neurons – NIH Director's Blog (original) (raw)
Energy-Producing Enzyme Fuels the Brain with Promise for Treating Parkinson’s Disease
Posted on September 12th, 2024 by Dr. Monica M. Bertagnolli

Credit: Donny Bliss/NIH
In Parkinson’s disease, neurons in parts of the brain gradually weaken and die, leading people to experience worsening problems with movement and other symptoms. While the causes of this disease aren’t fully known, studies have suggested the Parkinson’s brain lacks fuel to power dopamine-producing neurons that are essential for movement. When too many of those neurons are lost, Parkinson’s disease symptoms appear. But what if there was a way to boost energy levels in the brain and stop the neurodegenerative process in its tracks?
While the findings are preliminary, an NIH-supported study reported in Science Advances takes an encouraging step toward this goal. The key element, according to the new work, is an energy-producing enzyme known as phosphoglycerate kinase (PGK1). In fact, these latest preclinical findings in models of the disease suggest that boosting this enzyme in the brain even slightly may be enough to restore energy and afford some protection against Parkinson’s disease.
The team, led by Timothy Ryan and Alexandros Kokotos, Weill Cornell Medicine, New York City, was inspired by recent discoveries suggesting an unexpectedly important role for PGK1 in protecting the normal function of neurons. They knew PGK1 plays an essential role in the pathway through which cells use glucose to generate and store energy in the form of adenosine 5′-triphosphate (ATP) molecules. The surprise came when studies showed the drug terazosin, which is used to treat high blood pressure and enlarged prostate, has an unexpected side effect: it enhances PGK1 activity, although perhaps weakly.
Could the boost in PGK1 activity be enough to fuel and protect dopamine-producing neurons? Studies in Parkinson’s models including mice, rats, flies, and human cells treated with terazosin suggested that it could. A retrospective study in people taking terazosin for an enlarged prostate also showed that those taking the drug were less likely to develop Parkinson’s.
As promising as that sounded, it was hard to imagine that such a seemingly small increase in PGK1 activity was enough to explain the findings. To investigate further in the new study, the researchers ran more sensitive studies to see just how much of a difference PGK1 can make when it comes to energy production in neurons. Their new studies show that even a small increase in PGK1 activity keeps neurons firing, even when glucose levels are low.
The researchers report that the increases in PGK1 they saw were enough to protect neurons carrying mutations in genes with known links to familial forms of Parkinson’s disease. They found that effects of a PGK1 boost also depend on another protein, called DJ-1, which has also been implicated in Parkinson’s disease. When the researchers experimentally increased PGK1 levels in mouse models of the disease, it strongly protected their dopamine neurons.
For the approximately one million Americans with Parkinson’s disease today, current treatments help to relieve symptoms but don’t stop the disease from progressing. These new findings raise the possibility that terazosin or drugs that enhance PGK1 activity even more may fuel the brain, helping to protect essential dopamine-producing neurons to treat or even prevent Parkinson’s disease, as well as other neurodegenerative conditions where PGK1 may play a role.
Reference:
Kokotos AC, et al. Phosphoglycerate kinase is a central leverage point in Parkinson’s Disease driven neuronal metabolic deficits. Science Advances. DOI: 10.1126/sciadv.adn6016 (2024).
NIH Support: National Institute of Neurological Disorders and Stroke, National Institute of General Medical Sciences
New Clues for Healing Spinal Cord Injuries Found in Single-Cell Studies in Zebrafish
Posted on September 5th, 2024 by Dr. Monica M. Bertagnolli

Credit: Bigemrg/Adobe Stock, Mokalled Lab/WashU
Each year in the U.S. there are about 18,000 new spinal cord injuries, which damage the bundle of nerves and nerve fibers that send signals from the brain to other parts of the body and can affect feeling, movement, strength, and function below the injured site. A severe spinal cord injury can lead to immediate and permanent paralysis, as our spinal cords lack the capacity to regenerate the damaged tissues and heal.
So far, even the most groundbreaking regenerative therapies have yielded only modest improvements after spinal cord injuries. Now, an NIH-supported study reported in Nature Communications offers some new clues that may one day lead to ways to encourage healing of spinal cord injuries in people. The researchers uncovered these clues through detailed single-cell analysis in what might seem an unlikely place: the zebrafish spinal cord.
Why zebrafish? Unlike mammals, zebrafish have a natural ability to spontaneously heal and recover after spinal cord injuries, even when the injuries are severe. Remarkably, after a complete spinal cord injury, a zebrafish can reverse the paralysis and start swimming again within six to eight weeks. Earlier studies in zebrafish after spinal cord injury found that this regenerative response involves many types of cells, including immune cells, progenitor cells, neurons, and supportive glial cells, all of which work together to successfully repair damage.
