diet – NIH Director's Blog (original) (raw)
Changes in Human Microbiome Precede Alzheimer’s Cognitive Declines
Posted on June 27th, 2023 by Lawrence Tabak, D.D.S., Ph.D.
Caption: The human gut teems with bacteria and other microbes. They contribute to our health but also influence our susceptibility to certain diseases, including Alzheimer’s disease. Credit: Donny Bliss, NIH
In people with Alzheimer’s disease, the underlying changes in the brain associated with dementia typically begin many years—or even decades—before a diagnosis. While pinpointing the exact causes of Alzheimer’s remains a major research challenge, they likely involve a combination of genetic, environmental, and lifestyle factors. Now an NIH-funded study elucidates the role of another likely culprit that you may not have considered: the human gut microbiome, the trillions of diverse bacteria and other microbes that live primarily in our intestines [1].
Earlier studies had showed that the gut microbiomes of people with symptomatic Alzheimer’s disease differ from those of healthy people with normal cognition [2]. What this new work advances is that these differences arise early on in people who will develop Alzheimer’s, even before any obvious symptoms appear.
The science still has a ways to go before we’ll know if specific dietary changes can alter the gut microbiome and modify its influence on the brain in the right ways. But what’s exciting about this finding is it raises the possibility that doctors one day could test a patient’s stool sample to determine if what’s present from their gut microbiome correlates with greater early risk for Alzheimer’s dementia. Such a test would help doctors detect Alzheimer’s earlier and intervene sooner to slow or ideally even halt its advance.
The new findings, reported in the journal Science Translational Medicine, come from a research team led by Gautam Dantas and Beau Ances, Washington University School of Medicine, St. Louis. Ances is a clinician who treats and studies people with Alzheimer’s; Dantas is a basic researcher and expert on the gut microbiome.
The pair struck up a conversation one day about the possible connection between the gut microbiome and Alzheimer’s. While they knew about the earlier studies suggesting a link, they were surprised that nobody had looked at the gut microbiomes of people in the earliest, so-called preclinical, stages of the disease. That’s when dementia isn’t detectable, but the brain has formed amyloid-beta plaques, which are associated with Alzheimer’s.
To take a look, they enrolled 164 healthy volunteers, age 68 to 94, who performed normally on standard tests of cognition. They also collected stool samples from each volunteer and thoroughly analyzed them all the microbes from their gut microbiome. Study participants also kept food diaries and underwent extensive testing, including two types of brain scans, to look for signs of amyloid-beta plaques and tau protein accumulation that precede the onset of Alzheimer’s symptoms.
Among the volunteers, about a third (49 individuals) unfortunately had signs of early Alzheimer’s disease. And, as it turned out, their microbiomes showed differences, too.
The researchers found that those with preclinical Alzheimer’s disease had markedly different assemblages of gut bacteria. Their microbiomes differed in many of the bacterial species present. Those species-level differences also point to differences in the way their microbiomes would be expected to function at a metabolic level. These microbiome changes were observed even though the individuals didn’t seem to have any apparent differences in their diets.
The team also found that the microbiome changes correlated with amyloid-beta and tau levels in the brain. But they did not find any relationship to degenerative changes in the brain, which tend to happen later in people with Alzheimer’s.
The team is now conducting a five-year study that will follow volunteers to get a better handle on whether the differences observed in the gut microbiome are a cause or a consequence of the brain changes seen in Alzheimer’s. If it’s a cause, this discovery would raise the tantalizing possibility that specially formulated probiotics or fecal transplants that promote the growth of “good” bacteria over “bad” bacteria in the gut might slow the development of Alzheimer’s and its most devastating symptoms. It’s an exciting area of research and definitely one worth following in the years ahead.
References:
[1] Gut microbiome composition may be an indicator of preclinical Alzheimer’s disease. Ferreiro AL, Choi J, Ryou J, Newcomer EP, Thompson R, Bollinger RM, Hall-Moore C, Ndao IM, Sax L, Benzinger TLS, Stark SL, Holtzman DM, Fagan AM, Schindler SE, Cruchaga C, Butt OH, Morris JC, Tarr PI, Ances BM, Dantas G. Sci Transl Med. 2023 Jun 14;15(700):eabo2984. doi: 10.1126/scitranslmed.abo2984. Epub 2023 Jun 14. PMID: 37315112.
[2] Gut microbiome alterations in Alzheimer’s disease. Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE. Sci Rep. 2017 Oct 19;7(1):13537. doi: 10.1038/s41598-017-13601-y. PMID: 29051531; PMCID: PMC5648830.
Links:
Alzheimer’s Disease and Related Dementias (National Institute on Aging/NIH)
Video: How Alzheimer’s Changes the Brain (NIA)
Dantas Lab (Washington University School of Medicine. St. Louis)
Ances Bioimaging Laboratory (Washington University School of Medicine, St. Louis)
NIH Support: National Institute on Aging; National Institute of Diabetes and Digestive and Kidney Diseases
Posted In: News
Tags: aging, Alzheimer’s disease, amyloid plaques, beta amyloid, brain, dementia, diet, fecal transplant, gut, gut bacteria, gut microbiome, microbiome, probiotics, tau
Biology of Aging Study Shows Why Curbing Calories Counts
Posted on March 8th, 2022 by Richard J. Hodes, M.D., National Institute on Aging
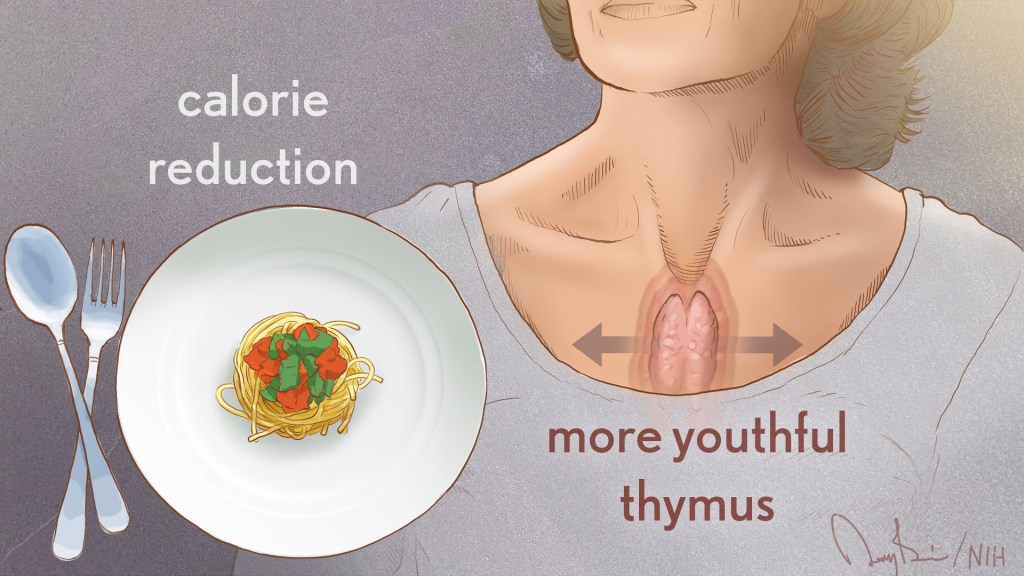
The NIH’s National Institute on Aging (NIA) broadly invests in research to find ways to help people live longer and healthier. As people age, they are more likely to have multiple chronic diseases, and NIA-supported research studies reflect a strong focus on geroscience. This advancing area of science seeks to understand the mechanisms that make aging a major risk factor and driver of common chronic conditions and diseases of older people.
More than 85 years ago, researchers at Cornell University, Ithaca, NY, observed that some lab rodents lived longer when fed a lower calorie diet that otherwise had the appropriate nutrients [1]. Since then, many scientists have studied calorie restriction to shed light on the various biological mechanisms that may explain its benefits and perhaps discover a way to extend healthy years of life, known as our healthspan.
Although there have been many studies of calorie restriction since the Cornell findings, the NIA-supported clinical trial CALERIE, which stands for Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy, provided critical data on the impact of this intervention in people. Completed in 2012, CALERIE was the first carefully controlled study to test whether study participants undergoing moderate calorie restriction would display any of the benefits observed in animal studies.
Volunteers for the CALERIE study were healthy, non-obese adults ages 25 to 45. People in one group were randomly assigned to continue their customary dietary choices, and those in the second group were trained by an expert team of psychologists and dietitians to restrict calories through specific strategies, such as eating smaller servings of food.
