Diego Sepulveda-Falla Lab (University Medical Center Hamburg-Eppendorf, Hamburg, Germany)
Francisco Lopera (University of Antioquia, Medellín, Colombia)
NIH Support: National Institute on Aging; National Eye Institute; National Institute of Neurological Disorders and Stroke; Office of the Director
Posted In: News
Tags: Alzheimer’s disease, APOE3, brain, Christchurch variant, cognitive resilience, Colombia, Colombia-Boston biomarker research study, dementia, genetics, genomics, global health, Paisa mutation, Paisa variant, Presinilin-1, Reelin-COLBOS gene variant, tau, tau protein
An Inflammatory View of Early Alzheimer’s Disease
Posted on February 21st, 2023 by Lawrence Tabak, D.D.S., Ph.D.
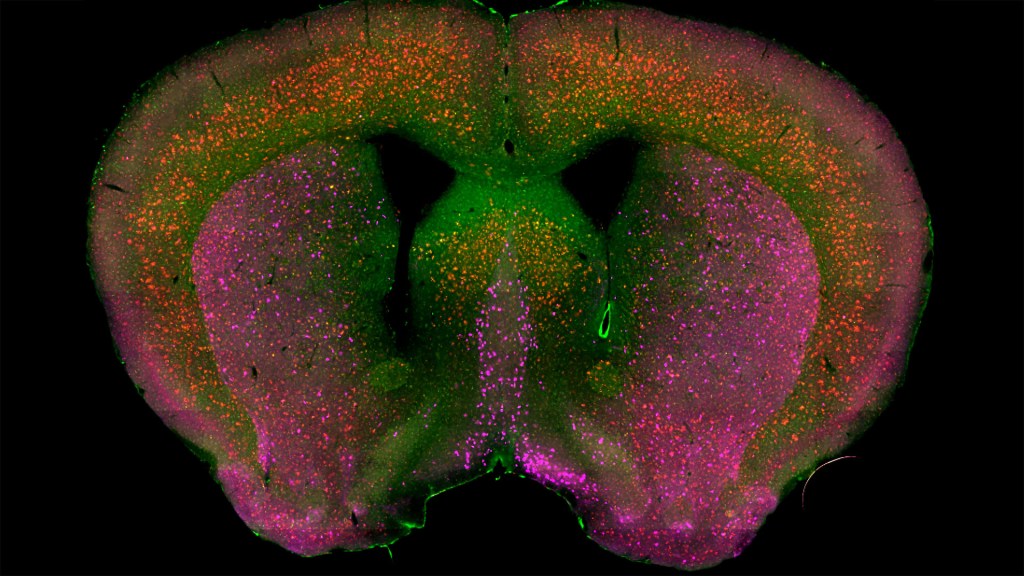
Credit: Sakar Budhathoki, Mala Ananth, Lorna Role, David Talmage, National Institute of Neurological Diseases and Stroke, NIH
Detecting the earliest signs of Alzheimer’s disease (AD) in middle-aged people and tracking its progression over time in research studies continue to be challenging. But it is easier to do in shorter-lived mammalian models of AD, especially when paired with cutting-edge imaging tools that look across different regions of the brain. These tools can help basic researchers detect telltale early changes that might point the way to better prevention or treatment strategies in humans.
That’s the case in this technicolor snapshot showing early patterns of inflammation in the brain of a relatively young mouse bred to develop a condition similar to AD. You can see abnormally high levels of inflammation throughout the front part of the brain (orange, green) as well as in its middle part—the septum that divides the brain’s two sides. This level of inflammation suggests that the brain has been injured.
What’s striking is that no inflammation is detectable in parts of the brain rich in cholinergic neurons (pink), a distinct type of nerve cell that helps to control memory, movement, and attention. Though these neurons still remain healthy, researchers would like to know if the inflammation also will destroy them as AD progresses.
