oncology – NIH Director's Blog (original) (raw)
Taking Inspiration from Art Created by a Patient’s Granddaughter
Posted on September 20th, 2024 by Dr. Monica M. Bertagnolli

NIH Director Dr. Monica Bertagnolli in her office with Suzi Grossman’s artwork. Credit: Chia-Chi Charlie Chang, NIH
I have a specific ritual when moving into a new office, as I did when I became NIH Director in 2023: I hang a very special framed screen print on the wall. This piece of art has followed me through several different offices, representing different positions I have held as a cancer surgeon, researcher, and educator. It’s always the first thing I want to see as I settle into a new workplace. It serves as an inspiration for my work, for what so many of us involved in health care strive to do. I’d like to take this opportunity to tell you the story behind it.
Many years ago, in my role as an oncologist and surgeon, I had a very memorable patient, an older woman who had advanced cancer. From the time that I met her, she had incurable disease, so our goal was to make every effort, through multiple surgeries and chemotherapy, to help her continue living a life that she enjoyed for as long as possible. She was very courageous and spirited in her attitude toward her disease; although realistic about her situation, she was determined not to let anything get in her way. She was in my care and remained undaunted for about a decade until she passed away as a result of her disease.
Sometime after that, an unexpected package arrived at my office. It was a screen print created and sent to me by my patient’s granddaughter, an emerging artist in the Boston area. She titled it, “We Are Not What You Have Taken: A Response to Cancer.” It is a very powerful piece of art.

“We Are Not What You Have Taken: A Response to Cancer,” by Suzi Grossman
As you can see in the piece, my patient’s granddaughter created an artistic equation. First, she shows us a widely used symbol of a woman. Then, she uses images to represent the many surgeries her grandmother underwent, including a double mastectomy and operations to remove a kidney and a tumor that I remember describing to her as “the size of a football.” She also underwent bowel resection and thyroid surgeries, and another image represents the loss of her hair with chemotherapy. But the product of the equation is the original symbol of a woman, telling us that after all that was taken from her, she was still the same person, with the same indomitable spirit and sense of self she had at the start.
Seeing this artwork every time I come into my office is a humbling experience for me. To me, this image recognizes the trauma that a surgeon inflicts to counteract the harm that cancer creates. People with cancer face incredible challenges and must make many difficult decisions concerning their treatment. As a feature of these decisions, I don’t believe there is anything more profound than the trust a patient puts in their surgeon, allowing the surgeon to perform potentially life-altering operations. This work of art reminds me of the amazing courage of the woman it represents, who trusted in me and was determined not to let the trauma of cancer treatment diminish or define her.
Receiving such a meaningful work of art from a family member is very special for me. It is so challenging for family members to see a loved one going through all it takes to persevere in the face of this kind of disease, but my patient must have conveyed a message of strength and optimism to her granddaughter that led her to create this beautiful artwork.
I am so grateful to my patient’s granddaughter for creating and sharing this gift with me. I know her grandmother would be very proud of her for conveying its inspirational message in such a wonderful, moving way.
A New Target to Improve the Health and Lives of Childhood Cancer Survivors: Diabetes Prevention
Posted on January 11th, 2024 by Dr. Monica M. Bertagnolli
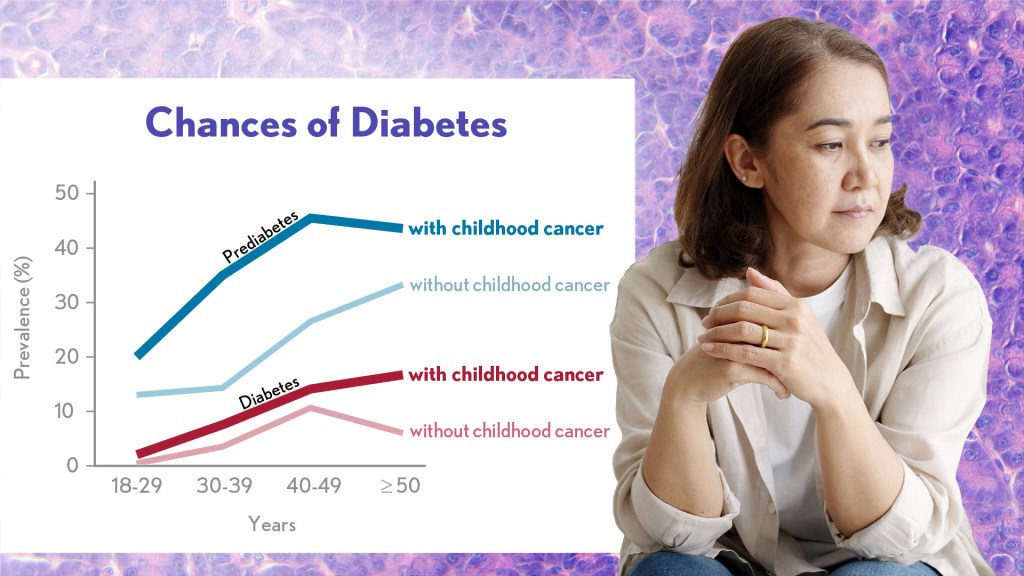
Researchers found that prediabetes and diabetes are highly prevalent in survivors of childhood cancer. Credit Donny Bliss/NIH. Modified from SB Dixon, et al.