In addition to demonstrating that people could sustain moderate calorie restriction for two years, the CALERIE study also showed that this intervention could diminish risk factors for age-related cardiovascular and metabolic diseases [2]. The CALERIE investigators also made their data and biological samples available for other research teams to study further.
Recently, a team led by Vishwa Dixit, Yale University, New Haven, CT, examined CALERIE data to investigate the effects of calorie restriction on immune function. The findings, published in the journal Science, suggest that calorie restriction may improve immune function and reduce chronic inflammation [3,4].
As people age, the size of the thymus, which is part of the immune system, tends to become smaller. As this organ shrinks, its output of T cells declines, which hampers the ability of the immune system to combat infectious diseases. This deficiency of T cells is one of the reasons people over age 40 are at increased susceptibility for a range of diseases.
Dixit’s team noted that MRI scans showed the thymus volume increased among people who reduced their calories for the two-year CALERIE study but was not significantly different in the control group. The increase in thymus size in the group restricting calories was accompanied by an increase in indicators of new T cell production.
Next, the team analyzed immune system effects in belly fat samples from people in the CALERIE study. The team discovered that the PLA2G7 gene—which codes for a protein involved in fat metabolism that is made by immune cells such as T cells—was suppressed after calorie restriction, with evidence that the suppression occurred in immune cells present in fat. They hypothesized that the PLA2G7 gene could have played a role in the improved thymus function resulting from calorie restriction.
To test this hypothesis, the team suppressed the Pla2g7 gene in lab mice. When these mice were two years old, which is equivalent to a human age of about 70, the thymus had not decreased in volume. In addition, the mice had decreased fat mass and lower levels of certain inflammation-promoting substances. These findings suggest that mice without the Pla2g7 gene might have been protected from age-related chronic inflammation, which has been linked to many conditions of old age.
Taken together, the findings extend our understanding of the power of calorie restriction and suggest that it might also improve immune function and reduce chronic inflammation in people. The results also indicate interventions that influence PLA2G7 gene function might have favorable health effects. Additional research is still needed to assess the health effects and to determine whether calorie restriction extends lifespan or healthspan in humans. The NIA is funding more studies to determine the benefits and risks of calorie restriction, as well as the mechanisms that account for its effects.
References:
[1] The effect of retarded growth upon the length of life span and upon the ultimate body size. McCay CM, Crowell MF, Maynard LA. J. Nutr. 1935 July 10(1): 63–79.
[2] A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. Ravussin E, Redman LM, Rochon J, Das SK, Fontana L, Kraus WE, Romashkan S, Williamson DA, Meydani SN, Villareal DT, Smith SR, Stein RI, Scott TM, Stewart TM, Saltzman E, Klein S, Bhapkar M, Martin CK, Gilhooly CH, Holloszy JO, Hadley EC, Roberts SB; CALERIE Study Group. J Gerontol A Biol Sci Med Sci. 2015 Sep;70(9):1097-104.
[3] Caloric restriction in humans reveals immunometabolic regulators of health span. Spadaro O, Youm Y, Shchukina I, Ryu S, Sidorov S, Ravussin A, Nguyen K, Aladyeva E, Predeus AN, Smith SR, Ravussin E, Galban C, Artyomov MN, Dixit VD. Science. 2022 Feb 11;375(6581):671-677.
[4] Caloric restriction has a new player. Rhoads TW and Anderson RM. Science. 2022 Feb 11;375(6581):620-621.
Links:
Dietary Restriction (National Institute on Aging, NIH)
What Do We Know About Healthy Aging? (NIA)
Calorie Restriction and Fasting Diets: What Do We Know? (NIA)
Live Long in Good Health: Could Calorie Restriction Mimetics Hold the Key? (NIA)
Geroscience: The Intersection of Basic Aging Biology, Chronic Disease, and Health (NIA)
Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy (CALERIE) (NIA)
CALERIE Intensive Intervention Database (NIA)
Research Highlights (NIA)
Vishwa Deep Dixit (Yale University, New Haven, CT)
CALERIE Research Network (Duke University, Durham, N.C.)
[Note: Acting NIH Director Lawrence Tabak has asked the heads of NIH’s Institutes and Centers (ICs) to contribute occasional guest posts to the blog to highlight some of the cool science that they support and conduct. This is the fourth in the series of NIH IC guest posts that will run until a new permanent NIH director is in place.]
Posted In: Generic
Tags: aging, CALERIE, calorie, calorie reduction, calorie restriction, calorie-restricted diet, cardiovascular disease, chronic disease, clinical research, Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy, diet, fat metabolism, geroscience, healthspan, immune system, inflammation, metabolic disease, mice, MRI, National Institute on Aging, NIA, PLA2G7, portion size, T cells, thymus
The People’s Picks for Best Posts
Posted on January 5th, 2021 by Dr. Francis Collins
It’s 2021—Happy New Year! Time sure flies in the blogosphere. It seems like just yesterday that I started the NIH Director’s Blog to highlight recent advances in biology and medicine, many supported by NIH. Yet it turns out that more than eight years have passed since this blog got rolling and we are fast approaching my 1,000th post!
I’m pleased that millions of you have clicked on these posts to check out some very cool science and learn more about NIH and its mission. Thanks to the wonders of social media software, we’ve been able to tally up those views to determine each year’s most-popular post. So, I thought it would be fun to ring in the New Year by looking back at a few of your favorites, sort of a geeky version of a top 10 countdown or the People’s Choice Awards. It was interesting to see what topics generated the greatest interest. Spoiler alert: diet and exercise seemed to matter a lot! So, without further ado, I present the winners:
2013: Fighting Obesity: New Hopes from Brown Fat. Brown fat, one of several types of fat made by our bodies, was long thought to produce body heat rather than store energy. But Shingo Kajimura and his team at the University of California, San Francisco, showed in a study published in the journal Nature, that brown fat does more than that. They discovered a gene that acts as a molecular switch to produce brown fat, then linked mutations in this gene to obesity in humans.
What was also nice about this blog post is that it appeared just after Kajimura had started his own lab. In fact, this was one of the lab’s first publications. One of my goals when starting the blog was to feature young researchers, and this work certainly deserved the attention it got from blog readers. Since highlighting this work, research on brown fat has continued to progress, with new evidence in humans suggesting that brown fat is an effective target to improve glucose homeostasis.
2014: In Memory of Sam Berns. I wrote this blog post as a tribute to someone who will always be very near and dear to me. Sam Berns was born with Hutchinson-Gilford progeria syndrome, one of the rarest of rare diseases. After receiving the sad news that this brave young man had passed away, I wrote: “Sam may have only lived 17 years, but in his short life he taught the rest of us a lot about how to live.”
Affecting approximately 400 people worldwide, progeria causes premature aging. Without treatment, children with progeria, who have completely normal intellectual development, die of atherosclerotic cardiovascular disease, on average in their early teens.
From interactions with Sam and his parents in the early 2000s, I started to study progeria in my NIH lab, eventually identifying the gene responsible for the disorder. My group and others have learned a lot since then. So, it was heartening last November when the Food and Drug Administration approved the first treatment for progeria. It’s an oral medication called Zokinvy (lonafarnib) that helps prevent the buildup of defective protein that has deadly consequences. In clinical trials, the drug increased the average survival time of those with progeria by more than two years. It’s a good beginning, but we have much more work to do in the memory of Sam and to help others with progeria. Watch for more about new developments in applying gene editing to progeria in the next few days.
2015: Cytotoxic T Cells on Patrol. Readers absolutely loved this post. When the American Society of Cell Biology held its first annual video competition, called CellDance, my blog featured some of the winners. Among them was this captivating video from Alex Ritter, then working with cell biologist Jennifer Lippincott-Schwartz of NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development. The video stars a roving, specialized component of our immune system called cytotoxic T cells. Their job is to seek out and destroy any foreign or detrimental cells. Here, these T cells literally convince a problem cell to commit suicide, a process that takes about 10 minutes from detection to death.
These cytotoxic T cells are critical players in cancer immunotherapy, in which a patient’s own immune system is enlisted to control and, in some cases, even cure the cancer. Cancer immunotherapy remains a promising area of research that continues to progress, with a lot of attention now being focused on developing immunotherapies for common, solid tumors like breast cancer. Ritter is currently completing a postdoctoral fellowship in the laboratory of Ira Mellman, Genentech, South San Francisco. His focus has shifted to how cancer cells protect themselves from T cells. And video buffs—get this—Ritter says he’s now created even cooler videos that than the one in this post.