In the new work, a team at Washington University School of Medicine in St. Louis led by Mayssa Mokalled took advantage of tools that make it possible to study the gene activity underlying this spinal healing response by sequencing RNA transcripts within individual neurons. The goal was to learn in much more detail about the zebrafish’s response to spinal cord injury in major neural cell types. The researchers also wanted to compare what they found in zebrafish to other single-cell studies in mice, which lack this regenerative capacity.
To do it, the researchers sequenced RNA from spinal cells at the time of injury and then again one, three, and six weeks later. Their analyses show that zebrafish neurons demonstrate a dramatic response indicated by changes in gene activity, followed by the growth of new neurons to restore essential connections. Importantly, the researchers also found that some of the existing injured neurons get reprogrammed, showing a regenerative pattern of gene activity after a week that encourages their survival and increased flexibility to allow healing. When those injury-responsive neurons, which the researchers call iNeurons, were disabled, the zebrafish didn’t regain the ability to swim, even when regenerative stem cells remained active.
In people or mice, by comparison, a spinal cord injury sets off a toxic chain of events that kills the existing neurons and prevents repair. Interestingly, the new study suggests that, in the zebrafish, the regeneration is not only due to stem cells sprouting new neurons as long suspected, and may be more related to the processes that protect and save the injured neurons and lend them more flexibility to heal. It’s possible that this kind of protective and regenerative capacity is present in mammalian neurons, even if the regenerative process doesn’t automatically switch on in the way seen in zebrafish, according to the researchers. Indeed, many of the genes that play important roles in the zebrafish healing process are also present in the human genome.
In future work, the researchers plan to conduct similar studies in the many other cell types known to play some role in spinal cord healing in zebrafish, including supportive glia and immune cells. They’re also continuing to explore how the activities they see in the zebrafish spinal cord compare to what happens in mice and humans. With much more study, these kinds of findings in zebrafish may lead to promising new ideas and even treatments that encourage neural protection, flexibility, and recovery in the human nervous system after spinal cord injuries.
Reference:
Muraleedharan Saraswathy V, et al. Single-cell analysis of innate spinal cord regeneration identifies intersecting modes of neuronal repair. Nature Communications. DOI: 10.1038/s41467-024-50628-y (2024).
NIH Support: National Institute of Neurological Disorders and Stroke
Machine Learning Study Offers Clues to Why Some People Have Rheumatoid Arthritis Pain Without Inflammation
Posted on May 2nd, 2024 by Dr. Monica M. Bertagnolli

Credit: Yakobchuk Olena/Adobe Stock
About 1.5 million adults in the U.S. are living with rheumatoid arthritis (RA), an autoimmune disease in which the immune system attacks joint tissue, causing inflammation, swelling, and pain. Treatments often do a good job fighting inflammation to slow or even stop joint damage and ease pain. But this doesn’t work for everyone. Many people with RA don’t find pain relief, even with the strongest anti-inflammatory, disease-modifying therapies now available.
Why is that? A new study supported in part by NIH and reported in Science Translational Medicine has an intriguing answer.1 The findings suggest that in some people with RA, the joint lining may direct the growth of pain-sensing neurons to cause pain in the absence of inflammation. This discovery, made possible with the help of machine learning, suggests potential new ways to treat this painful disease.
The findings come from a team led by Fei Wang, Weill Cornell Medicine, New York City, and Dana E. Orange, Rockefeller University, New York City. They were inspired by recent studies showing that RA pain and inflammation don’t always go together. In fact, people with RA who have limited inflammation in some cases report just as much pain as those who have extreme inflammation. As a result, they also tend to get less benefit from anti-inflammatory drugs.
To find out why, the researchers studied the soft tissue, or synovium, lining the spaces of the joints from people with this less common form of RA. They were in search of underlying differences in gene activity to explain the pain without inflammation. They knew it wouldn’t be easy, given the variation in the way people experience and report pain and the limited availability of surgically removed tissue samples. To overcome those roadblocks, they developed a machine learning approach that could pinpoint pain-associated patterns of gene activity in the complex data that would otherwise be too difficult to discern.
Their RNA sequencing analysis turned up 815 genes that were expressed at unusually high levels in the joint tissue of 22 people who had RA pain with low inflammation. They also confirmed this same pattern of gene activity in a second group of patients with early untreated RA and little inflammation.
The researchers went on to find that this pattern was clearest in fibroblast cells (a major cell type of the synovium) which provide the structural framework of the joint space, but become a key driver of inflammation and joint damage in RA. Those fibroblasts also expressed a gene that encodes a protein called netrin-4, which is related to a family of proteins that play a role in the growth of neurons. It led them to wonder whether the joint tissue might be producing substances that could alter pain-sensing nerves to cause pain.
