liquid biopsy – NIH Director's Blog (original) (raw)
New Approach to ‘Liquid Biopsy’ Relies on Repetitive RNA in the Bloodstream
Posted on September 12th, 2023 by Lawrence Tabak, D.D.S., Ph.D.
Researchers have identified segments of noncoding RNA circulating in the blood that are early signs of cancer. Credit: Modified from Adobe Stock/ Andrey Popov; Donny Bliss, NIH
It’s always best to diagnose cancer at an early stage when treatment is most likely to succeed. Unfortunately, far too many cancers are still detected only after cancer cells have escaped from a primary tumor and spread to distant parts of the body. This explains why there’s been so much effort in recent years to develop liquid biopsies, which are tests that can pick up on circulating cancer cells or molecular signs of cancer in blood or other bodily fluids and reliably trace them back to the organ in which a potentially life-threatening tumor is growing.
Earlier methods to develop liquid biopsies for detecting cancers often have relied on the presence of cancer-related proteins and/or DNA in the bloodstream. Now, an NIH-supported research team has encouraging evidence to suggest that this general approach to detecting cancers—including aggressive pancreatic cancers—may work even better by taking advantage of signals from a lesser-known form of genetic material called noncoding RNA.
The findings reported in Nature Biomedical Engineering suggest that the new liquid biopsy approach may aid in the diagnosis of many forms of cancer [1]. The studies show that the sensitivity of the tests varies—a highly sensitive test is one that rarely misses cases of disease. However, they already have evidence that millions of circulating RNA molecules may hold promise for detecting cancers of the liver, esophagus, colon, stomach, and lung.
How does it work? The human genome contains about 3 billion paired DNA letters. Most of those letters are transcribed, or copied, into single-stranded RNA molecules. While RNA is best known for encoding proteins that do the work of the cell, most RNA never gets translated into proteins at all. This noncoding RNA includes repetitive RNA that can be transcribed from millions of repeat elements—patterns of the same few DNA letters occurring multiple times in the genome.
Common approaches to studying RNA don’t analyze repetitive RNA, so its usefulness as a diagnostic tool has been unclear—until recently. Last year, the lab of Daniel Kim at the University of California, Santa Cruz reported [2] that a key genetic mutation that occurs early on in some cancers causes repetitive RNA molecules to be secreted in large quantities from cancer cells, even at the earliest stages of cancer. Non-cancerous cells, by comparison, release much less repetitive RNA.
The findings suggested that liquid biopsy tests that look for this repetitive, noncoding RNA might offer a powerful new way to detect cancers sooner, according to the authors. But first they needed a method capable of measuring it. Due to its oftentimes uncertain functions, the researchers have referred to repetitive, noncoding RNA as “dark matter.”
Using a liquid biopsy platform they developed called COMPLETE-seq, Kim’s team trained computers to detect cancers by looking for patterns in RNA data. The platform enables sequencing and analysis of all protein coding and noncoding RNAs—including any RNA from more than 5 million repeat elements—present in a blood sample. They found that their classifiers worked better when repetitive RNAs were included. The findings lend support to the idea that repetitive, noncoding RNA in the bloodstream is a rich source of information for detecting cancers, which has previously been overlooked.
In a study comparing blood samples from healthy people to those with pancreatic cancer, the COMPLETE-seq technology showed that nearly all people in the study with pancreatic cancer had more repetitive, noncoding RNA in their blood samples compared to healthy people, according to the researchers. They used the COMPLETE-seq test on blood samples from people with other types of cancer as well. For example, their test accurately detected 91% of colorectal cancer samples and 93% of lung cancer samples.
They now plan to look at many more cancer types with samples from additional patients representing a broad range of cancer stages. The goal is to develop a single RNA liquid biopsy test that could detect multiple forms of cancer with a high degree of accuracy and specificity. They note that such a test might also be used to guide treatment decisions and more readily detect a cancer’s recurrence. The hope is that one day a comprehensive liquid biopsy test including coding and noncoding RNA will catch many more cancers sooner, when treatment can be most successful.
References:
[1] RE Reggiardo et al. Profiling of repetitive RNA sequences in the blood plasma of patients with cancer. Nature Biomedical Engineering DOI: 10.1038/s41551-023-01081-7 (2023).
[2] RE Reggiardo et al. Mutant KRAS regulates transposable element RNA and innate immunity via KRAB zinc-finger genes. Cell Reports DOI: 10.1016/j.celrep.2022.111104 (2022).
Links:
Daniel Kim Lab (UC Santa Cruz)
Cancer Screening Overview (National Cancer Institute/NIH)
Early Detection (National Cancer Institute/NIH)
NIH Support: National Cancer Institute, National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases
Posted In: Health, News, Science, technology
Tags: cancer, colorectal cancer, Daniel Kim, diagnosing cancer, liquid biopsy, lung cancer, noncoding RNA, pancreatic cancer, repetitive rna, RNA
Looking for Answers to Epilepsy in a Blood Test
Posted on April 18th, 2019 by Dr. Francis Collins

Gemma Carvill (second from right) with members of her lab. Courtesy of Gemma Carvill
Millions of people take medications each day for epilepsy, a diverse group of disorders characterized by seizures. But, for about a third of people with epilepsy, current drug treatments don’t work very well. What’s more, the medications are designed to treat symptoms of these disorders, basically by suppressing seizure activity. The medications don’t really change the underlying causes, which are wired deep within the brain.