2016: Exercise Releases Brain-Healthy Protein. The research literature is pretty clear: exercise is good for the brain. In this very popular post, researchers led by Hyo Youl Moon and Henriette van Praag of NIH’s National Institute on Aging identified a protein secreted by skeletal muscle cells to help explore the muscle-brain connection. In a study in Cell Metabolism, Moon and his team showed that this protein called cathepsin B makes its way into the brain and after a good workout influences the development of new neural connections. This post is also memorable to me for the photo collage that accompanied the original post. Why? If you look closely at the bottom right, you’ll see me exercising—part of my regular morning routine!
2017: Muscle Enzyme Explains Weight Gain in Middle Age. The struggle to maintain a healthy weight is a lifelong challenge for many of us. While several risk factors for weight gain, such as counting calories, are within our control, there’s a major one that isn’t: age. Jay Chung, a researcher with NIH’s National Heart, Lung, and Blood Institute, and his team discovered that the normal aging process causes levels of an enzyme called DNA-PK to rise in animals as they approach middle age. While the enzyme is known for its role in DNA repair, their studies showed it also slows down metabolism, making it more difficult to burn fat.
Since publishing this paper in Cell Metabolism, Chung has been busy trying to understand how aging increases the activity of DNA-PK and its ability to suppress renewal of the cell’s energy-producing mitochondria. Without renewal of damaged mitochondria, excess oxidants accumulate in cells that then activate DNA-PK, which contributed to the damage in the first place. Chung calls it a “vicious cycle” of aging and one that we’ll be learning more about in the future.
2018: Has an Alternative to Table Sugar Contributed to the C. Diff. Epidemic? This impressive bit of microbial detective work had blog readers clicking and commenting for several weeks. So, it’s no surprise that it was the runaway People’s Choice of 2018.
Clostridium difficile (C. diff) is a common bacterium that lives harmlessly in the gut of most people. But taking antibiotics can upset the normal balance of healthy gut microbes, allowing C. diff. to multiply and produce toxins that cause inflammation and diarrhea.
In the 2000s, C. diff. infections became far more serious and common in American hospitals, and Robert Britton, a researcher at Baylor College of Medicine, Houston, wanted to know why. He and his team discovered that two subtypes of C. diff have adapted to feed on the sugar trehalose, which was approved as a food additive in the United States during the early 2000s. The team’s findings, published in the journal Nature, suggested that hospitals and nursing homes battling C. diff. outbreaks may want to take a closer look at the effect of trehalose in the diet of their patients.
2019: Study Finds No Benefit for Dietary Supplements. This post that was another one that sparked a firestorm of comments from readers. A team of NIH-supported researchers, led by Fang Fang Zhang, Tufts University, Boston, found that people who reported taking dietary supplements had about the same risk of dying as those who got their nutrients through food. What’s more, the mortality benefits associated with adequate intake of vitamin A, vitamin K, magnesium, zinc, and copper were limited to amounts that are available from food consumption. The researchers based their conclusion on an analysis of the well-known National Health and Nutrition Examination Survey (NHANES) between 1999-2000 and 2009-2010 survey data. The team, which reported its data in the Annals of Internal Medicine, also uncovered some evidence suggesting that certain supplements might even be harmful to health when taken in excess.
2020: Genes, Blood Type Tied to Risk of Severe COVID-19. Typically, my blog focuses on research involving many different diseases. That changed in 2020 due to the emergence of a formidable public health challenge: the coronavirus disease 2019 (COVID-19) pandemic. Since last March, the blog has featured 85 posts on COVID-19, covering all aspects of the research response and attracting more visitors than ever. And which post got the most views? It was one that highlighted a study, published last June in the New England Journal of Medicine, that suggested the clues to people’s variable responses to COVID-19 may be found in our genes and our blood types.
The researchers found that gene variants in two regions of the human genome are associated with severe COVID-19 and correspondingly carry a greater risk of COVID-19-related death. The two stretches of DNA implicated as harboring risks for severe COVID-19 are known to carry some intriguing genes, including one that determines blood type and others that play various roles in the immune system.
In fact, the findings suggest that people with blood type A face a 50 percent greater risk of needing oxygen support or a ventilator should they become infected with the novel coronavirus. In contrast, people with blood type O appear to have about a 50 percent reduced risk of severe COVID-19.
That’s it for the blog’s year-by-year Top Hits. But wait! I’d also like to give shout outs to the People’s Choice winners in two other important categories—history and cool science images.
Top History Post: HeLa Cells: A New Chapter in An Enduring Story. Published in August 2013, this post remains one of the blog’s greatest hits with readers. The post highlights science’s use of cancer cells taken in the 1950s from a young Black woman named Henrietta Lacks. These “HeLa” cells had an amazing property not seen before: they could be grown continuously in laboratory conditions. The “new chapter” featured in this post is an agreement with the Lacks family that gives researchers access to the HeLa genome data, while still protecting the family’s privacy and recognizing their enormous contribution to medical research. And the acknowledgments rightfully keep coming from those who know this remarkable story, which has been chronicled in both book and film. Recently, the U.S. Senate and House of Representatives passed the Henrietta Lacks Enhancing Cancer Research Act to honor her extraordinary life and examine access to government-funded cancer clinical trials for traditionally underrepresented groups.
Top Snapshots of Life: A Close-up of COVID-19 in Lung Cells. My blog posts come in several categories. One that you may have noticed is “Snapshots of Life,” which provides a showcase for cool images that appear in scientific journals and often dominate Science as Art contests. My blog has published dozens of these eye-catching images, representing a broad spectrum of the biomedical sciences. But the blog People’s Choice goes to a very recent addition that reveals exactly what happens to cells in the human airway when they are infected with the coronavirus responsible for COVID-19. This vivid image, published in the New England Journal of Medicine, comes from the lab of pediatric pulmonologist Camille Ehre, University of North Carolina at Chapel Hill. This image squeezed in just ahead of another highly popular post from Steve Ramirez, Boston University, in 2019 that showed “What a Memory Looks Like.”
As we look ahead to 2021, I want to thank each of my blog’s readers for your views and comments over the last eight years. I love to hear from you, so keep on clicking! I’m confident that 2021 will generate a lot more amazing and bloggable science, including even more progress toward ending the COVID-19 pandemic that made our past year so very challenging.
Posted In: Generic
Tags: aging, blood type, brown fat, C. diff, cancer, cancer immunotherapy, cathepsin, cathepsin B, Clostridium difficile, coronavirus, COVID-19, cytotoxic T cells, diet, dietary supplements, DNA-PK, exercise, FDA, genes, HeLa cells, Henrietta Lacks, Henrietta Lacks Enhancing Cancer Research Act, Hutchinson-Gilford progeria syndrome, Ira Mellman, Jennifer Lippincott-Schwartz, lonafarnib, lung cells, memory, mitochondria, muscle, obesity, progeria, sam berns, T cells, trehalose, weight gain, Zokinvy
Celebrating 2019 Biomedical Breakthroughs
Posted on January 2nd, 2020 by Dr. Francis Collins

Happy New Year! As we say goodbye to the Teens, let’s take a look back at 2019 and some of the groundbreaking scientific discoveries that closed out this remarkable decade.
Each December, the reporters and editors at the journal Science select their breakthrough of the year, and the choice for 2019 is nothing less than spectacular: An international network of radio astronomers published the first image of a black hole, the long-theorized cosmic singularity where gravity is so strong that even light cannot escape [1]. This one resides in a galaxy 53 million light-years from Earth! (A light-year equals about 6 trillion miles.)
Though the competition was certainly stiff in 2019, the biomedical sciences were well represented among _Science_’s “runner-up” breakthroughs. They include three breakthroughs that have received NIH support. Let’s take a look at them:
In a first, drug treats most cases of cystic fibrosis: Last October, two international research teams reported the results from phase 3 clinical trials of the triple drug therapy Trikafta to treat cystic fibrosis (CF). Their data showed Trikafta effectively compensates for the effects of a mutation carried by about 90 percent of people born with CF. Upon reviewing these impressive data, the Food and Drug Administration (FDA) approved Trikafta, developed by Vertex Pharmaceuticals.
The approval of Trikafta was a wonderful day for me personally, having co-led the team that isolated the CF gene 30 years ago. A few years later, I wrote a song called “Dare to Dream” imagining that wonderful day when “the story of CF is history.” Though we’ve still got more work to do, we’re getting a lot closer to making that dream come true. Indeed, with the approval of Trikafta, most people with CF have for the first time ever a real chance at managing this genetic disease as a chronic condition over the course of their lives. That’s a tremendous accomplishment considering that few with CF lived beyond their teens as recently as the 1980s.