This colorful image comes from medical student Sakar Budhathoki, who earlier worked in the NIH labs of Lorna Role and David Talmage, National Institute of Neurological Disorders and Stroke (NINDS). Budhathoki, teaming with postdoctoral scientist Mala Ananth, used a specially designed wide-field scanner that sweeps across brain tissue to light up fluorescent markers and capture the image. It’s one of the scanning approaches pioneered in the Role and Talmage labs [1,2].
The two NIH labs are exploring possible links between abnormal inflammation and damage to the brain’s cholinergic signaling system. In fact, medications that target cholinergic function remain the first line of treatment for people with AD and other dementias. And yet, researchers still haven’t adequately determined when, why, and how the loss of these cholinergic neurons relates to AD.
It’s a rich area of basic research that offers hope for greater understanding of AD in the future. It’s also the source of some fascinating images like this one, which was part of the 2022 Show Us Your BRAIN! Photo and Video Contest, supported by NIH’s Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative.
References:
[1] NeuRegenerate: A framework for visualizing neurodegeneration. Boorboor S, Mathew S, Ananth M, Talmage D, Role LW, Kaufman AE. IEEE Trans Vis Comput Graph. 2021;Nov 10;PP.
[2] NeuroConstruct: 3D reconstruction and visualization of neurites in optical microscopy brain images. Ghahremani P, Boorboor S, Mirhosseini P, Gudisagar C, Ananth M, Talmage D, Role LW, Kaufman AE. IEEE Trans Vis Comput Graph. 2022 Dec;28(12):4951-4965.
Links:
Alzheimer’s Disease & Related Dementias (National Institute on Aging/NIH)
Role Lab (National Institute of Neurological Disorders and Stroke/NIH)
Talmage Lab (NINDS)
The Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
Show Us Your BRAINs! Photo and Video Contest (BRAIN Initiative)
NIH Support: National Institute of Neurological Disorders and Stroke
Posted In: Snapshots of Life
Tags: Alzheimer’s disease, attention, brain, BRAIN Initiative, Brain Research through Advancing Innovative Neurotechnologies Initiative, cholinergic neurons, dementia, imaging, inflammation, memory, mouse study, movement, neurodegenerative disorders, Show Us Your BRAINs!, wide-field scanner
This Is Why NIH Invests in Global Health Research
Posted on December 13th, 2022 by Roger I. Glass, M.D., Ph.D., Fogarty International Center
Caption: Global partnerships fostered by NIH’s Fogarty International Center speed translation of scientific discoveries into lifesaving biomedical products. Credit: Gabe Bienczycki, PATH, Seattle
Efforts over the past few years to end the COVID-19 pandemic clearly reveal how global health impacts individual wellbeing and national security. At NIH, the Fogarty International Center helps the other institutes become engaged with global health research, which investigates the dual burden of infectious disease and non-communicable disease.
Global health research also encompasses data science, economics, genetics, climate change science, and many other disciplines. For more than 50 years, Fogarty has been building partnerships among institutions in the U.S. and abroad, while training the next generation of scientists focused on universal health needs.
America’s investment in Fogarty has paid rich dividends
During the pandemic, in particular, we’ve seen researchers trained by our programs make scientific discoveries that contributed to international security. Take Jessica Manning, a former Fogarty fellow who now conducts malaria research in Phnom Penh, Cambodia. Her team at the Ministry of Health sequenced the viral strain of SARS-CoV-2, the cause of COVID-19, infecting the first Cambodian patient and documented early the spread of this novel coronavirus outside of China.
Similarly, Christian Happi, director of the African Centre of Excellence for the Genomics of Infectious Disease, Ede, Nigeria, sequenced the first SARS-CoV-2 genome in Africa. Happi was able to do it by adapting the sequencing and analytical pipelines that he’d created back when he was a Fogarty grantee studying Ebola.