To learn more, they turned to studies in mice. They found that fluid collected from joint fibroblast cell cultures and netrin-4 made mouse neurons sprout new branches carrying pain receptors in the lab. The findings suggested that the RA joint lining was indeed producing substances that could lead to the growth of pain-sensing neurons.
To see if this might play a role in people with RA and little inflammation, they looked closely at the joints. Those images revealed an abundance of blood vessels that could nurture tissue growth. Those vessels were also surrounded by pain-sensing nerve fibers extending toward the joint lining in places where there was an abnormal amount of tissue growth.
The researchers think this process explains why painful, arthritic joints sometimes feel squishy and swollen even when they aren’t inflamed. In future studies, they want to learn more about which sensory neurons are specifically affected, noting that there are about a dozen different types. While much more study is needed, their goal is to find promising new ways to treat RA by targeting this underlying process, giving more people with RA much needed pain relief.
Reference:
[1] Bai Z, et al. Synovial fibroblast gene expression is associated with sensory nerve growth and pain in rheumatoid arthritis. Science Translational Medicine. DOI: 10.1126/scitranslmed.adk3506 (2024).
NIH Support: National Institute of Arthritis and Musculoskeletal and Skin Diseases
Posted In: Health, News, Science
Tags: autoimmune disease, basic research, gene expression, genetics, inflammation, machine learning, neurons, pain, rheumatoid arthritis, treatment
Study Suggests Treatments that Unleash Immune Cells in the Brain Could Help Combat Alzheimer’s
Posted on April 25th, 2024 by Dr. Monica M. Bertagnolli
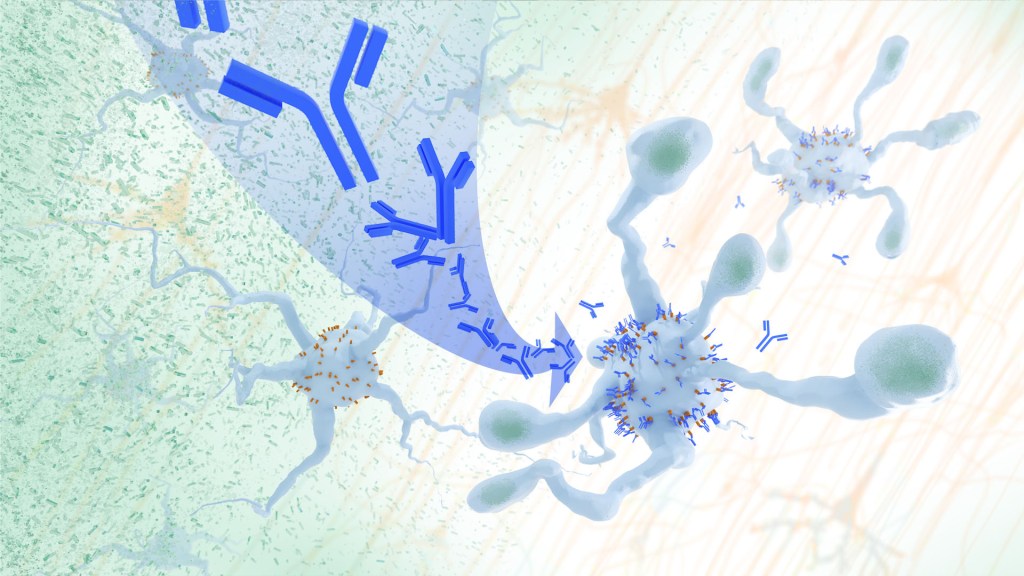
In a study, an antibody treatment blocked interaction between APOE proteins and LILRB4 receptors in the brain, enabling microglia immune cells to clear amyloid plaques, a feature of Alzheimer’s. Credit: Donny Bliss/NIH
In Alzheimer’s disease, a buildup of sticky amyloid proteins in the brain clump together to form plaques, causing damage that gradually leads to worsening dementia symptoms. A promising way to change the course of this disease is with treatments that clear away damaging amyloid plaques or stop them from forming in the first place. In fact, the Food and Drug Administration recently approved the first drug for early Alzheimer’s that moderately slows cognitive decline by reducing amyloid plaques.1 Still, more progress is needed to combat this devastating disease that as many as 6.7 million Americans were living with in 2023.