Such progress has been made possible by decades of work involving a vast number of researchers, many funded by NIH, as well as by more than two decades of visionary and collaborative efforts between the Cystic Fibrosis Foundation and Aurora Biosciences (now, Vertex) that built upon that fundamental knowledge of the responsible gene and its protein product. Not only did this innovative approach serve to accelerate the development of therapies for CF, it established a model that may inform efforts to develop therapies for other rare genetic diseases.
Hope for Ebola patients, at last: It was just six years ago that news of a major Ebola outbreak in West Africa sounded a global health emergency of the highest order. Ebola virus disease was then recognized as an untreatable, rapidly fatal illness for the majority of those who contracted it. Though international control efforts ultimately contained the spread of the virus in West Africa within about two years, over 28,600 cases had been confirmed leading to more than 11,000 deaths—marking the largest known Ebola outbreak in human history. Most recently, another major outbreak continues to wreak havoc in northeastern Democratic Republic of Congo (DRC), where violent civil unrest is greatly challenging public health control efforts.
As troubling as this news remains, 2019 brought a needed breakthrough for the millions of people living in areas susceptible to Ebola outbreaks. A randomized clinical trial in the DRC evaluated four different drugs for treating acutely infected individuals, including an antibody against the virus called mAb114, and a cocktail of anti-Ebola antibodies referred to as REGN-EB3. The trial’s preliminary data showed that about 70 percent of the patients who received either mAb114 or the REGN-EB3 antibody cocktail survived, compared with about half of those given either of the other two medicines.
So compelling were these preliminary results that the trial, co-sponsored by NIH’s National Institute of Allergy and Infectious Diseases (NIAID) and the DRC’s National Institute for Biomedical Research, was halted last August. The results were also promptly made public to help save lives and stem the latest outbreak. All Ebola patients in the DRC treatment centers now are treated with one or the other of these two options. The trial results were recently published.
The NIH-developed mAb114 antibody and the REGN-EB3 cocktail are the first therapeutics to be shown in a scientifically rigorous study to be effective at treating Ebola. This work also demonstrates that ethically sound clinical research can be conducted under difficult conditions in the midst of a disease outbreak. In fact, the halted study was named Pamoja Tulinde Maisha (PALM), which means “together save lives” in Kiswahili.
To top off the life-saving progress in 2019, the FDA just approved the first vaccine for Ebola. Called Ervebo (earlier rVSV-ZEBOV), this single-dose injectable vaccine is a non-infectious version of an animal virus that has been genetically engineered to carry a segment of a gene from the Zaire species of the Ebola virus—the virus responsible for the current DRC outbreak and the West Africa outbreak. Because the vaccine does not contain the whole Zaire virus, it can’t cause Ebola. Results from a large study in Guinea conducted by the WHO indicated that the vaccine offered substantial protection against Ebola virus disease. Ervebo, produced by Merck, has already been given to over 259,000 individuals as part of the response to the DRC outbreak. The NIH has supported numerous clinical trials of the vaccine, including an ongoing study in West Africa.
Microbes combat malnourishment: Researchers discovered a few years ago that abnormal microbial communities, or microbiomes, in the intestine appear to contribute to childhood malnutrition. An NIH-supported research team followed up on this lead with a study of kids in Bangladesh, and it published last July its groundbreaking finding: that foods formulated to repair the “gut microbiome” helped malnourished kids rebuild their health. The researchers were able to identify a network of 15 bacterial species that consistently interact in the gut microbiomes of Bangladeshi children. In this month-long study, this bacterial network helped the researchers characterize a child’s microbiome and/or its relative state of repair.
But a month isn’t long enough to determine how the new foods would help children grow and recover. The researchers are conducting a similar study that is much longer and larger. Globally, malnutrition affects an estimated 238 million children under the age 5, stunting their normal growth, compromising their health, and limiting their mental development. The hope is that these new foods and others adapted for use around the world soon will help many more kids grow up to be healthy adults.
Measles Resurgent: The staff at Science also listed their less-encouraging 2019 Breakdowns of the Year, and unfortunately the biomedical sciences made the cut with the return of measles in the U.S. Prior to 1963, when the measles vaccine was developed, 3 to 4 million Americans were sickened by measles each year. Each year about 500 children would die from measles, and many more would suffer lifelong complications. As more people were vaccinated, the incidence of measles plummeted. By the year 2000, the disease was even declared eliminated from the U.S.
But, as more parents have chosen not to vaccinate their children, driven by the now debunked claim that vaccines are connected to autism, measles has made a very preventable comeback. Last October, the Centers for Disease Control and Prevention (CDC) reported an estimated 1,250 measles cases in the United States at that point in 2019, surpassing the total number of cases reported annually in each of the past 25 years.
The good news is those numbers can be reduced if more people get the vaccine, which has been shown repeatedly in many large and rigorous studies to be safe and effective. The CDC recommends that children should receive their first dose by 12 to 15 months of age and a second dose between the ages of 4 and 6. Older people who’ve been vaccinated or have had the measles previously should consider being re-vaccinated, especially if they live in places with low vaccination rates or will be traveling to countries where measles are endemic.
Despite this public health breakdown, 2019 closed out a memorable decade of scientific discovery. The Twenties will build on discoveries made during the Teens and bring us even closer to an era of precision medicine to improve the lives of millions of Americans. So, onward to 2020—and happy New Year!
Reference:
[1] 2019 Breakthrough of the Year. Science, December 19, 2019.
NIH Support: These breakthroughs represent the culmination of years of research involving many investigators and the support of multiple NIH institutes.
Posted In: News
Tags: 2019 Biomedical Breakdown, 2019 Breakthroughs of the Year, Africa, antibody, Bangladesh, breakdown, breakthroughs, Breakthroughs of 2019, CDC, CF, child health, childhood vaccines, children, clinical trials, Congo, cystic fibrosis, Cystic Fibrosis Foundation, diet, Ebola, Ebola vaccine, Ebola Virus Disease, Ervebo, FDA, gene-based therapy, genetic diseases, global health, Guinea, gut microbiome, hemorrhagic fever, infectious disease, mAB114, malnutrition, measles, measles outbreak, Merck, microbiology, microbiome, PALM, pandemic, precision medicine, public health, rare disease, REGN-EB3, Trikafta, Vertex, Vertex Pharmaceuticals, virus, West Africa
Why When You Eat Might Be as Important as What You Eat
Posted on December 10th, 2019 by Dr. Francis Collins

Adapted from Wilkinson MJ, Cell Metab, 2019
About 1 in 3 American adults have metabolic syndrome, a group of early warning signs for increased risk of type 2 diabetes, heart disease, and stroke. To help avoid such health problems, these folks are often advised to pay close attention to the amount and type of foods they eat. And now it seems there may be something else to watch: how food intake is spaced over a 24-hour period.
In a three-month pilot study, NIH-funded researchers found that when individuals with metabolic syndrome consumed all of their usual daily diet within 10 hours—rather than a more customary span of about 14 hours—their early warning signs improved. Not only was a longer stretch of daily fasting associated with moderate weight loss, in some cases, it was also tied to lower blood pressure, lower blood glucose levels, and other improvements in metabolic syndrome.
The study, published in Cell Metabolism, is the result of a joint effort by Satchidananda Panda, Salk Institute for Biological Sciences, La Jolla, CA, and Pam R. Taub, University of California, San Diego [1]. It was inspired by Panda’s earlier mouse studies involving an emerging dietary intervention, called time-restricted eating (TRE), which attempts to establish a consistent daily cycle of feeding and fasting to create more stable rhythms for the body’s own biological clock [2, 3].
But would observations in mice hold true for humans? To find out, Panda joined forces with Taub, a cardiologist and physician-scientist. The researchers enlisted 19 men and women with metabolic syndrome, defined as having three or more of five specific risk factors: high fasting blood glucose, high blood pressure, high triglyceride levels, low “good” cholesterol, and/or extra abdominal fat. Most participants were obese and taking at least one medication to help manage their metabolic risk factors.
In the study, participants followed one rule: eat anything that you want, just do so over a 10-hour period of your own choosing. So, for the next three months, these folks logged their eating times and tracked their sleep using a special phone app created by the research team. They also wore activity and glucose monitors.
By the pilot study’s end, participants following the 10-hour limitation had lost on average 3 percent of their weight and about 3 percent of their abdominal fat. They also lowered their cholesterol and blood pressure. Although this study did not find 10-hour TRE significantly reduced blood glucose levels in all participants, those with elevated fasting blood glucose did have improvement. In addition, participants reported other lifestyle improvements, including better sleep.