Before joining NIH, I conducted research on how inflammation drives colon cancer. I eventually led a trial to see if certain anti-inflammatory drugs could prevent the colon polyps that can can turn into cancer. The drugs worked; however, they also increased the risk of strokes and heart attacks, so they were not safe for people at high risk of cardiovascular disease.
The trial gave us valuable insight about the risks of these drugs and serves as an example of how clinicians and researchers must consider the needs of the whole patient rather than focusing on one organ system or disease. We have to recognize how certain interventions might improve one health issue but exacerbate another. This is especially important in adult survivors of childhood cancer. We know this population—about 500,000 people living in the U.S. according to 2020 estimates from the National Cancer Institute—faces an increased risk of developing chronic health conditions, including diabetes.
NIH supports numerous researchers working to understand better the health outcomes in childhood cancer survivors. One team at St. Jude Children’s Research Hospital in Memphis has been following more than 3,500 adults who had been diagnosed with childhood cancer. Known as the St. Jude LIFE cohort, the participants undergo regular health screenings and researchers use the information to determine the prevalence and predictors of health issues and to identify interventions to reduce risks.
A new analysis published in the Journal of Clinical Oncology1 shows that prediabetes is highly prevalent in the St. Jude LIFE cohort: about one in every three survivors in the study had prediabetes by a median age of 30, compared to about one in five similarly aged adults without a cancer history. Prediabetes means that a person has higher than normal blood sugar levels but not high enough for a diagnosis of diabetes. Without intervention, many people with prediabetes will later develop diabetes.
By the time survivors in the study entered their 40s, more than half of them had prediabetes or diabetes, putting their future health at more risk compared to the general population. While these findings aren’t good news, they suggest that efforts to detect prediabetes and encourage lifestyle or treatment interventions before survivors go on to have more serious health complications can ensure that more people will live longer, healthier lives.
The research team, led by Stephanie Dixon, found that among 695 survivors with prediabetes who were followed over time, 10 percent progressed to diabetes within five years. The researchers also noted an association between radiation exposure to the pancreas and increased risk of prediabetes and diabetes. The pancreas produces insulin, and people are diagnosed with diabetes when the pancreas does not produce enough insulin to keep up with demand.
Their findings further suggest that, compared to survivors with normal blood sugar levels, those with prediabetes also had higher risk for future heart attack and chronic kidney disease. Those who progressed to diabetes also had more risk for developing stroke or cardiomyopathy (a condition where the heart pumps inefficiently and can lead to heart failure), in the future.
In the general population, prediabetes can be successfully managed through lifestyle changes, such as a healthy diet and exercise, as well as medication to prevent progression to diabetes and related health conditions. While this study is only a first step in identifying the consequences of prediabetes in survivors, it suggests that efforts to identify prediabetes and offer counseling on the importance of diabetes prevention may help more survivors of childhood cancers live long and healthy lives.
References:
[1] SB Dixon, et al. Prediabetes and Associated Risk of Cardiovascular Events and Chronic Kidney Disease Among Adult Survivors of Childhood Cancer in the St Jude Lifetime Cohort. Journal of Clinical Oncology DOI: 10.1200/JCO.23.01005 (2023).
NIH Support: National Cancer Institute
NCI Support for Basic Science Paves Way for Kidney Cancer Drug Belzutifan
Posted on January 25th, 2022 by Norman "Ned" Sharpless, M.D., National Cancer Institute

There’s exciting news for people with von Hippel-Lindau (VHL) disease, a rare genetic disorder that can lead to cancerous and non-cancerous tumors in multiple organs, including the brain, spinal cord, kidney, and pancreas. In August 2021, the U.S. Food and Drug Administration (FDA) approved belzutifan (Welireg), a new drug that has been shown in a clinical trial led by National Cancer Institute (NCI) researchers to shrink some tumors associated with VHL disease [1], which is caused by inherited mutations in the VHL tumor suppressor gene.