In Botswana, Sikhulile Moyo leveraged the skills he’d acquired while supported by a Fogarty HIV research training grant with Max Essex, Harvard School of Public Health, Cambridge, MA, to track COVID-19 mutations for his country’s Ministry of Health. Last November, he alerted the world of a new Omicron variant. Within six weeks, Omicron became the dominant global strain, challenging the ability of COVID vaccines to control its spread. In the Dominican Republic, William Duke, a national commission member, used what he’d learned as a Fogarty trainee to help create a national COVID-19 intervention plan to prevent and control the disease.
Fogarty’s fostering of global health leaders is one way we advance scientific expertise while ensuring our nation’s biosecurity. Another is by finding effective ways to study abroad the same health conditions that affect our own population.
Research conducted in Colombia, for example, may provide clues for preventing Alzheimer’s disease in the U.S. Fogarty support brought together neuroscientists Kenneth Kosik, University of California, Santa Barbara, and Francisco Lopera, University of Antioquia, Colombia, to study members of the largest-known family with an early-onset, rapidly progressive form of the disease. Over the years, Kosik and Lopera have trained local scientists, explored gene therapy targets, investigated biomarkers to monitor disease progression, and conducted drug trials in search of a cure for Alzheimer’s.
Researchers in other fields also discover unique opportunities to investigate populations with high rates of disease. Siana Nkya, a Fogarty grantee based in Tanzania, has devoted her career to studying the genetic determinants of sickle cell disease, which affects many people around the world, including in the U.S. We hope that US-African partnerships might develop improved, affordable treatments and a cure for all patients with this devastating disease. Similarly, people in the U.S. have access to state-of-the-art HIV treatment studies in places around the globe where incidence rates are higher.
Fogarty has supported many milestone achievements in HIV research over the years. Among them is a study that took place in nine countries. The research, led by Myron Cohen of the University of North Carolina at Chapel Hill, established that antiretroviral therapy can prevent sexual transmission of HIV-1 among couples in which one person is infected and the other is not. In fact, this research informs current HIV treatment recommendations worldwide, including in the U.S.
Americans will also undoubtedly benefit from projects funded by Fogarty’s Global Brain and Nervous System Disorders Research across the Lifespan program. For example, psychologist Tatiana Balachova, University of Oklahoma, Oklahoma City, has designed an intervention for women in Russia to prevent fetal alcohol spectrum disorders. In another project in South Africa, Sandra and Joseph Jacobson, Wayne State University, Detroit, conducted the first-ever prospective longitudinal study of the syndrome. Findings from both projects are ripe for translation within an American context.
Other examples of Global Brain program investigations with broad implications in our own country include studying early psychosis in China; capacity building for schizophrenia research in Macedonia; exploring family consequences from the Zika virus in Brazil; and studying dementia and related health and social challenges in Lebanon.
These are just a few examples of Fogarty’s work and its unique mission. What is most remarkable about Fogarty is that just under 90 percent of our grants are co-funded by at least one other NIH institute, center, or office. Collaboration, both within borders and across them, is Fogarty’s formula for success.
Links:
Fogarty International Center (NIH)
Overview of Brain Disorders: Research Across the Lifespan (Fogarty)
Former Fogarty Scholar Dr Jessica Manning Helps Cambodia Respond to COVID (Fogarty)
Christian Happi: Former Fogarty Grantee Leads COVID-19 Genomics Work in Africa (Fogarty)
Sikhulile Moyo: Fogarty Fellow Recognized for Omicron Discovery (Fogarty)
William Duke: Former Fogarty HIV Trainee Helps Lead Dominican Republic’s COVID Response (Fogarty)
Kenneth Kosic and Francisco Lopera: NIH Support Spurs Alzheimer’s Research in Colombia (Fogarty)
Former Fogarty fellow Siana Nkya Tackles Sickle Cell Disease in Tanzania (Fogarty)
Tatiana Balachova: Researchers Tackle Fetal Alcohol Syndrome in Russia (Fogarty)
Sandra and Joseph Jacobson: Fetal Alcohol Exposure Research Supported by NIAAA in South Africa, Ukraine and Russia Improves Prevention, Outcomes (Fogarty)
Note: Dr. Lawrence Tabak, who performs the duties of the NIH Director, has asked the heads of NIH’s Institutes and Centers (ICs) to contribute occasional guest posts to the blog to highlight some of the interesting science that they support and conduct. This is the 22nd in the series of NIH IC guest posts that will run until a new permanent NIH director is in place.