Recent findings from a study in mice, supported in part by NIH and reported in Science Translational Medicine, offer another potential way to clear amyloid plaques in the brain. The key component of this strategy is using the brain’s built-in cleanup crew for amyloid plaques and other waste products: immune cells known as microglia that naturally help to limit the progression of Alzheimer’s. The findings suggest it may be possible to develop immunotherapies—treatments that use the body’s immune system to fight disease—to activate microglia in the brains of people with Alzheimer’s and clear amyloid plaques more effectively.2
In their report, the research team—including Marco Colonna, Washington University School of Medicine in St. Louis, and Jinchao Hou, now at Children’s Hospital of Zhejiang University School of Medicine in Zhejiang Province, China—wrote that microglia in the brain surround plaques to create a barrier that controls their spread. Microglia can also destroy amyloid plaques directly. But how microglia work in the brain depends on a fine-tuned balance of signals that activate or inhibit them. In people with Alzheimer’s, microglia don’t do their job well enough.
The researchers suspected this might have something to do with a protein called apolipoprotein E (APOE). This protein normally helps carry cholesterol and other fats in the bloodstream. But the gene encoding the protein is known for its role in influencing a person’s risk for developing Alzheimer’s, and in the Alzheimer’s brain, the protein is a key component of amyloid plaques. The protein can also inactivate microglia by binding to a receptor called LILRB4 found on the immune cells’ surfaces.
Earlier studies in mouse models of Alzheimer’s showed that the LILRB4 receptor is expressed at high levels in microglia when amyloid plaques build up. This suggested that treatments targeting this receptor on microglia might hold promise for treating Alzheimer’s. In the new study, the research team looked for evidence that an increase in LILRB4 receptors on microglia plays an important role in the brains of people with Alzheimer’s.
To do this, the researchers first studied brain tissue samples from people who died with this disease and discovered unusually high amounts of the LILRB4 receptor on the surfaces of microglia, similar to what had been seen in the mouse models. This could help explain why microglia struggle to control amyloid plaques in the Alzheimer’s brain.
Next, the researchers conducted studies of mouse brains with accumulating amyloid plaques that express the LILRB4 receptor to see if an antibody targeting the receptor could lower amyloid levels by boosting activity of immune microglia. Their findings suggest that the antibody treatment blocked the interaction between APOE proteins and LILRB4 receptors and enabled microglia to clear amyloid plaques. Intriguingly, the team’s additional studies found that this clearing process also changed the animals’ behavior, making them less likely to take risks. That’s important because people with Alzheimer’s may engage in risky behaviors as they lack memories of earlier experiences that they could use to make decisions.
There’s plenty more to learn. For instance, the researchers don’t know yet whether this approach will affect the tau protein, which forms damaging tangles inside neurons in the Alzheimer’s brain. They also want to investigate whether this strategy of clearing amyloid plaques might come with other health risks.
But overall, these findings add to evidence that immunotherapies of this kind could be a promising way to treat Alzheimer’s. This strategy may also have implications for treating other neurodegenerative conditions characterized by toxic debris in the brain, such as Parkinson’s disease, amyotrophic lateral sclerosis (ALS), and Huntington’s disease. The hope is that this kind of research will ultimately lead to more effective treatments for Alzheimer’s and other conditions affecting the brain.
References:
[1] FDA Converts Novel Alzheimer’s Disease Treatment to Traditional Approval. U.S. Food and Drug Administration (2023).
[2] Hou J, et al. Antibody-mediated targeting of human microglial leukocyte Ig-like receptor B4 attenuates amyloid pathology in a mouse model. Science Translational Medicine. DOI: 10.1126/scitranslmed.adj9052 (2024).
NIH Support: National Institute of General Medical Sciences, National Institute on Aging
Study Suggests During Sleep, Neural Process Helps Clear the Brain of Damaging Waste
Posted on March 14th, 2024 by Dr. Monica M. Bertagnolli
Artist’s rendering of neural activity clearing waste products through tight spaces of the brain. Credit: Donny Bliss/NIH
We’ve long known that sleep is a restorative process necessary for good health. Research has also shown that the accumulation of waste products in the brain is a leading cause of numerous neurological disorders, including Alzheimer’s and Parkinson’s diseases. What hasn’t been clear is how the healthy brain “self-cleans,” or flushes out that detrimental waste.
But a new study by a research team supported in part by NIH suggests that a neural process that happens while we sleep helps cleanse the brain, leading us to wake up feeling rested and restored. Better understanding this process could one day lead to methods that help people function well on less sleep. It could also help researchers find potential ways to delay or prevent neurological diseases related to accumulated waste products in the brain.
The findings, reported in Nature, show that, during sleep, neural networks in the brain act like an array of miniature pumps, producing large and rhythmic waves through synchronous bursts of activity that propel fluids through brain tissue. Much like the process of washing dishes, where you use a rhythmic motion of varying speeds and intensity to clear off debris, this process that takes place during sleep clears accumulated metabolic waste products out.