As exciting as this news is, relatively few people have this rare disease. The greater public health implication of this advancement is for people with sporadic, or non-inherited, clear cell kidney cancer, which is by far the most common subtype of kidney cancer, with more than 70,000 cases and about 14,000 deaths per year. Most cases of sporadic clear cell kidney cancer are caused by spontaneous mutations in the VHL gene.
This advancement is also a great story of how decades of support for basic science through NCI’s scientists in the NIH Intramural Research Program and its grantees through extramural research funding has led to direct patient benefit. And it’s a reminder that we never know where basic science discoveries might lead.
Belzutifan works by disrupting the process by which the loss of VHL in a tumor turns on a series of molecular processes. These processes involve the hypoxia-inducible factor (HIF) transcription factor and one of its subunits, HIF-2α, that lead to tumor formation.
The unraveling of the complex relationship among VHL, the HIF pathway, and cancer progression began in 1984, when Bert Zbar, Laboratory of Immunobiology, NCI-Frederick; and Marston Linehan, NCI’s Urologic Oncology Branch, set out to find the gene responsible for clear cell kidney cancer. At the time, there were no effective treatments for advanced kidney cancer, and 80 percent of patients died within two years.
Zbar and Linehan started by studying patients with sporadic clear cell kidney cancer, but then turned their focus to investigations of people affected with VHL disease, which predisposes a person to developing clear cell kidney cancer. By studying the patients and the genetic patterns of tumors collected from these patients, the researchers hypothesized that they could find genes responsible for kidney cancer.
Linehan established a clinical program at NIH to study and manage VHL patients, which facilitated the genetic studies. It took nearly a decade, but, in 1993, Linehan, Zbar, and Michael Lerman, NCI-Frederick, identified the VHL gene, which is mutated in people with VHL disease. They soon discovered that tumors from patients with sporadic clear cell kidney cancer also have mutations in this gene.
Subsequently, with NCI support, William G. Kaelin Jr., Dana-Farber Cancer Institute, Boston, discovered that VHL is a tumor suppressor gene that, when inactivated, leads to the accumulation of HIF.
Another NCI grantee, Gregg L. Semenza, Johns Hopkins School of Medicine, Baltimore, identified HIF as a transcription factor. And Peter Ratcliffe, University of Oxford, United Kingdom, discovered that HIF plays a role in blood vessel development and tumor growth.
Kaelin and Ratcliffe simultaneously showed that the VHL protein tags a subunit of HIF for destruction when oxygen levels are high. These results collectively answered a very old question in cell biology: How do cells sense the intracellular level of oxygen?
Subsequent studies by Kaelin, with NCI’s Richard Klausner and Linehan, revealed the critical role of HIF in promoting the growth of clear cell kidney cancer. This work ultimately focused on one member of the HIF family, the HIF-2α subunit, as the key mediator of clear cell kidney cancer growth.
The fundamental work of Kaelin, Semenza, and Ratcliffe earned them the 2019 Nobel Prize in Physiology or Medicine. It also paved the way for drug discovery efforts that target numerous points in the pathway leading to clear cell kidney cancer, including directly targeting the transcriptional activity of HIF-2α with belzutifan.
Clinical trials of belzutifan, including several supported by NCI, demonstrated potent anti-cancer activity in VHL-associated kidney cancer, as well as other VHL-associated tumors, leading to the aforementioned recent FDA approval. This is an important development for patients with VHL disease, providing a first-in-class therapy that is effective and well-tolerated.
We believe this is only the beginning for belzutifan’s use in patients with cancer. A number of trials are now studying the effectiveness of belzutifan for sporadic clear cell kidney cancer. A phase 3 trial is ongoing, for example, to look at the effectiveness of belzutifan in treating people with advanced kidney cancer. And promising results from a phase 2 study show that belzutifan, in combination with cabozantinib, a widely used agent to treat kidney cancer, shrinks tumors in patients previously treated for metastatic clear cell kidney cancer [2].
This is a great scientific story. It shows how studies of familial cancer and basic cell biology lead to effective new therapies that can directly benefit patients. I’m proud that NCI’s support for basic science, both intramurally and extramurally, is making possible many of the discoveries leading to more effective treatments for people with cancer.
References:
[1] Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. Jonasch E, Donskov F, Iliopoulos O, Rathmell WK, Narayan VK, Maughan BL, Oudard S, Else T, Maranchie JK, Welsh SJ, Thamake S, Park EK, Perini RF, Linehan WM, Srinivasan R; MK-6482-004 Investigators. N Engl J Med. 2021 Nov 25;385(22):2036-2046.