The participants generally saw their metabolic health improve without skipping meals. Most chose to delay breakfast, waiting about two hours after they got up in the morning. They also ate dinner earlier, about three hours before going to bed—and then did no late night snacking.
After the study, more than two-thirds reported that they stuck with the 10-hour eating plan at least part-time for up to a year. Some participants were able to cut back or stop taking cholesterol and/or blood-pressure-lowering medications.
Following up on the findings of this small study, Taub will launch a larger NIH-supported clinical trial involving 100 people with metabolic syndrome. Panda is now exploring in greater detail the underlying biology of the metabolic benefits observed in the mice following TRE.
For people looking to improve their metabolic health, it’s a good idea to consult with a doctor before making significant changes to one’s eating habits. But the initial data from this study indicate that, in addition to exercising and limiting portion size, it might also pay to watch the clock.
References:
[1] Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleisher JG, Panda S, Taub PR. Cell Metab. 2019 Jan 7; 31: 1-13. Epub 2019 Dec 5.
[2] Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, Ellisman MH, Panda S. Cell Metab. 2012 Jun 6;15(6):848-60.
[3] Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Chaix A, Zarrinpar A, Miu P, Panda S. Cell Metab. 2014 Dec 2;20(6):991-1005.
Links:
Metabolic Syndrome (National Heart, Lung, and Blood Institute/NIH)
Obesity (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Body Weight Planner (NIDDK/NIH)
Satchidananda Panda (Salk Institute for Biological Sciences, La Jolla, CA)
Taub Research Group (University of California, San Diego)
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases
Posted In: News
Tags: bad cholesterol, biological clock, blood glucose, blood pressure, circadian rhythms, diet, fasting, fat, food, lipids, metabolic syndrome, metabolism, obesity, pilot study, sleep, time-restricted eating, TRE, triglycerides, weight loss
Americans Are Still Eating Too Much Added Sugar, Fat
Posted on October 1st, 2019 by Dr. Francis Collins

Credit: iStock/happy_lark
Most of us know one of the best health moves we can make is to skip the junk food and eat a nutritious, well-balanced diet. But how are we doing at putting that knowledge into action? Not so great, according to a new analysis that reveals Americans continue to get more than 50 percent of their calories from low-quality carbohydrates and artery-clogging saturated fat.
In their analysis of the eating habits of nearly 44,000 adults over 16 years, NIH-funded researchers attributed much of our nation’s poor dietary showing to its ongoing love affair with heavily processed fast foods and snacks. But there were a few bright spots. The analysis also found that, compared to just a few decades ago, Americans are eating more foods with less added sugar, as well as more whole grains (e.g., brown rice, quinoa, rolled oats), plant proteins (e.g., nuts, beans), and sources of healthy fats (e.g., olive oil).
Over the last 20-plus years, research has generated new ideas about eating a proper diet. In the United States, the revised thinking led to the 2015-2020 Dietary Guidelines for Americans. They recommend eating more fruits, vegetables, whole grains, and other nutrient-dense foods, while limiting foods containing added sugars, saturated fats, and salt.
In the report published in JAMA, a team of researchers wanted to see how Americans are doing at following the new guidelines. The team was led by Shilpa Bhupathiraju, Harvard T. H. Chan School of Public Health, Boston, and Fang Fang Zhang, Tufts University, Boston.
To get the answer, the researchers looked to the National Health and Nutrition Examination Survey (NHANES). The survey includes a nationally representative sample of U.S. adults, age 20 or older, who had answered questions about their food and beverage intake over a 24-hour period at least once during nine annual survey cycles between 1999-2000 and 2015-2016.
The researchers assessed the overall quality of the American diet using the Healthy Eating Index-2015 (HEI-2015), which measures adherence to the 2015-2020 Dietary Guidelines. The HEI-2015 scores range from 0 to 100, with the latter number being a perfect, A-plus score. The analysis showed the American diet barely inching up over the last two decades from a final score of 55.7 to 57.7.
That, of course, is still far from a passing grade. Some of the common mistakes identified:
• Refined grains, starchy vegetables, and added sugars still account for 42 percent of the average American’s daily calories.
• Whole grains and fruits provide just 9 percent of daily calories.
• Saturated fat consumption remains above 10 percent of daily calories, as many Americans continue to eat more red and processed meat.
Looking on the bright side, the data do indicate more Americans are starting to lean toward the right choices. They are getting slightly more of their calories from healthier whole grains and a little less from added sugar. Americans are also now looking a little more to whole grains, nuts, and beans as a protein source. It’s important to note, though, these small gains weren’t seen in lower income groups or older adults.
The bottom line is most Americans still have an awfully long way to go to shape up their diets. The question is: how to get there? There are plenty of good choices that can help to turn things around, from reading food labels and limiting calories or portion sizes to exercising and finding healthy recipes that suit your palate.
Meanwhile, nutrition research is poised for a renaissance. Tremendous progress is being made in studying the microbial communities, or microbiomes, helping to digest our foods. The same is true for studies of energy metabolism, genetic variation influencing our dietary preferences, and the effects of aging.
This is an optimum time to enhance the science and evidence base for human nutrition. That may result in some updating of the scoring system for the nation’s dietary report card. But it will be up to all of us to figure out how to ace it.
References:
[1] Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality Among US Adults, 1999-2016. Shan Z, Rehm CD, Rogers G, Ruan M, Wang DD, Hu FB, Mozaffarian D, Zhang FF, Bhupathiraju SN. JAMA. 2019 Sep 24;322(12):1178-1187.
Links:
Eat Right (National Heart, Lung, and Blood Institute/NIH)
Dietary Fats (MedlinePlus, National Library of Medicine/NIH)
ChooseMyPlate (U.S. Department of Agriculture)
Healthy Eating Index (Department of Agriculture)
NIH Nutrition Research Task Force (National Institute of Diabetes and Digestive and Kidney Disease/NIH)
Dietary Guidelines for Americans (U.S. Department of Health and Human Services)
Shilpa Bhupathiraju (Harvard T. H. Chan School of Public Health, Boston)
Fang Fang Zhang (Tufts University, Boston)
NIH Support: National Institute on Minority Health and Health Disparities; National Institute of Diabetes and Digestive and Kidney Diseases
Posted In: News
Tags: 2015-2020 Dietary Guidelines for Americans, aging, carbohydrates, diet, dietary guidelines, eating, energy metabolism, fat, food, fruits, Healthy Eating Index, high-fat diet, junk food, low-quality carbohydrates, microbiome, National Health and Nutrition Examination Survey, NHANES, nutrition, plant proteins, refined grains, salt, saturated fat, sugar, vegetables, whole grains
Targeting the Microbiome to Treat Malnutrition
Posted on July 23rd, 2019 by Dr. Francis Collins

Caption: A Bangladeshi mother and child in the Nutritional Rehabilitation Unit.
Credit: International Centre for Diarrhoeal Disease Research, Bangladesh
A few years ago, researchers discovered that abnormalities in microbial communities, or microbiomes, in the intestine appear to contribute to childhood malnutrition. Now comes word that this discovery is being translated into action, with a new study showing that foods formulated to repair the “gut microbiome” may help malnourished kids rebuild their health [1].
In a month-long clinical trial in Bangladesh, 63 children received either regular foods to treat malnutrition or alternative formulations for needed calories and nutrition that also encouraged growth of beneficial microbes in the intestines. The kids who ate the microbiome-friendly diets showed improvements in their microbiome, which helps to extract and metabolize nutrients in our food to help the body grow. They also had significant improvements in key blood proteins associated with bone growth, brain development, immunity, and metabolism; those who ate standard therapeutic food did not experience the same benefit.
Globally, malnutrition affects an estimated 238 million children under the age 5, stunting their normal growth, compromising their health, and limiting their mental development [2]. Malnutrition can arise not only from a shortage of food but from dietary imbalances that don’t satisfy the body’s need for essential nutrients. Far too often, especially in impoverished areas, the condition can turn extremely severe and deadly. And the long term effects on intellectual development can limit the ability of a country’s citizens to lift themselves out of poverty.
Jeffrey Gordon, Washington University School of Medicine in St. Louis, and his NIH-supported research team have spent decades studying what constitutes a normal microbiome and how changes can affect health and disease. Their seminal studies have revealed that severely malnourished kids have “immature” microbiomes that don’t develop in the intestine like the microbial communities seen in well nourished, healthy children of the same age.