The research team, led by Jonathan Kipnis and Li-Feng Jiang-Xie at Washington University School of Medicine in St. Louis, wanted to better understand how the brain manages its waste. This is not an easy task, given that the human brain’s billions of neurons inevitably produce plenty of junk during cognitive processes that allow us to think, feel, move, and solve problems. Those waste products also build in a complex environment, including a packed maze of interconnected neurons, blood vessels, and interstitial spaces, surrounded by a protective blood-brain barrier that limits movement of substances in or out.
So, how does the brain move fluid through those tight spaces with the force required to get waste out? Earlier research suggested that neural activity during sleep might play an important role in those waste-clearing dynamics. But previous studies hadn’t pinned down the way this works.
To learn more in the new study, the researchers recorded brain activity in mice. They also used an ultrathin silicon probe to measure fluid dynamics in the brain’s interstitial spaces. In awake mice, they saw irregular neural activity and only minor fluctuations in the interstitial spaces. But when the animals were resting under anesthesia, the researchers saw a big change. Brain recordings showed strongly enhanced neural activity, with two distinct but tightly coupled rhythms. The research team realized that the structured wave patterns could generate strong energy that could move small molecules and peptides, or waste products, through the tight spaces within brain tissue.
To make sure that the fluid dynamics were really driven by neurons, the researchers used tools that allowed them to turn neural activity off in some areas. Those experiments showed that, when neurons stopped firing, the waves also stopped. They went on to show similar dynamics during natural sleep in the animals and confirmed that disrupting these neuron-driven fluid dynamics impaired the brain’s ability to clear out waste.
These findings highlight the importance of this cleansing process during sleep for brain health. The researchers now want to better understand how specific patterns and variations in those brain waves lead to changes in fluid movement and waste clearance. This could help researchers eventually find ways to speed up the removal of damaging waste, potentially preventing or delaying certain neurological diseases and allowing people to need less sleep.
Reference:
[1] Jiang-Xie LF, et al. Neuronal dynamics direct cerebrospinal fluid perfusion and brain clearance. Nature. DOI: 10.1038/s41586-024-07108-6 (2024).
NIH Support: National Center for Complementary and Integrative Health
How Neurons Make Connections
Posted on August 8th, 2023 by Lawrence Tabak, D.D.S., Ph.D.

Credit: Emily Heckman, Doe Lab, University of Oregon, Eugene
For many people, they are tiny pests. These fruit flies that sometimes hover over a bowl of peaches or a bunch of bananas. But for a dedicated community of researchers, fruit flies are an excellent model organism and source of information into how neurons self-organize during the insect’s early development and form a complex, fully functioning nervous system.
That’s the scientific story on display in this beautiful image of a larval fruit fly’s developing nervous system. Its subtext is: fundamental discoveries in the fruit fly, known in textbooks as Drosophila melanogaster, provide basic clues into the development and repair of the human nervous system. That’s because humans and fruit flies, though very distantly related through the millennia, still share many genes involved in their growth and development. In fact, 60 percent of the Drosophila genome is identical to ours.
Once hatched, as shown in this image, a larval fly uses neurons (magenta) to sense its environment. These include neurons that sense the way its body presses against the surrounding terrain, as needed to coordinate the movements of its segmented body parts and crawl in all directions.
This same set of neurons will generate painful sensations, such as the attack of a parasitic wasp. Paintbrush-like neurons in the fly’s developing head (magenta, left side) allow the insect to taste the sweetness of a peach or banana.
There is a second subtype of neurons, known as proprioceptors (green). These neurons will give the young fly its “sixth sense” understanding about where its body is positioned in space. The complete collection of developing neurons shown here are responsible for all the fly’s primary sensations. They also send these messages on to the insect’s central nervous system, which contains thousands of other neurons that are hidden from view.
Emily Heckman, now a postdoctoral researcher at the Michigan Neuroscience Institute, University of Michigan, Ann Arbor, captured this image during her graduate work in the lab of Chris Doe, University of Oregon, Eugene. For her keen eye, she received a trainee/early-career BioArt Award from the Federation of American Societies for Experimental Biology (FASEB), which each year celebrates the art of science.
The image is one of many from a much larger effort in the Doe lab that explores the way neurons that will partner find each other and link up to drive development. Heckman and Doe also wanted to know how neurons in the developing brain interconnect into integrated neural networks, or circuits, and respond when something goes wrong. To find out, they disrupted sensory neurons or forced them to take alternate paths and watched to see what would happen.
As published in the journal eLife [1], the system has an innate plasticity. Their findings show that developing sensory neurons instruct one another on how to meet up just right. If one suddenly takes an alternate route, its partner can still reach out and make the connection. Once an electrically active neural connection, or synapse, is made, the neural signals themselves slow or stop further growth. This kind of adaptation and crosstalk between neurons takes place only during a particular critical window during development.