Gemma Carvill, a researcher at Northwestern University Feinberg School of Medicine, Chicago, wants to help change that in the years ahead. She’s dedicated her research career to discovering the genetic causes of epilepsy in hopes of one day designing treatments that can control or even cure some forms of the disorder [1].
It certainly won’t be easy. A recent paper put the number of known genes associated with epilepsy at close to 1,000 [2]. However, because some disease-causing genetic variants may arise during development, and therefore occur only within the brain, it’s possible that additional genetic causes of epilepsy are still waiting to be discovered within the billions of cells and their trillions of interconnections.
To find these new leads, Carvill won’t have to rely only on biopsies of brain tissue. She’s received a 2018 NIH Director’s New Innovator Award in search of answers hidden within “liquid biopsies”—tiny fragments of DNA that research in other forms of brain injury and neurological disease [3] suggests may spill into the bloodstream and cerebrospinal fluid (CSF) from dying neurons or other brain cells following a seizure.
Carvill and team will start with mouse models of epilepsy to test whether it’s possible to detect DNA fragments from the brain in bodily fluids after a seizure. They’ll also attempt to show DNA fragments carry telltale signatures indicating from which cells and tissues in the brain those molecules originate. The hope is these initial studies will also tell them the best time after a seizure to collect blood samples.
In people, Carvill’s team will collect the DNA fragments and begin searching for genetic alterations to explain the seizures, capitalizing on Carvill’s considerable expertise in the use of next generation DNA sequencing technology for ferreting out disease-causing variants. Importantly, if this innovative work in epilepsy pans out, it also can be applied to any other neurological condition in which DNA spills from dying brain cells, including Alzheimer’s disease and Parkinson’s disease.
References:
[1] Unravelling the genetic architecture of autosomal recessive epilepsy in the genomic era. Calhoun JD, Carvill GL. J Neurogenet. 2018 Sep 24:1-18.
[2] Epilepsy-associated genes. Wang J, Lin ZJ, Liu L, Xu HQ, Shi YW, Yi YH, He N, Liao WP. Seizure. 2017 Jan;44:11-20.
[3] Identification of tissue-specific cell death using methylation patterns of circulating DNA. Lehmann-Werman R, Neiman D, Zemmour H, Moss J, Magenheim J, Vaknin-Dembinsky A, Rubertsson S, Nellgård B, Blennow K, Zetterberg H, Spalding K, Haller MJ, Wasserfall CH, Schatz DA, Greenbaum CJ, Dorrell C, Grompe M, Zick A, Hubert A, Maoz M, Fendrich V, Bartsch DK, Golan T, Ben Sasson SA, Zamir G, Razin A, Cedar H, Shapiro AM, Glaser B, Shemer R, Dor Y. Proc Natl Acad Sci U S A. 2016 Mar 29;113(13):E1826-34.
Links:
Epilepsy Information Page (National Institute of Neurological Disorders and Stroke/NIH)
Gemma Carvill Lab (Northwestern University Feinberg School of Medicine, Chicago)
Carvill Project Information (NIH RePORTER)
NIH Director’s New Innovator Award (Common Fund)
NIH Support: Common Fund; National Institute of Neurological Disorders and Stroke
Posted In: Creative Minds
Tags: 2018 NIH Director’s New Innovator Award, Alzheimer’s disease, biomarkers, blood test, brain, DNA, epilepsy, gene variants, genetics, liquid biopsy, neurology, Parkinson's disease
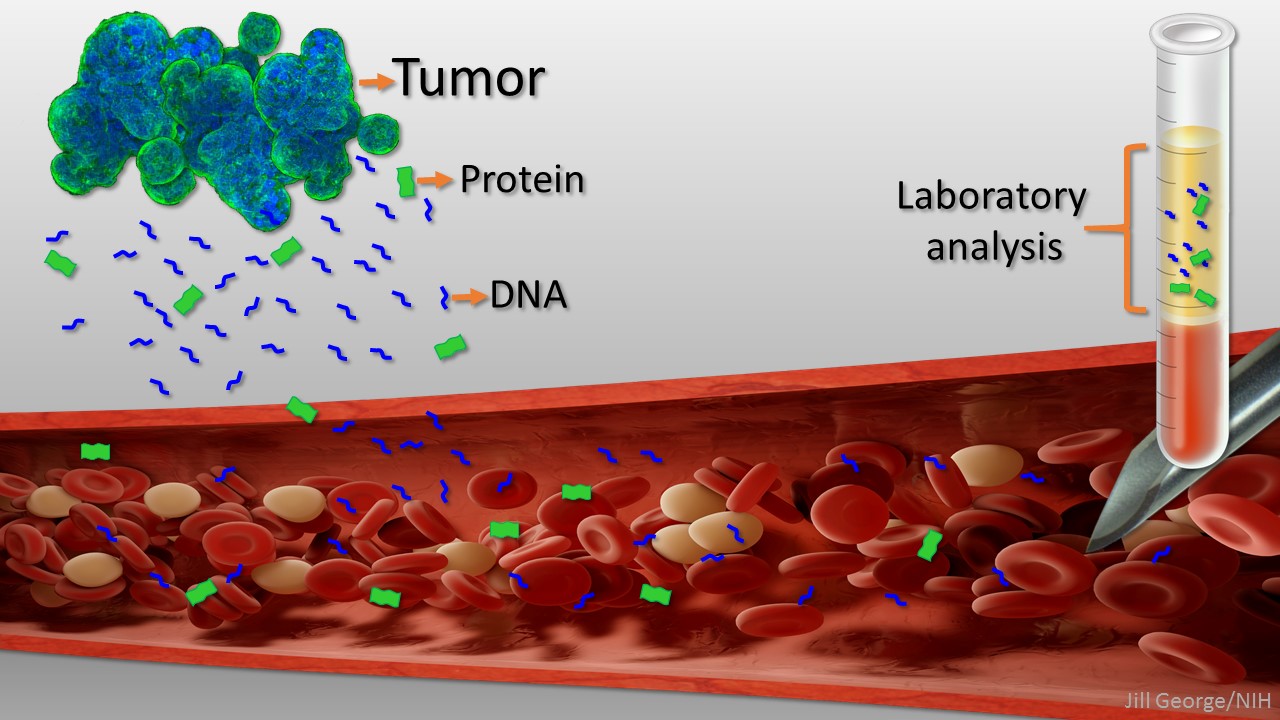
New ‘Liquid Biopsy’ Shows Early Promise in Detecting Cancer
Posted on January 30th, 2018 by Dr. Francis Collins
Caption: Liquid biopsy. Tumor cells shed protein and DNA into bloodstream for laboratory analysis and early cancer detection.