Gordon and team have also found that this microbial immaturity doesn’t resolve when kids consume the usual supplemental foods [3]. In another study, they turned to mice raised under sterile conditions and with no microbes of their own to demonstrate this cause and effect. The researchers colonized the intestines of the germ-free mice with microbes from malnourished children, and the rodents developed similar abnormalities in weight gain, bone growth, and metabolism [4].
All of this evidence raised a vital question: Could the right combination of foods “mature” the microbiome and help to steer malnourished children toward a healthier state?
To get the answer, Gordon and his colleagues at the International Centre for Diarrhoeal Disease Research, Dhaka, Bangladesh, led by Tahmeed Ahmed, first had to formulate the right, microbiome-friendly food supplements, and that led to some interesting science. They carefully characterized over time the immature microbiomes found in Bangladeshi children treated for severe malnutrition. This allowed them to test their new method for analyzing how individual microbial species fluctuate over time and in relationship to one another in the intestine [5]. The team then paired up these data with measurements of a set of more than 1,300 blood proteins from the children that provide “readouts” of their biological state.
Their investigation identified a network of 15 bacterial species that consistently interact in the gut microbiomes of Bangladeshi children. This network became their means to characterize sensitively and accurately the development of a child’s microbiome and/or its relative state of repair.
Next, they turned to mice colonized with the same collections of microbes found in the intestines of the Bangladeshi children. Gordon’s team then tinkered with the animals’ diets in search of ingredients commonly consumed by young children in Bangladesh that also appeared to encourage a healthier, more mature microbiome. They did similar studies in young pigs, whose digestive and immune systems more closely resemble humans.
The Gordon team settled on three candidate microbiome-friendly formulations. Two included chickpea flour, soy flour, peanut flour, and banana at different concentrations; one of these two also included milk powder. The third combined chickpea flour and soy flour. All three contained similar amounts of protein, fat, and calories.
The researchers then launched a randomized, controlled clinical trial with children from a year to 18 months old with moderate acute malnutrition. These young children were enrolled into one of four treatment groups, each including 14 to 17 kids. Three groups received one of the newly formulated foods. The fourth group received standard rice-and-lentil-based meals.
The children received these supplemental meals twice a day for four weeks at the International Centre for Diarrhoeal Disease Research followed by two-weeks of observation. Mothers were encouraged throughout the study to continue breastfeeding their children.
The formulation containing chickpea, soy, peanut, and banana, but no milk powder, stood out above the rest in the study. Children taking this supplement showed a dramatic shift toward a healthier state as measured by those more than 1,300 blood proteins. Their gut microbiomes also resembled those of healthy children their age.
Their new findings published in the journal Science offer the first evidence that a therapeutic food, developed to support the growth and development of a healthy microbiome, might come with added benefits for children suffering from malnutrition. Importantly, the researchers took great care to design the supplements with foods that are readily available, affordable, culturally acceptable, and palatable for young children in Bangladesh.
A month isn’t nearly long enough to see how the new foods would help children grow and recover over time. So, the researchers are now conducting a much larger study of their leading supplement in children with histories of malnutrition, to explore its longer-term health effects for them and their microbiomes. The hope is that these new foods and others adapted for use around the world soon will help many more kids grow up to be healthy adults.
References:
[1] Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Gehrig JL, Venkatesh S, Chang HW, Hibberd MC, Kung VL, Cheng J, Chen RY, Subramanian S, Cowardin CA, Meier MF, O’Donnell D, Talcott M, Spears LD, Semenkovich CF, Henrissat B, Giannone RJ, Hettich RL, Ilkayeva O, Muehlbauer M, Newgard CB, Sawyer C, Head RD, Rodionov DA, Arzamasov AA, Leyn SA, Osterman AL, Hossain MI, Islam M, Choudhury N, Sarker SA, Huq S, Mahmud I, Mostafa I, Mahfuz M, Barratt MJ, Ahmed T, Gordon JI. Science. 2019 Jul 12;365(6449).
[2] Childhood Malnutrition. World Health Organization
[3] Persistent gut microbiota immaturity in malnourished Bangladeshi children. Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD, Barratt MJ, VanArendonk LG, Zhang Q, Province MA, Petri WA Jr, Ahmed T, Gordon JI. Nature. 2014 Jun 19;510(7505):417-21.
[4] Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, Fan YM, Henrissat B, Leyn SA, Rodionov DA, Osterman AL, Maleta KM, Newgard CB, Ashorn P, Dewey KG, Gordon JI. Science. 2016 Feb 19;351(6275).
[5] A sparse covarying unit that describes healthy and impaired human gut microbiota development. Raman AS, Gehrig JL, Venkatesh S, Chang HW, Hibberd MC, Subramanian S, Kang G, Bessong PO, Lima AAM, Kosek MN, Petri WA Jr, Rodionov DA, Arzamasov AA, Leyn SA, Osterman AL, Huq S, Mostafa I, Islam M, Mahfuz M, Haque R, Ahmed T, Barratt MJ, Gordon JI. Science. 2019 Jul 12;365(6449).
Links:
Childhood Nutrition Facts (Centers for Disease Control and Prevention)
Gordon Lab (Washington University School of Medicine in St. Louis)
International Centre for Diarrhoeal Disease Research (Dhaka, Bangladesh)
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases; National Institute of General Medical Sciences; National Institute of Arthritis and Musculoskeletal and Skin Diseases; National Center for Advancing Translational Sciences; National Cancer Institute
Posted In: News
Tags: bacteria, banana, Bangladesh, chickpeas, childiren, clinical trial, development, diet, food, global health, gut microbiome, immature microbiome, infants, International Centre for Diarrhoeal Disease Researc, intestine, malnutrition, metabolism, microbiome, microbiota, nutrition, peanut, poverty, soy
Fundamental Knowledge of Microbes Shedding New Light on Human Health
Posted on May 29th, 2019 by Dr. Francis Collins

Caption: Human microbiome research requires teamwork. Kimberly Jefferson (second from left), a leader of the Multi-Omic Microbiome Study—Pregnancy Initiative, joins some of the team at Virginia Commonwealth University, Richmond. Credit: Courtesy of Kimberly Jefferson
Basic research in biology generates fundamental knowledge about the nature and behavior of living systems. It is generally impossible to predict exactly where this line of scientific inquiry might lead, but history shows that basic science almost always serves as the foundation for dramatic breakthroughs that advance human health. Indeed, many important medical advances can be traced back to basic research that, at least at the outset, had no clear link at all to human health.
One exciting example of NIH-supported basic research is the Human Microbiome Project (HMP), which began 12 years ago as a quest to use DNA sequencing to identify and characterize the diverse collection of microbes—including trillions of bacteria, fungi, and viruses—that live on and in the healthy human body.
The HMP researchers have subsequently been using those vast troves of fundamental data as a tool to explore how microbial communities interact with human cells to influence health and disease. Today, these explorers are reporting their latest findings in a landmark set of papers in the Nature family of journals. Among other things, these findings shed new light on the microbiome’s role in prediabetes, inflammatory bowel disease, and preterm birth. The studies are part of the Integrative Human Microbiome Project.
If you’d like to keep up on the microbiome and other basic research journeys, here’s a good way to do so. Consider signing up for basic research updates from the NIH Director’s Blog and NIH Research Matters. Here’s how to do it: Go to Email Updates, type in your email address, and enter. That’s it. If you’d like to see other update possibilities, including clinical and translational research, hit the “Finish” button to access Subscriber Preferences.
As for the recent microbiome findings, let’s start with the prediabetes study [1]. An estimated 1 in 3 American adults has prediabetes, detected by the presence of higher than normal fasting blood glucose levels. If uncontrolled and untreated, prediabetes can lead to the more-severe type 2 diabetes (T2D) and its many potentially serious side effects [2].
George Weinstock, The Jackson Laboratory for Genomic Medicine, Farmington, CT, Michael Snyder, Stanford University, Palo Alto, CA, and colleagues report that they have assembled a rich new data set covering the complex biology of prediabetes. That includes a comprehensive analysis of the human microbiome in prediabetes.
The data come from monitoring the health of 106 people with and without prediabetes for nearly four years. The researchers met with participants every three months, drawing blood, assessing the gut microbiome, and performing 51 laboratory tests. All this work generated millions of molecular and microbial measurements that provided a unique biological picture of prediabetes.
The picture showed specific interactions between cells and microbes that were different for people who are sensitive to insulin and those whose cells are resistant to it (as is true of many of those with prediabetes). The data also pointed to extensive changes in the microbiome during respiratory viral infections. Those changes showed clear differences in people with and without prediabetes. Some aspects of the immune response also appeared abnormal in people who were prediabetic.