[2] Phase 2 study of the oral hypoxia-inducible factor 2α (HIF-2α) inhibitor MK-6482 in combination with cabozantinib in patients with advanced clear cell renal cell carcinoma (ccRCC). Choueiri TK et al. J Clin Oncol. 2021 Feb 20;39(6_suppl): 272-272.
Links:
Von Hippel-Lindau Disease (Genetic and Rare Diseases Information Center/National Center for Advancing Translational Sciences/NIH)
The Long Road to Understanding Kidney Cancer (Intramural Research Program/NIH)
[Note: Acting NIH Director Lawrence Tabak has asked the heads of NIH’s institutes and centers to contribute occasional guest posts to the blog as a way to highlight some of the cool science that they support and conduct. This is the first in the series of NIH institute and center guest posts that will run until a new permanent NIH director is in place.]
Posted In: Generic
Tags: 2019 Nobel Prize in Physiology or Medicine, basic research, belzutifan, cancer, cancer drugs, cell biology, clear cell kidney cancer, clinical trials, FDA, genetics, HIF, kidney cancer, NCI, NCI-MATCH trial, oncology, rare disease, tumor suppressor gene, VHL, von Hippel-Lindau disease, Welireg
Insurance Status Helps Explain Racial Disparities in Cancer Diagnosis
Posted on January 21st, 2020 by Dr. Francis Collins

Credit: iStock/jmangostock
Women have the best odds of surviving breast cancer if their disease is caught at an early stage, when treatments are most likely to succeed. Major strides have been made in the early detection of breast cancer in recent years. But not all populations have benefited equally, with racial and ethnic minorities still more likely to be diagnosed with later-stage breast cancer than non-Hispanic whites. Given that recent observance of Martin Luther King Day, I thought that it would be particularly appropriate to address a leading example of health disparities.
A new NIH-funded study of more than 175,000 U.S. women diagnosed with breast cancer from 2010-2016 has found that nearly half of the troubling disparity in breast cancer detection can be traced to lack of adequate health insurance. The findings suggest that improving insurance coverage may help to increase early detection and thereby reduce the disproportionate number of breast cancer deaths among minority women.
Naomi Ko, Boston University School of Medicine, has had a long interest in understanding the cancer disparities she witnesses first-hand in her work as a medical oncologist. For the study published in JAMA Oncology, she teamed up with epidemiologist Gregory Calip, University of Illinois Cancer Center, Chicago [1]. Their goal was to get beyond documenting disparities in breast cancer and take advantage of available data to begin to get at why such disparities exist and what to do about them.
Disparities in breast cancer outcomes surely stem from a complicated mix of factors, including socioeconomic factors, culture, diet, stress, environment, and biology. Ko and Calip focused their attention on insurance, thinking of it as a factor that society can collectively modify.
Many earlier studies had shown a link between insurance and cancer outcomes [2]. It also stood to reason that broad differences among racial and ethnic minorities in their access to adequate insurance might drive some of the observed cancer disparities. But, Ko and Calip asked, just how big a factor was it?
To find out, they looked to the NIH’s Surveillance Epidemiology, and End Results (SEER) Program, run by the National Cancer Institute. The SEER Program is an authoritative source of information on cancer incidence and survival in the United States.
The researchers focused their attention on 177,075 women of various races and ethnicities, ages 40 to 64. All had been diagnosed with invasive stage I to III breast cancer between 2010 and 2016.
The researchers found that a higher proportion of women receiving Medicaid or who were uninsured received a diagnosis of advanced stage III breast cancer compared with women with health insurance. Black, American Indian, Alaskan Native, and Hispanic women also had higher odds of receiving a late-stage diagnosis.
Overall, their sophisticated statistical analyses traced up to 47 percent of the racial/ethnic differences in the risk of locally advanced disease to differences in health insurance. Such late-stage diagnoses and the more extensive treatment regimens that go with them are clearly devastating for women with breast cancer and their families. But, the researchers note, they’re also costly for society, due to lost productivity and escalating treatment costs by stage of breast cancer.
These researchers surely aren’t alone in recognizing the benefit of early detection. Last week, an independent panel convened by NIH called for enhanced research to assess and explore how to reduce health disparities that lead to unequal access to health care and clinical services that help prevent disease.
References:
[1] Association of Insurance Status and Racial Disparities With the Detection of Early-Stage Breast Cancer. Ko NY, Hong S, Winn RA, Calip GS. JAMA Oncol. 2020 Jan 9.
[2] The relation between health insurance coverage and clinical outcomes among women with breast cancer. Ayanian JZ, Kohler BA, Abe T, Epstein AM. N Engl J Med. 1993 Jul 29;329(5):326-31.