Heckman says part of what she enjoys about the image is how it highlights that many sensory neurons develop simultaneously and in a coordinated process. What’s also great about visualizing these events in the fly embryo is that she and other researchers can track many individual neurons from the time they’re budding stem cells to when they become a fully functional and interconnected neural circuit.
So, the next time you see fruit flies hovering in the kitchen, just remember there’s more to their swarm than you think. Our lessons learned studying them will help point researchers toward new ways in people to restore or rebuild neural connections after devastating disruptions from injury or disease.
Reference:
Presynaptic contact and activity opposingly regulate postsynaptic dendrite outgrowth. Heckman EL, Doe CQ. Elife. 2022 Nov 30;11:e82093.
Links:
Research Organisms (National Institute of General Medical Sciences/NIH)
Doe Lab (University of Oregon, Eugene)
Emily Heckman (University of Michigan, Ann Arbor)
BioArt Awards (Federation of American Societies for Experimental Biology, Rockville, MD)
NIH Support: Eunice Kennedy Shriver National Institute of Child Health and Human Development
Posted In: Snapshots of Life
Tags: 2022 BioArt Awards, development, Drosophila melanogaster, fruit fly, insects, model organisms, nervous system, neural circuits, neurons, neuroplasticity, neuroscience, proprioception, sensory neurons
Celebrating the Power of Connection This Holiday Season
Posted on December 20th, 2022 by Lawrence Tabak, D.D.S., Ph.D.
Happy holidays to one and all! This short science video brings to mind all those twinkling lights now brightening the night, as we mark the beginning of winter and shortest day of the year. This video also helps to remind us about the power of connection this holiday season.
It shows a motor neuron in a mouse’s primary motor cortex. In this portion of the brain, which controls voluntary movement, heavily branched neural projections interconnect, sending and receiving signals to and from distant parts of the body. A single motor neuron can receive thousands of inputs at a time from other branching sensory cells, depicted in the video as an array of blinking lights. It’s only through these connections—through open communication and cooperation—that voluntary movements are possible to navigate and enjoy our world in all its wonder. One neuron, like one person, can’t do it all alone.
This power of connection, captured in this award-winning video from the 2022 Show Us Your Brains Photo and Video contest, comes from Forrest Collman, Allen Institute for Brain Science, Seattle. The contest is part of NIH’s Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative.
In the version above, we’ve taken some liberties with the original video to enhance the twinkling lights from the synaptic connections. But creating the original was quite a task. Collman sifted through reams of data from high-resolution electron microscopy imaging of the motor cortex to masterfully reconstruct this individual motor neuron and its connections.
Those data came from The Machine Intelligence from Cortical Networks (MICrONS) program, supported by the Intelligence Advanced Research Projects Activity (IARPA). It’s part of the Office of the Director of National Intelligence, one of NIH’s governmental collaborators in the BRAIN Initiative.
The MICrONS program aims to better understand the brain’s internal wiring. With this increased knowledge, researchers will develop more sophisticated machine learning algorithms for artificial intelligence applications, which will in turn advance fundamental basic science discoveries and the practice of life-saving medicine. For instance, these applications may help in the future to detect and evaluate a broad range of neural conditions, including those that affect the primary motor cortex.
Pretty cool stuff. So, as you spend this holiday season with friends and family, let this video and its twinkling lights remind you that there’s much more to the season than eating, drinking, and watching football games.
The holidays are very much about the power of connection for people of all faiths, beliefs, and traditions. It’s about taking time out from the everyday to join together to share memories of days gone by as we build new memories and stronger bonds of cooperation for the years to come. With this in mind, happy holidays to one and all.
Links:
“NIH BRAIN Initiative Unveils Detailed Atlas of the Mammalian Primary Motor Cortex,” NIH News Release, October 6, 2021
Forrest Collman (Allen Institute for Brain Science, Seattle)
Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
Show Us Your Brains Photo and Video Contest (BRAIN Initiative)
Posted In: Generic
Tags: brain, brain connectivity, brain imaging, BRAIN Initiative, electron microscopy, IARPA, imaging, motor neurons, movement, neurons, neuroscience, primary motor cortex, Show Us Your Brain, Show Us Your BRAINs!, synapse
The Amazing Brain: Where Thoughts Trigger Body Movement
Posted on August 30th, 2022 by Lawrence Tabak, D.D.S., Ph.D.

Credit: Nicolas Antille, SUNY Downstate Health Sciences University, Brooklyn, NY
You’re looking at a section of a mammalian motor cortex (left), the part of the brain where thoughts trigger our body movements. Part of the section is also shown (right) in higher resolution to help you see the intricate details.