As demonstrated in a landmark NIH study several years ago [2], people with prediabetes can do a lot to reduce their chances of developing T2D, such as exercising, eating healthy, and losing a modest amount of body weight. But this study offers some new leads to define the biological underpinnings of T2D in its earliest stages. These insights potentially point to high value targets for slowing or perhaps stopping the systemic changes that drive the transition from prediabetes to T2D.
The second study features the work of the Inflammatory Bowel Disease Multi’omics Data team. It’s led by Ramnik Xavier and Curtis Huttenhower, Broad Institute of MIT and Harvard, Cambridge, MA. [4]
Inflammatory bowel disease (IBD) is an umbrella term for chronic inflammations of the body’s digestive tract, such as Crohn’s disease and ulcerative colitis. These disorders are characterized by remissions and relapses, and the most severe flares can be life-threatening. Xavier, Huttenhower, and team followed 132 people with and without IBD for a year, collecting samples of their gut microbiomes every other week along with biopsies and blood samples for a total of nearly 3,000 samples.
By integrating DNA, RNA, protein, and metabolic analyses, they followed precisely which microbial species were present. They could also track which biochemical functions those microbes were capable of performing, and which functions they actually were performing over the course of the study.
These data now offer the most comprehensive view yet of functional imbalances associated with changes in the microbiome during IBD flares. These data also show how those imbalances may be altered when a person with IBD goes into remission. It’s also noteworthy that participants completed questionnaires on their diet. This dataset is the first to capture associations between diet and the gut microbiome in a relatively large group of people over time.
The evidence showed that the gut microbiomes of people with IBD were significantly less stable than the microbiomes of those without IBD. During IBD activity, the researchers observed increases in certain groups of microbes at the expense of others. Those changes in the microbiome also came with other telltale metabolic and biochemical disruptions along with shifts in the functioning of an individual’s immune system. The shifts, however, were not significantly associated with people taking medications or their social status.
By presenting this comprehensive, “multi-omic” view on the microbiome in IBD, the researchers were able to single out a variety of new host and microbial features that now warrant further study. For example, people with IBD had dramatically lower levels of an unclassified Subdoligranulum species of bacteria compared to people without the condition.
The third study features the work of The Vaginal Microbiome Consortium (VMC). The study represents a collaboration between Virginia Commonwealth University, Richmond, and Global Alliance to Prevent Prematurity and Stillbirth (GAPPS). The VMC study is led by Gregory Buck, Jennifer Fettweis, Jerome Strauss,and Kimberly Jefferson of Virginia Commonwealth and colleagues.
In this study, part of the Multi-Omic Microbiome Study: Pregnancy Initiative, the team followed up on previous research that suggested a potential link between the composition of the vaginal microbiome and the risk of preterm birth [5]. The team collected various samples from more than 1,500 pregnant women at multiple time points in their pregnancies. The researchers sequenced the complete microbiomes from the vaginal samples of 45 study participants, who gave birth prematurely and 90 case-matched controls who gave birth to full-term babies. Both cases and controls were primarily of African ancestry.
Those data reveal unique microbial signatures early in pregnancy in women who went on to experience a preterm birth. Specifically, women who delivered their babies earlier showed lower levels of Lactobacillus crispatus, a bacterium long associated with health in the female reproductive tract. Those women also had higher levels of several other microbes. The preterm birth-associated signatures also were associated with other inflammatory molecules.
The findings suggest a link between the vaginal microbiome and preterm birth, and raise the possibility that a microbiome test, conducted early in pregnancy, might help to predict a woman’s risk for preterm birth. Even more exciting, this might suggest a possible way to modify the vaginal microbiome to reduce the risk of prematurity in susceptible individuals.
Overall, these landmark HMP studies add to evidence that our microbial inhabitants have important implications for many aspects of our health. We are truly a “superorganism.” In terms of the implications for biomedicine, this is still just the beginning of what is sure to be a very exciting journey.
References:
[1] Longitudinal multi-omics of host-microbe dynamics in prediabetes. Zhou W, Sailani MR, Contrepois K, Sodergren E, Weinstock GM, Snyder M, et. al. Nature. 2019 May 29.
[2] National Diabetes Statistics Report, 2017, Center for Disease Control and Prevention (Atlanta, GA)
[3] Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Diabetes Prevention Program Research Group.Lancet Diabetes Endocrinol.2015 Nov;3(11):866-875.
[4] Multi-omics of the gut microbial ecosystem in inflammatory bowel disease. Lloyd-Price J, Arze C. Ananthakrishnan AN, Vlamakis H, Xavier RJ, Huttenhower C, et. al. Nature. 2019 May 29.
[5] The vaginal microbiome and preterm birth. Fettweis JM, Serrano MG, Brooks, JP, Jefferson KK, Strauss JF, Buck GA, et al. Nature Med. 2019 May 29.
Links:
Insulin Resistance & Prediabetes (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Crohn’s Disease (NIDDK/NIH)
Ulcerative colitis (NIDDK/NIH)
Preterm Labor and Birth: Condition Information (Eunice Kennedy Shriver National Institute of Child Health and Human Development/NIH)
Global Alliance to Prevent Prematurity and Stillbirth (Seattle, WA)
NIH Integrative Human Microbiome Project
NIH Support:
Prediabetes Study: Common Fund; National Institute of Dental and Craniofacial Research; National Institute of Diabetes and Digestive and Kidney Diseases; National Institute of Human Genome Research; National Center for Advancing Translational Sciences
Inflammatory Bowel Disease Study: Common Fund; National Institute of Diabetes and Digestive and Kidney Diseases; National Center for Advancing Translational Sciences; National Institute of Human Genome Research; National Institute of Dental and Craniofacial Research
Preterm Birth Study: Common Fund; National Institute of Allergy and Infectious Diseases; Eunice Kennedy Shriver National Institute of Child Health and Human Development
Posted In: News
Tags: bacteria, basic research, basic science, Crohn's disease, diabetes, diet, digestive tract, gut microbiome, HMP, Human Microbiome Project, inflammatory bowel disease, Inflammatory Bowel Disease Multi’omics Data team, Integrative Human Microbiome Project, Lactobacillus crispatus, microbiome, multi-omic, Multi-Omic Microbiome Study: Pregnancy Initiative, prediabetes, pregnancy, preterm births, T2D, The Vaginal Microbiome Consortium, type 2 diabetes, ulcerative colitis, vaginal microbiome
Ultra-Processed Diet Leads to Extra Calories, Weight Gain
Posted on May 21st, 2019 by Dr. Francis Collins

Credit: Hall et al., Cell Metabolism, 2019
If you’ve ever tried to lose a few pounds or just stay at a healthy weight, you’ve likely encountered a dizzying array of diets, each with passionate proponents: low carb, low fat, keto, paleo, vegan, Mediterranean, and so on. Yet most nutrition experts agree on one thing: it’s best to steer clear of ultra-processed foods. Now, there’s some solid scientific evidence to back up that advice.
In the first randomized, controlled study to compare the effects of ultra-processed with unprocessed foods, NIH researchers found healthy adults gained about a pound per week when they were given a daily diet high in ultra-processed foods, which often contain ingredients such as hydrogenated fats, high fructose corn syrup, flavoring agents, emulsifiers, and preservatives. In contrast, when those same people ate unprocessed whole foods, they lost weight.
Intriguingly, the weight differences on the two diets occurred even though both kinds of foods had been carefully matched from a nutritional standpoint, including calorie density, fiber, fat, sugar, and salt. For example, breakfast for the ultra-processed group might consist of a bagel with cream cheese and turkey bacon, while the unprocessed group might be offered oatmeal with bananas, walnuts, and skim milk.
The explanation for the differences appears to lie in the fact that study participants were free to eat as little or as much food as they wished at mealtimes and to snack between meals. It turns out that when folks were on the ultra-processed diet they ate significantly more—about 500 extra calories per day on average—than when they were on the unprocessed diet. And, as you probably know, more calories without more exercise usually leads to more weight!
This might not seem new to you. After all, it has been tempting for some time to suggest a connection between the rise of packaged, ultra-processed foods and America’s growing waistlines. But as plausible as it might seem that such foods may encourage overeating, perhaps because of their high salt, sugar, and fat content, correlation is not causation and controlled studies of what people actually eat are tough to do. As a result, definitive evidence directly tying ultra-processed foods to weight gain has been lacking.