[3] Cancer Stat Facts: Female Breast Cancer. National Cancer Institute Surveillance, Epidemiology, and End Results Program.
Links:
Cancer Disparities (National Cancer Institute/NIH)
Breast Cancer (National Cancer Institute/NIH)
Naomi Ko (Boston University)
Gregory Calip (University of Illinois Cancer Center, Chicago)
NIH Support: National Center for Advancing Translational Sciences; National Cancer Institute; National Institute on Minority Health and Health Disparities
Posted In: News
Tags: African American health, Alaskan Natives, American Indian, black, breast cancer, cancer, cancer diagnosis, health disparities, health insurance, Hispanic, insurance, oncology, race, racial disparities, SEER, women's health
Caught on Video: Cancer Cells in Act of Cannibalism
Posted on October 29th, 2019 by Dr. Francis Collins
Tumors rely on a variety of tricks to grow, spread, and resist our best attempts to destroy them. Now comes word of yet another of cancer’s surprising stunts: when chemotherapy treatment hits hard, some cancer cells survive by cannibalizing other cancer cells.
Researchers recently caught this ghoulish behavior on video. In what, during this Halloween season, might look a little bit like The Blob, you can see a down-for-the-count breast cancer cell (green), treated earlier with the chemotherapy drug doxorubicin, gobbling up a neighboring cancer cell (red). The surviving cell delivers its meal to internal compartments called lysosomes, which digest it in a last-ditch effort to get some nourishment and keep going despite what should have been a lethal dose of a cancer drug.
Crystal Tonnessen-Murray, a postdoctoral researcher in the lab of James Jackson, Tulane University School of Medicine, New Orleans, captured these dramatic interactions using time-lapse and confocal microscopy. When Tonnessen-Murray saw the action, she almost couldn’t believe her eyes. Tumor cells eating tumor cells wasn’t something that she’d learned about in school.
As the NIH-funded team described in the Journal of Cell Biology, these chemotherapy-treated breast cancer cells were not only cannibalizing their neighbors, they were doing it with remarkable frequency [1]. But why?
A possible explanation is that some cancer cells resist chemotherapy by going dormant and not dividing. The new study suggests that while in this dormant state, cannibalism is one way that tumor cells can keep going.
The study also found that these acts of cancer cell cannibalism depend on genetic programs closely resembling those of immune cells called macrophages. These scavenging cells perform their important protective roles by gobbling up invading bacteria, viruses, and other infectious microbes. Drug-resistant breast cancer cells have apparently co-opted similar programs in response to chemotherapy but, in this case, to eat their own neighbors.
Tonnessen-Murray’s team confirmed that cannibalizing cancer cells have a survival advantage. The findings suggest that treatments designed to block the cells’ cannibalistic tendencies might hold promise as a new way to treat otherwise hard-to-treat cancers. That’s a possibility the researchers are now exploring, although they report that stopping the cells from this dramatic survival act remains difficult.
Reference:
[1] Chemotherapy-induced senescent cancer cells engulf other cells to enhance their survival. Tonnessen-Murray CA, Frey WD, Rao SG, Shahbandi A, Ungerleider NA, Olayiwola JO, Murray LB, Vinson BT, Chrisey DB, Lord CJ, Jackson JG. J Cell Biol. 2019 Sep 17.
Links:
Breast Cancer (National Cancer Institute/NIH)
James Jackson (Tulane University School of Medicine, New Orleans)
NIH Support: National Institute of General Medical Sciences
Posted In: Cool Videos
Tags: breast cancer, cancer, cancer drug resistance, cannibalism, cannibalizing cancer cells, cell biology, chemotherapy, confocal microscopy, doxorubicin, drug resistance, macrophage, oncology, senescent cells, tumor cells
Using MicroRNA to Starve a Tumor?