Posted In: Generic
Tags: Africa, Alzheimer’s disease, antiretroviral therapy, Botswana, brain, Brazil, Cambodia, China, Colombia, COVID-19, COVID-19 vaccine, dementia, Dominican Republic, early psychosis, early-onset Alzheimer's disease, Ebola, fetal alcohol spectrum disorders, Fogarty, Fogarty International Center, global health, HIV, HIV-1, international security, Lebanon, Macedonia, neuroscience, Nigeria, novel coronavirus, Omicron variant, pandemic, SARS-CoV-2, schizophrenia, sickle cell disease, South Africa, Tanzania, Zika virus
Dedicating Roy Blunt Center for Alzheimer’s Disease and Related Dementias
Posted on September 20th, 2022 by Lawrence Tabak, D.D.S., Ph.D.

On September 19, I welcomed everyone to the dedication of the brand-new Roy Blunt Center for Alzheimer’s Disease and Related Dementias, which is located on the main NIH campus, Bethesda, MD. Seated behind me (l-r) are former NIH director Francis Collins; Dawn Beraud, NIH’s National Institute on Aging; Senator Roy Blunt of Missouri; and Congressman Tom Cole of Oklahoma. The 24,000-square foot building, which includes 12,000 square feet of laboratory space, will house the NIH Intramural Research Program’s Center for Alzheimer’s and Related Dementias (CARD). The facility is named in honor of Senator Blunt, who will be retiring from Congress, to recognize his extraordinary leadership and unwavering commitment to speed medical progress in this important area that touches far too many lives and families. Credit: NIH
Getting Closer to a Blood Test for Alzheimer’s Disease?
Posted on March 31st, 2020 by Dr. Francis Collins
iStock/ericsphotography
As research on Alzheimer’s disease (AD) advances, a desperate need remains for an easy blood test to help diagnose the condition as early as possible. Ideally, such a test could also distinguish AD from other forms of dementia that produce similar symptoms. As published recently in Nature Medicine, an NIH-funded research team has designed a simple blood test that is on course to meet these criteria [1].
The latest work builds on a large body of work showing that one secret to predicting a person’s cognitive decline and treatment response in AD lies in a protein called tau. Using the powerful, but expensive, approach of PET scan imaging, we know that tau builds up in the brain as Alzheimer’s disease progresses. We also know that some tau spills from the brain into the bloodstream.
The trouble is that the circulating tau protein breaks down far too quickly for a blood test to offer a reliable measure of what’s happening in a person’s brain. A few years ago, researchers discovered a possible solution: test for blood levels of a slightly different and more stable version of the protein called pTau181 [2]. (The “p” in its name comes from the addition of phosphorus in a particular part of the protein’s structure.)
In the latest study, researchers in the lab of Adam Boxer, University of California, San Francisco, followed up further on this compelling lead. Boxer’s team measured pTau181 levels in blood samples from 362 people between the ages of 58 and 70. Those samples included 56 people with an Alzheimer’s diagnosis, along with 47 people with mild cognitive impairment and 69 healthy controls.
The researchers also included another 190 people diagnosed with frontotemporal lobar degeneration (FTLD). It is a relatively rare form of dementia that leads to a gradual decline in behavior, language, and movement, often in connection with a buildup of tau in the brain.
The study found that levels of pTau181 were roughly 3.5-times higher in the blood of people with AD compared to people without AD. Those with mild cognitive impairment due to underlying AD also showed an intermediate increase in blood levels of pTau181.
Importantly, people with FLTD had normal blood levels of pTau181. As a result, the blood test could reliably distinguish between a person with AD and a person with FLTD. That’s important because, while FLTD is a relatively rare condition, its prevalence is similar to AD in people under the age of 65. But both conditions have similar symptoms, making it often challenging to distinguish them.