To explore the possible connection in the study now reported in Cell Metabolism, researchers at NIH’s National Institute of Diabetes and Digestive and Kidney Diseases took advantage of the Metabolic Clinical Research Unit at the NIH Clinical Center, Bethesda, MD. The unit is specially equipped to study issues involving diet and metabolism.
The researchers asked 20 healthy men and women of stable weight to stay at the center for 28 days. Each volunteer was randomly assigned to eat either an ultra-processed or unprocessed diet for two consecutive weeks. At that point, they switched to the other diet for another two weeks.
Both diets consisted of three daily meals, and volunteers were given permission to eat as much food as they liked. Importantly, a team of dieticians had carefully designed the ultra-processed and unprocessed meals such that they were well matched for total calories, calorie density, macronutrients, fiber, sugars, and salt.
At lunch, for example, one of the study’s processed meals consisted of quesadillas, refried beans, and diet lemonade. An unprocessed lunch consisted of a spinach salad with chicken breast, apple slices, bulgur, and sunflower seeds with a side of grapes.
The main difference between each diet was the proportion of calories derived from ultra-processed versus unprocessed foods as defined by the NOVA diet classification system. This system categorizes food based on the nature, extent, and purpose of food processing, rather than its nutrient content.
Each week, researchers measured the energy expenditure, weight, and changes in body composition of all volunteers. After two weeks on the ultra-processed diet, volunteers gained about two pounds on average. That’s compared to a loss of about two pounds for those on the unprocessed diet.
Metabolic testing showed that people expended more energy on the ultra-processed diet. However, that wasn’t enough to offset the increased consumption of calories. As a result, participants gained pounds and body fat. The study does have some limitations, such as slight differences in the protein content of the two diets. and the researchers plan to address such issues in their future work.
During this relatively brief study, the researchers did not observe other telltale changes associated with poor metabolic health, such as a rise in blood glucose levels or fat in the liver. While a couple of pounds might not sound like much, the extra calories and weight associated with an ultra-processed diet would, over time, add up.
So, it appears that a good place to start in reaching or maintaining a healthy weight is to follow the advice shared by all those otherwise conflicting diet plans: work to eliminate or at least reduce ultra-processed foods in your diet in favor of a balanced variety of unprocessed, nutrient-packed foods.
Reference:
[1] Ultra-processed diets cause excess calorie intake and weight gain: An inpatient randomized controlled trial of ad libitum food intake. Hall KD et al. Cell Metab. 2019 May 16.
Links:
Obesity (National Institute of Diabetes and Digestive and Kidney Diseases/NIH)
Healthy Eating Plan (National Heart, Lung, and Blood Institute/NIH)
Body Weight Planner (NIDDK/NIH)
Kevin D. Hall (NIDDK/NIH)
Metabolic Clinical Research Unit (NIDDK/NIH)
NIH Support: National Institute of Diabetes and Digestive and Kidney Diseases
Posted In: News
Tags: calories, clinical study, diet, fat, food, Metabolic Clinical Research Unit, metabolism, NIH Clinical Center, NOVA, NOVA Diet Classification System, processed foods, sugar, ultra-processed food, weight, weight gain, whole foods
Study Finds No Benefit for Dietary Supplements
Posted on April 16th, 2019 by Dr. Francis Collins

Credit: iStock/Artfully79
More than half of U.S. adults take dietary supplements [1]. I don’t, but some of my family members do. But does popping all of these vitamins, minerals, and other substances really lead to a longer, healthier life? A new nationwide study suggests it doesn’t.
Based on an analysis of survey data gathered from more than 27,000 people over a six-year period, the NIH-funded study found that individuals who reported taking dietary supplements had about the same risk of dying as those who got their nutrients through food. What’s more, the mortality benefits associated with adequate intake of vitamin A, vitamin K, magnesium, zinc, and copper were limited to food consumption.
The study, published in the Annals of Internal Medicine, also uncovered some evidence suggesting that certain supplements might even be harmful to health when taken in excess [2]. For instance, people who took more than 1,000 milligrams of supplemental calcium per day were more likely to die of cancer than those who didn’t.
The researchers, led by Fang Fang Zhang, Tufts University, Boston, were intrigued that so many people take dietary supplements, despite questions about their health benefits. While the overall evidence had suggested no benefits or harms, results of a limited number of studies had suggested that high doses of certain supplements could be harmful in some cases.
To take a broader look, Zhang’s team took advantage of survey data from tens of thousands of U.S. adults, age 20 or older, who had participated in six annual cycles of the National Health and Nutrition Examination Survey (NHANES) between 1999-2000 and 2009-2010. NHANES participants were asked whether they’d used any dietary supplements in the previous 30 days. Those who answered yes were then asked to provide further details on the specific product(s) and how long and often they’d taken them.
Just over half of participants reported use of dietary supplements in the previous 30 days. Nearly 40 percent reported use of multivitamins containing three or more vitamins.
Nutrient intake from foods was also assessed. Each year, the study’s participants were asked to recall what they’d eaten over the last 24 hours. The researchers then used that information to calculate participants’ nutrient intake from food. Those calculations indicated that more than half of the study’s participants had inadequate intake of vitamins D, E, and K, as well as choline and potassium.
Over the course of the study, more than 3,600 of the study’s participants died. Those deaths included 945 attributed to cardiovascular disease and 805 attributed to cancer. The next step was to look for any association between the nutrient intake and the mortality data.
The researchers found the use of dietary supplements had no influence on mortality. People with adequate intake of vitamin A, vitamin K, magnesium, zinc, and copper were less likely to die. However, that relationship only held for nutrient intake from food consumption.
People who reported taking more than 1,000 milligrams of calcium per day were more likely to die of cancer. There was also evidence that people who took supplemental vitamin D at a dose exceeding 10 micrograms (400 IU) per day without a vitamin D deficiency were more likely to die from cancer.
It’s worth noting that the researchers did initially see an association between the use of dietary supplements and a lower risk of death due to all causes. However, those associations vanished when they accounted for other potentially confounding factors.
For example, study participants who reported taking dietary supplements generally had a higher level of education and income. They also tended to enjoy a healthier lifestyle. They ate more nutritious food, were less likely to smoke or drink alcohol, and exercised more. So, it appears that people who take dietary supplements are likely to live a longer and healthier life for reasons that are unrelated to their supplement use.
While the study has some limitations, including the difficulty in distinguishing association from causation, and a reliance on self-reported data, its findings suggest that the regular use of dietary supplements should not be recommended for the general U.S. population. Of course, this doesn’t rule out the possibility that certain subgroups of people, including perhaps those following certain special diets or with known nutritional deficiencies, may benefit.
These findings serve up a reminder that dietary supplements are no substitute for other evidence-based approaches to health maintenance and eating nutritious food. Right now, the best way to live a long and healthy life is to follow the good advice offered by the rigorous and highly objective reviews provided by the U.S. Preventive Services Task Force [3]. Those tend to align with what I hope your parents offered: eat a balanced diet, including plenty of fruits, veggies, and healthy sources of calcium and protein. Don’t smoke. Use alcohol in moderation. Avoid recreational drugs. Get plenty of exercise.
References:
[1] Trends in Dietary Supplement Use Among US Adults From 1999-2012. Kantor ED, Rehm CD, Du M, White E, Giovannucci EL. JAMA. 2016 Oct 11;316(14):1464-1474.
[2] Association among dietary supplement use, nutrient intake, and mortality among U.S. adults. Chen F, Du M, Blumberg JB, Ho Chui KK, Ruan M, Rogers G, Shan Z, Zeng L, Zhang. Ann Intern Med. 2019 Apr 9. [Epub ahead of print].
[3] Vitamin Supplementation to Prevent Cancer and CVD: Preventive Medication. U.S. Preventive Services Task Force, February 2014.
Links:
Office of Dietary Supplements (NIH)
Healthy Eating Plan (National Heart, Lung, and Blood Institute/NIH)
National Health and Nutrition Examination Survey (Centers for Disease Control and Prevention, Atlanta)
U.S. Preventive Services Task Force (Rockville, MD)
Fang Fang Zhang (Tufts University, Boston)
NIH Support: National Institute on Minority Health and Health Disparities
Posted In: News
Tags: calcium, cancer, cardiovascular disease, copper, diet, dietary supplements, food, magnesium, minerals, multivitamins, NHANES, nutrition, potassium, supplementation, supplements, U. S. Preventive Services Task Force, vitamin A, vitamin D, vitamin E, vitamin K, vitamins, zinc