Posted on September 5th, 2019 by Dr. Francis Collins
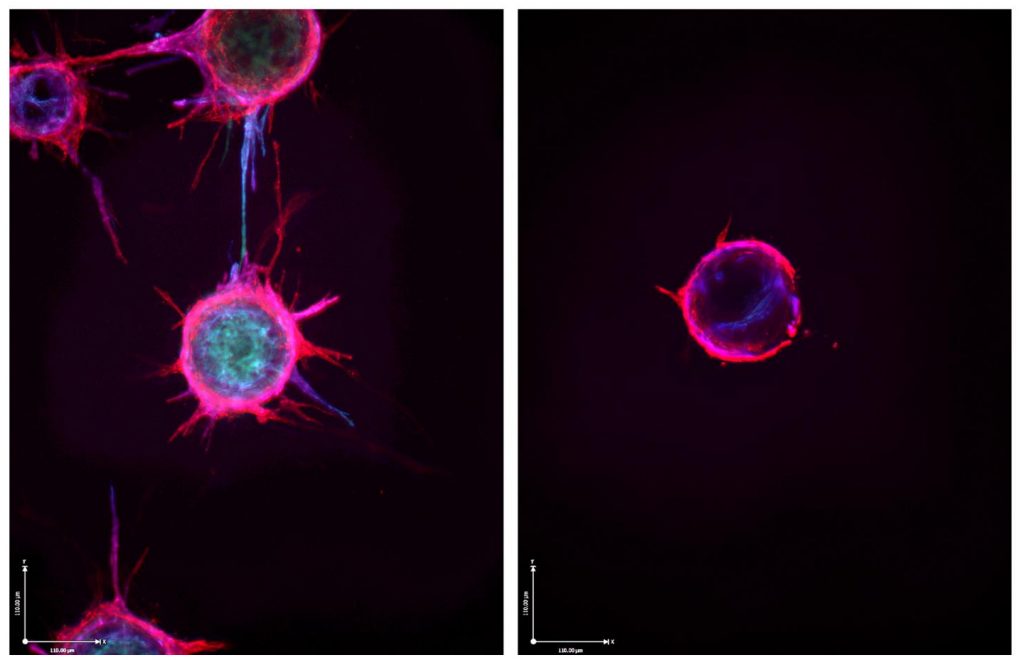
Credit: Dudley Lab, University of Virginia School of Medicine, Charlottesville
Tumor cells thrive by exploiting the willingness of normal cells in their neighborhood to act as accomplices. One of their sneakier stunts involves tricking the body into helping them form new blood vessels. This growth-enabling process of sprouting new blood vessels, called tumor angiogenesis, remains a vital area of cancer research and continues to yield important clues into how to beat this deadly disease.
The two-panel image above shows one such promising lead from recent lab studies with endothelial cells, specialized cells that line the inside of all blood vessels. In tumors, endothelial cells are induced to issue non-stop SOS signals that falsely alert the body to dispatch needed materials to rescue these cells. The endothelial cells then use the help to replicate and sprout new blood vessels.
The left panel demonstrates the basics of this growth process under normal conditions. Endothelial cells (red and blue) were cultured under special conditions that help them grow in the lab. When given the right cues, those cells sprout spiky extensions to form new vessels.
But in the right panel, the cells can’t sprout. The reason is because the cells are bathed in a molecule called miR-30c, which isn’t visible in the photo. These specialized microRNA molecules—and humans make a few thousand different versions of them—control protein production by binding to and disabling longer RNA templates, called messenger RNA.
This new anti-angiogenic lead, published in the Journal of Clinical Investigation, comes from a research team led by Andrew Dudley, University of Virginia Medical School, Charlottesville [1]. The team made its discovery while studying a protein called TGF-beta that tumors like to exploit to fuel their growth.
Their studies in mice showed that loss of TGF-beta signals in endothelial cells blocked the growth of new blood vessels and thus tumors. Further study showed that those effects were due in part to elevated levels of miR-30c. The two interact in endothelial cells as part of a previously unrecognized signaling pathway that coordinates the growth of new blood vessels in tumors.
Dudley’s team went on to show that levels of miR-30c vary widely amongst endothelial cells, even when those cells come from the very same tumor. Cells rich in miR-30c struggled to sprout new vessels, while those with less of this microRNA grew new vessels with ease.
Intriguingly, they found that levels of this microRNA also predicted the outcomes for patients with breast cancer. Those whose cancers had high levels of the vessel-stunting miR-30c fared better than those with lower miR-30c levels. While more research is needed, it does offer a potentially promising new lead in the fight against cancer.
Reference:
[1] Endothelial miR-30c suppresses tumor growth via inhibition of TGF-β-induced Serpine1. McCann JV, Xiao L, Kim DJ, Khan OF, Kowalski PS, Anderson DG, Pecot CV, Azam SH, Parker JS, Tsai YS, Wolberg AS, Turner SD, Tatsumi K, Mackman N, Dudley AC. J Clin Invest. 2019 Mar 11;130:1654-1670.