The findings add to evidence that the new blood test can help in diagnosing AD and in distinguishing it from other neurodegenerative conditions. In fact, it does so with an accuracy that often rivals more expensive PET scans and more invasive cerebrospinal fluid tests, which are now the only reliable ways to measure tau.
There’s still plenty of work to do before this blood test is ready for a doctor’s office. But these initial findings are very promising in helping to simplify the diagnosis of this devastating condition that now affects an estimated 5.5 million Americans [3].
References:
[1] Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, Bourakova V, Cobigo Y, Heuer H, Spina S, VandeVrede L, Chai X, Proctor NK, Airey DC, Shcherbinin S, Duggan Evans C, Sims JR, Zetterberg H, Blennow K, Karydas AM, Teunissen CE, Kramer JH, Grinberg LT, Seeley WW, Rosen H, Boeve BF, Miller BL, Rabinovici GD, Dage JL, Rojas JC, Boxer AL; Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) investigators. Nat Med. 2020 Mar 2.
[2] Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, Airey DC, Knopman DS, Roberts RO, Machulda MM, Jack CR Jr, Petersen RC, Dage JL. Alzheimers Dement. 2018 Aug;14(8):989-997.
[3] Alzheimer’s Disease Fact Sheet. National Institute on Aging, May 22, 2019.
Links:
Alzheimer’s Disease & Related Dementias (National Institute on Aging/NIH)
What Are Frontotemporal Disorders? (NIA)
Accelerating Medicines Partnership: Alzheimer’s Disease (NIH)
Adam Boxer (University of California, San Francisco)
NIH Support: National Institute on Aging; National Institute of Neurological Disorders and Stroke; National Center for Advancing Translational Sciences
Posted In: News
Tags: aging, Alzheimer's blood test, Alzheimer’s disease, blood test, brain, cognition, cognitive decline, dementia, diagnostics, Frontotemporal disorders, frontotemporal lobar degeneration, FTLD, neurology, PET scans, pTau191, senior health, tau, tauopathy
Discovering the Brain’s Nightly “Rinse Cycle”
Posted on March 5th, 2020 by Dr. Francis Collins
Getting plenty of deep, restful sleep is essential for our physical and mental health. Now comes word of yet another way that sleep is good for us: it triggers rhythmic waves of blood and cerebrospinal fluid (CSF) that appear to function much like a washing machine’s rinse cycle, which may help to clear the brain of toxic waste on a regular basis.
The video above uses functional magnetic resonance imaging (fMRI) to take you inside a person’s brain to see this newly discovered rinse cycle in action. First, you see a wave of blood flow (red, yellow) that’s closely tied to an underlying slow-wave of electrical activity (not visible). As the blood recedes, CSF (blue) increases and then drops back again. Then, the cycle—lasting about 20 seconds—starts over again.
The findings, published recently in the journal Science, are the first to suggest that the brain’s well-known ebb and flow of blood and electrical activity during sleep may also trigger cleansing waves of blood and CSF. While the experiments were conducted in healthy adults, further study of this phenomenon may help explain why poor sleep or loss of sleep has previously been associated with the spread of toxic proteins and worsening memory loss in people with Alzheimer’s disease.
In the new study, Laura Lewis, Boston University, MA, and her colleagues at the Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston. recorded the electrical activity and took fMRI images of the brains of 13 young, healthy adults as they slept. The NIH-funded team also built a computer model to learn more about the fluid dynamics of what goes on in the brain during sleep. And, as it turns out, their sophisticated model predicted exactly what they observed in the brains of living humans: slow waves of electrical activity followed by alternating waves of blood and CSF.
Lewis says her team is now working to come up with even better ways to capture CSF flow in the brain during sleep. Currently, people who volunteer for such experiments have to be able to fall asleep while wearing an electroencephalogram (EEG) cap inside of a noisy MRI machine—no easy feat. The researchers are also recruiting older adults to begin exploring how age-related changes in brain activity during sleep may affect the associated fluid dynamics.