These views are incredibly detailed, and they also can’t be produced on a microscope or any current state-of-the-art imaging device. They were created on a supercomputer. Researchers input vast amounts of data covering the activity of the motor cortex to model this highly detailed and scientifically accurate digital simulation.
The vertical section (left) shows a circuit within a column of motor neurons. The neurons run from the top, where the brain meets the skull, downward to the point that the motor cortex connects with other brain areas.
The various colors represent different layers of the motor cortex, and the bright spots show where motor neurons are firing. Notice the thread-like extensions of the motor neurons, some of which double back to connect cells from one layer with others some distance away. All this back and forth makes it appear as though the surface is unraveling.
This unique imaging was part of this year’s Show Us Your Brain Photo and Video contest, supported by NIH’s Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative. Nicolas Antille, an expert in turning scientific data into accurate and compelling visuals, created the images using a scientific model developed in the lab of Salvador Dura-Bernal, SUNY Downstate Health Sciences University, Brooklyn, NY. In the Dura-Bernal lab, scientists develop software and highly detailed computational models of neural circuits to better understand how they give rise to different brain functions and behavior [1].
Antille’s images make the motor neurons look densely packed, but in life the density would be five times as much. Antille has paused the computer simulation at a resolution that he found scientifically and visually interesting. But the true interconnections among neurons, or circuits, inside a real brain—even a small portion of a real brain—are more complex than the most powerful computers today can fully process.
While Antille is invested in revealing brain circuits as close to reality as possible, he also has the mind of an artist. He works with the subtle interaction of light with these cells to show how many individual neurons form this much larger circuit. Here’s more of his artistry at work. Antille wants to invite us all to ponder—even if only for a few moments—the wondrous beauty of the mammalian brain, including this remarkable place where thoughts trigger movements.
Reference:
[1] NetPyNE, a tool for data-driven multiscale modeling of brain circuits. Dura-Bernal S, Suter BA, Gleeson P, Cantarelli M, Quintana A, Rodriguez F, Kedziora DJ, Chadderdon GL, Kerr CC, Neymotin SA, McDougal RA, Hines M, Shepherd GM, Lytton WW. Elife. 2019 Apr 26;8:e44494.
Links:
Dura-Bernal Lab (State University of New York Downstate, Brooklyn)
Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
Show Us Your BRAINs Photo & Video Contest (BRAIN Initiative)
NIH Support: National Institute of Biomedical Imaging and Bioengineering; National Institute of Neurological Disorders and Stroke; BRAIN Initiative
The Amazing Brain: Capturing Neurons in Action
Posted on August 16th, 2022 by Lawrence Tabak, D.D.S., Ph.D.
Credit: Andreas Tolias, Baylor College of Medicine, Houston
With today’s powerful imaging tools, neuroscientists can monitor the firing and function of many distinct neurons in our brains, even while we move freely about. They also possess another set of tools to capture remarkable, high-resolution images of the brain’s many thousands of individual neurons, tracing the form of each intricate branch of their tree-like structures.
Most brain imaging approaches don’t capture neural form and function at once. Yet that’s precisely what you’re seeing in this knockout of a movie, another winner in the Show Us Your BRAINs! Photo and Video Contest, supported by NIH’s Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative.
This first-of-its kind look into the mammalian brain produced by Andreas Tolias, Baylor College of Medicine, Houston, and colleagues features about 200 neurons in the visual cortex, which receives and processes visual information. First, you see a colorful, tightly packed network of neurons. Then, those neurons, which were colorized by the researchers in vibrant pinks, reds, blues, and greens, pull apart to reveal their finely detailed patterns and shapes. Throughout the video, you can see neural activity, which appears as flashes of white that resemble lightning bolts.
Making this movie was a multi-step process. First, the Tolias group presented laboratory mice with a series of visual cues, using a functional imaging approach called two-photon calcium imaging to record the electrical activity of individual neurons. While this technique allowed the researchers to pinpoint the precise locations and activity of each individual neuron in the visual cortex, they couldn’t zoom in to see their precise structures.
So, the Baylor team sent the mice to colleagues Nuno da Costa and Clay Reid, Allen Institute for Brain Science, Seattle, who had the needed electron microscopes and technical expertise to zoom in on these structures. Their data allowed collaborator Sebastian Seung’s team, Princeton University, Princeton, NJ, to trace individual neurons in the visual cortex along their circuitous paths. Finally, they used sophisticated machine learning algorithms to carefully align the two imaging datasets and produce this amazing movie.
This research was supported by Intelligence Advanced Research Projects Activity (IARPA), part of the Office of the Director of National Intelligence. The IARPA is one of NIH’s governmental collaborators in the BRAIN Initiative.