Links:
Angiogenesis Inhibitors (National Cancer Institute/NIH)
Dudley Lab (University of Virginia School of Medicine, Charlottesville)
NIH Support: National Cancer Institute; National Heart, Lung, and Blood Institute
Posted In: Snapshots of Life
Tags: angiogenesis, blood vessels, breast cancer, cancer, endothelial cells, messenger RNA, MicroRNA, miR-30c, oncology, RNA, TGF-beta, tumor angiogenesis, tumor microenvironment
Personalized Combination Therapies Yield Better Cancer Outcomes
Posted on April 30th, 2019 by Dr. Francis Collins

Credit: NIH National Cancer Institute Visuals Online/Daniel Sone
Gratifying progress has been made recently in an emerging area of cancer medicine called precision oncology. It’s a bold attempt to target treatment to the very genes and molecules driving a cancer, aiming to slow or even halt its growth. But there’s always more to learn. Now comes evidence that, while a single well-matched drug might be good, a tailored combination of drugs that attack a cancer in multiple ways at once might be even better.
The findings come from the I-PREDICT clinical trial, which treated people with advanced cancer who hadn’t benefited from previous therapy [1]. The NIH-funded team found that analyzing a tumor’s unique genetic and molecular profile provided enough information to recommend individualized combination therapies to patients. What’s more, patients who followed their individualized combination therapies most closely lived longer, with longer periods of progression-free disease, than did those who took fewer of the recommended drugs.
In most previous clinical trials of precision oncology, researchers have relied on a tumor’s unique profile to identify a single, well-matched drug to treat each patient. But cancer is complex, and, just as with certain infectious diseases, tumors commonly develop resistance to a single drug.
In the trial reported in Nature Medicine, researchers led by Razelle Kurzrock and Jason Sicklick, University of California, San Diego, wondered if they could improve treatment responses by tailoring combinations of cancer drugs to target as many molecular and genetic changes in a person’s cancer as possible.
To test the potential for this strategy to work, the researchers enrolled 83 people with various cancers that had advanced despite previous treatment. Tumor tissue from each patient was run through a comprehensive battery of tests, and researchers sequenced hundreds of genes to look for telltale alterations in their DNA.
They also looked for evidence that a cancer had defects affecting the DNA “mismatch repair” pathway, which causes some tumors to generate larger numbers of mutations than others. Mismatch repair defects have been shown to predict better responses to immunotherapies, which are designed to harness the immune system against cancer .
With all the data in hand, a special panel of oncologists, pharmacologists, cancer biologists, geneticists, surgeons, radiologists, pathologists, and bioinformatics experts consulted to arrive at the right customized combination of drugs for each patient.
The panel’s findings were presented to the health care team working with each patient. The physician for each patient then had the final decision on whether to recommend the treatment regimen, balancing the panel’s suggestions with other real-world factors, such as a patient’s insurance coverage, availability of drugs, and his or her treatment preference.
Ten patients decided to stick with unmatched treatment. But 73 participants received a customized combination therapy. As no two molecular profiles were identical, the customized treatment regimens varied from person to person.
Many people received designer drugs targeting particular genetic alterations. Some also received checkpoint inhibitor immunotherapies to unleash the immune system against cancer. Four people also were treated with hormone therapies in combination with molecularly targeted drugs. In all, most regimens combined two to five drugs to target each cancer profile.
Participants were followed until their cancer progressed, they could no longer take treatment, or they died. For each person, the researchers calculated a “matching score,” roughly defined as the number of molecular alterations matched to administered drug(s), with some further calculations.
The evidence showed that those with matching scores greater than 50 percent, meaning more than half of a tumor’s identified aberrations had been targeted, were more likely to have stopped the progression of their cancers. Importantly, half of patients with the higher matching scores had prolonged stable disease (six months or longer) or a complete or partial remission. Similar results were attained in only 22 percent of those with low or no matching scores.
These encouraging results suggest that customized combinations of targeted treatments will help to advance precision oncology. However, there are still many challenges. For example, many of the combinations used in the study have not yet been safety tested. The researchers managed the potential risk of toxicities by starting patients on an initial low dose and having their physicians follow them closely while the dose was increased to a level well-tolerated by each individual patient.
And indeed, they saw no evidence that those receiving a greater proportion of “matched” drugs (i.e. those with a higher matching score) were more likely to experience adverse effects than those who took fewer drugs. So, that’s an encouraging sign.
The researchers are now enrolling patients in a new version of the I-PREDICT trial. Unlike the initial plan, patients are now being enrolled prior to receiving any treatment for a recently diagnosed aggressive, often-lethal form of cancer. The hope is that treating patients with well-matched, multi-drug treatment combinations early will yield even better results than waiting until standard treatment has failed. If correct, it would mark significant progress in building the future of precision oncology.