Reference:
[1] Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, Lewis LD. Science. 2019 Nov 1;366(6465):628-631.
Links:
Sleep and Memory (National Institute of Mental Health/NIH)
Sleep Deprivation and Deficiency (National Heart, Lung, and Blood Institute/NIH)
Alzheimer’s Disease and Related Dementias (National Institute on Aging/NIH)
NIH Support: National Institute of Mental Health; National Institute of Biomedical Imaging and Bioengineering; National Institute of Neurological Disorders and Stroke
Posted In: Cool Videos
Tags: Alzheimer’s disease, blood flow, brain, cerebrospinal fluid, CSF, dementia, electrophysiology, fMRI, Functional magnetic resonance imaging, imaging, memory, memory retrieval, neurology, sleep, waste
A Real-Time Look at Value-Based Decision Making
Posted on January 16th, 2020 by Dr. Francis Collins
All of us make many decisions every day. For most things, such as which jacket to wear or where to grab a cup of coffee, there’s usually no right answer, so we often decide using values rooted in our past experiences. Now, neuroscientists have identified the part of the mammalian brain that stores information essential to such value-based decision making.
Researchers zeroed in on this particular brain region, known as the retrosplenial cortex (RSC), by analyzing movies—including the clip shown about 32 seconds into this video—that captured in real time what goes on in the brains of mice as they make decisions. Each white circle is a neuron, and the flickers of light reflect their activity: the brighter the light, the more active the neuron at that point in time.
All told, the NIH-funded team, led by Ryoma Hattori and Takaki Komiyama, University of California at San Diego, La Jolla, made recordings of more than 45,000 neurons across six regions of the mouse brain [1]. Neural activity isn’t usually visible. But, in this case, researchers used mice that had been genetically engineered so that their neurons, when activated, expressed a protein that glowed.
Their system was also set up to encourage the mice to make value-based decisions, including choosing between two drinking tubes, each with a different probability of delivering water. During this decision-making process, the RSC proved to be the region of the brain where neurons persistently lit up, reflecting how the mouse evaluated one option over the other.
The new discovery, described in the journal Cell, comes as something of a surprise to neuroscientists because the RSC hadn’t previously been implicated in value-based decisions. To gather additional evidence, the researchers turned to optogenetics, a technique that enabled them to use light to inactivate neurons in the RSC’s of living animals. These studies confirmed that, with the RSC turned off, the mice couldn’t retrieve value information based on past experience.
The researchers note that the RSC is heavily interconnected with other key brain regions, including those involved in learning, memory, and controlling movement. This indicates that the RSC may be well situated to serve as a hub for storing value information, allowing it to be accessed and acted upon when it is needed.
The findings are yet another amazing example of how advances coming out of the NIH-led Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative are revolutionizing our understanding of the brain. In the future, the team hopes to learn more about how the RSC stores this information and sends it to other parts of the brain. They note that it will also be important to explore how activity in this brain area may be altered in schizophrenia, dementia, substance abuse, and other conditions that may affect decision-making abilities. It will also be interesting to see how this develops during childhood and adolescence.
Reference:
[1] Area-Specificity and Plasticity of History-Dependent Value Coding During Learning. Hattori R, Danskin B, Babic Z, Mlynaryk N, Komiyama T. Cell. 2019 Jun 13;177(7):1858-1872.e15.
Links:
Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
Komiyama Lab (UCSD, La Jolla)
NIH Support: National Institute of Neurological Disorders and Stroke; National Eye Institute; National Institute on Deafness and Other Communication Disorders
Posted In: Cool Videos
Tags: brain, BRAIN Initiative, connectomics, decision making, dementia, learning, mammalian brain, memory, neurology, neurons, neuroscience, optogenetics, retrosplenial cortex, schizophrenia, substance abuse, value-based decision making