Tolias and team already are making use of their imaging data to learn more about the precise ways in which individual neurons and groups of neurons in the mouse visual cortex integrate visual inputs to produce a coherent view of the animals’ surroundings. They’ve also collected an even-larger data set, scaling their approach up to tens of thousands of neurons. Those data are now freely available to other neuroscientists to help advance their work. As researchers make use of these and similar data, this union of neural form and function will surely yield new high-resolution discoveries about the mammalian brain.
Links:
Tolias Lab (Baylor College of Medicine, Houston)
Nuno da Costa (Allen Institute for Brain Science, Seattle)
R. Clay Reid (Allen Institute)
H. Sebastian Seung (Princeton University, Princeton, NJ)
Machine Intelligence from Cortical Networks (MICrONS) Explorer
Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
Show Us Your BRAINs Photo & Video Contest (BRAIN Initiative)
NIH Support: BRAIN Initiative; Common Fund
Posted In: Cool Videos
Tags: Allen Institute, Andreas Tolias, Baylor, brain, Clay Reid, COVID-19, electron microscopy, IARPA, imaging, MICrONS, MICrONS Explorer, motor neurons, neurons, neuroscience, neuroscientists, Nuno da Costa, Show Us Your BRAINs!, spinal neurons, structural biology, visual cortex
The Amazing Brain: Seeing Two Memories at Once
Posted on August 2nd, 2022 by Lawrence Tabak, D.D.S., Ph.D.

Credit: Stephanie Grella, Boston University, MA
The NIH’s Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative is revolutionizing our understanding of the human brain. As described in the initiative’s name, the development of innovative imaging technologies will enable researchers to see the brain in new and increasingly dynamic ways. Each year, the initiative celebrates some standout and especially creative examples of such advances in the “Show Us Your BRAINs! Photo & Video Contest. During most of August, I’ll share some of the most eye-catching developments in our blog series, The Amazing Brain.
In this fascinating image, you’re seeing two stored memories, which scientists call engrams, in the hippocampus region of a mouse’s brain. The engrams show the neural intersection of a good memory (green) and a bad memory (pink). You can also see the nuclei of many neurons (blue), including nearby neurons not involved in the memory formation.
This award-winning image was produced by Stephanie Grella in the lab of NIH-supported neuroscientist Steve Ramirez, Boston University, MA. It’s also not the first time that the blog has featured Grella’s technical artistry. Grella, who will soon launch her own lab at Loyola University, Chicago, previously captured what a single memory looks like.
To capture two memories at once, Grella relied on a technology known as optogenetics. This powerful method allows researchers to genetically engineer neurons and selectively activate them in laboratory mice using blue light. In this case, Grella used a harmless virus to label neurons involved in recording a positive experience with a light-sensitive molecule, known as an opsin. Another molecular label was used to make those same cells appear green when activated.
After any new memory is formed, there’s a period of up to about 24 hours during which the memory is malleable. Then, the memory tends to stabilize. But with each retrieval, the memory can be modified as it restabilizes, a process known as memory reconsolidation.
Grella and team decided to try to use memory reconsolidation to their advantage to neutralize an existing fear. To do this, they placed their mice in an environment that had previously startled them. When a mouse was retrieving a fearful memory (pink), the researchers activated with light associated with the positive memory (green), which for these particular mice consisted of positive interactions with other mice. The aim was to override or disrupt the fearful memory.
As shown by the green all throughout the image, the experiment worked. While the mice still showed some traces of the fearful memory (pink), Grella explained that the specific cells that were the focus of her study shifted to the positive memory (green).
What’s perhaps even more telling is that the evidence suggests the mice didn’t just trade one memory for another. Rather, it appears that activating a positive memory actually suppressed or neutralized the animal’s fearful memory. The hope is that this approach might one day inspire methods to help people overcome negative and unwanted memories, such as those that play a role in post-traumatic stress disorder (PTSD) and other mental health issues.
Links:
Stephanie Grella (Boston University, MA)
Ramirez Group (Boston University)
Brain Research through Advancing Innovative Neurotechnologies® (BRAIN) Initiative (NIH)
Show Us Your BRAINs Photo & Video Contest (BRAIN Initiative)
NIH Support: BRAIN Initiative; Common Fund
Posted In: Snapshots of Life
Tags: brain, brain imaging, BRAIN Initiative, Brain Research through Advancing Innovative Neurotechnologies Initiative, engrams, fear, hippocampus, memories, memory, mental health, mental illness, mouse study, neurons, neuroscience, opsin, optogenetics, post-traumatic stress disorder, PTSD, Show Us Your BRAINs!