Early detection usually offers the best chance to beat cancer. Unfortunately, many tumors aren’t caught until they’ve grown relatively large and spread to other parts of the body. That’s why researchers have worked so tirelessly to develop new and more effective ways of screening for cancer as early as possible. One innovative approach, called “liquid biopsy,” screens for specific molecules that tumors release into the bloodstream.
Recently, an NIH-funded research team reported some encouraging results using a “universal” liquid biopsy called CancerSEEK [1]. By analyzing samples of a person’s blood for eight proteins and segments of 16 genes, CancerSEEK was able to detect most cases of eight different kinds of cancer, including some highly lethal forms—such as pancreatic, ovarian, and liver—that currently lack screening tests.
In a study of 1,005 people known to have one of eight early-stage tumor types, CancerSEEK detected the cancer in blood about 70 percent of the time, which is among the best performances to date for a blood test. Importantly, when CancerSEEK was performed on 812 healthy people without cancer, the test rarely delivered a false-positive result. The test can also be run relatively cheaply, at an estimated cost of less than $500.
Posted In: Health, Science, technology
Tags: blood test, breast cancer, cancer, cancer blood test, cancer detection, cancer diagnostics, CancerSEEK, clinical study, colorectal cancer, early detection, esophageal cancer, liquid biopsy, liver cancer, lung cancer, machine learning, ovarian cancer, pancreatic cancer, stomach cancer, universal liquid biopsy
A New Tool in the Toolbox: New Method Traces Free-Floating DNA Back to Its Source
Posted on February 16th, 2016 by Dr. Francis Collins
Caption: DNA (blue) loops around nucleosomes (gray) and is bound by transcription factors (red), proteins that switch genes on and off and act in a tissue-specific manner. When cells die, enzymes (scissors) chop up areas between the nucleosomes and transcription factors, releasing DNA fragments in unique patterns. By gathering the released DNA fragments in blood, researchers can tell which types of cells produced them.
Credit: Shendure Lab/University of Washington
When cells die, scissor-like enzymes snip their DNA into tiny fragments that leak into the bloodstream and other bodily fluids. Researchers have been busy in recent years working on ways to collect these free-floating bits of DNA and explore their potential use in clinical care.
These approaches, sometimes referred to as “liquid biopsies,” hinge on the ability to distinguish specific DNA fragments from the body’s normal background of “cell-free” DNA, most of which comes from dying white blood cells. Seeking other sources for cell-free DNA in particular situations is beginning to bear fruit, however. Current applications include: 1) a test in maternal blood to look for DNA from the fetus (actually from the fetal component of the placenta), which provides a means of detecting a possible genetic abnormality; 2) a test in a cancer patient’s blood to look for cancer-specific mutations, as a way of assessing response to treatment or early signs of relapse; and 3) a test in an organ transplant recipient, where increasing abundance of DNA fragments from the donor can be an early sign of rejection.
But recent proposals have been floated about looking for cell-free DNA in healthy individuals, as an early sign of some health problems. Suppose something was found—how could you know the source? Now a team of NIH-funded researchers has devised a new method that uses distinctive features of DNA packaging to provide an additional layer of information about the origins of free-floating DNA, vastly expanding the potential uses for such tests [1].