Reference:
[1] Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Sicklick JK, Kato S, Okamura R, Schwaederle M, Hahn ME, Williams CB, De P, Krie A, Piccioni DE, Miller VA, Ross JS, Benson A, Webster J, Stephens PJ, Lee JJ, Fanta PT, Lippman SM, Leyland-Jones B, Kurzrock R. Nat Med. 2019 Apr 22.
Links:
Precision Medicine in Cancer Treatment (National Cancer Institute/NIH)
Study of Molecular Profile-Related Evidence to Determine Individualized Therapy for Advanced or Poor Prognosis Cancers (I-PREDICT) (Clinicaltrials.gov)
Razelle Kurzrock (University of California, San Diego)
Jason Sicklick (University of California, San Diego)
NIH Support: National Cancer Institute
Posted In: News
Tags: cancer, chemotherapy, clinical trial, combination therapy, gene profile, hormone therapy, I-PREDICT, immunotherapy, matched drugs, mismatch repair, molecular profiling, oncology, personalized medicine, precision medicine, precision oncology
Most Women with Early-Stage Breast Cancer Don’t Need Chemo
Posted on June 12th, 2018 by Dr. Francis Collins

Credit: National Cancer Institute, NIH
In the last few days, you may have heard that there’s been a significant development in the management of breast cancer. So here’s the NIH Director’s blog description of what’s happened. Each year, as many as 135,000 American women who’ve undergone surgery for the most common form of early-stage breast cancer face a difficult decision: whether or not to undergo chemotherapy. Genetic testing of tumor tissue has helped to inform some of these decisions, with women whose tumors score high on the breast cancer recurrence scale likely to benefit from chemo, and those with low-scoring tumors able to skip the cost and potentially serious side effects. But there’s been a catch: most tumors score somewhere in the middle, leaving women and their doctors uncertain about what to do.
Now, thanks to the long-awaited results of a large, NIH-funded clinical trial, we finally have an answer. About 70 percent of women with hormone receptor (HR)-positive, HER2-negative, axillary lymph node-negative breast cancer—including those with mid-range scores on the cancer recurrence scale—do not benefit from chemotherapy [1]. These findings promise to spare a great many women with breast cancer from unnecessary exposure to costly and potentially toxic chemotherapy.
Posted In: News
Tags: adjuvant chemotherapy, American Society of Clinical Oncology, ASCO, axillary lymph node-negative breast cancer, breast cancer, cancer, cancer recurrence, chemo, chemotherapy, clinical trial, early-stage breast cancer, gene test, genetic testing, HER2-negative, hormone therapy, HR-positive, lymph node, oncology, Oncotype DX Breast Cancer Assay, TAILORx, Trial Assigning Individualized Options for Treatment
Optimizing Radio-Immunotherapy for Cancer
Posted on April 5th, 2018 by Dr. Francis Collins

Zachary Morris
Credit: Alan Leon
Zachary Morris has certainly done some memorable things. As a Rhodes Scholar, he once attended an evening reception at Buckingham Palace, played a game of pick-up football with former President Bill Clinton, and traveled to South Africa to take a Robben Island Prison tour, led by the late Nelson Mandela. But something the young radiation oncologist did during his medical residency could prove even more momentous. He received a special opportunity from the American Board of Radiology to join others in studying how to pair radiation therapy with the emerging cancer treatment strategy of immunotherapy.
Morris’s studies in animals showed that the two treatments have a unique synergy, generating a sustained tumor-specific immune response that’s more potent than either therapy alone. But getting this combination therapy just right to optimize its cancer-fighting abilities remains complicated. Morris, now a researcher and clinician at the University of Wisconsin School of Medicine and Public Health, Madison, has received a 2017 NIH Director’s Early Independence Award to look deeper into this promising approach. He and his collaborators will use what they learn to better inform their future early stage clinical trials of radio-immunotherapy starting with melanoma, head and neck cancers, and neuroblastoma.
Posted In: Creative Minds, Health, Science
Tags: 2017 NIH Director’s Early Independence Award, cancer, dinutuximab, head and neck cancer, IL-2, immunotherapy, low-dose radiation, lymphoma, melanoma, neuroblastoma, oncology, radiation, radiation oncology, radiation therapy, radio-immunotherapy, Tregs
Working Toward Greater Precision in Childhood Cancers
Posted on March 6th, 2018 by Dr. Francis Collins

Credit: National Cancer Institute, NIH
Each year, more than 15,000 American children and teenagers will be diagnosed with cancer. While great progress has been made in treating many types of childhood cancer, it remains the leading cause of disease-related death among kids who make it past infancy in the United States [1]. One reason for that sobering reality is our relatively limited knowledge about the precise biological mechanisms responsible for childhood cancers—information vital for designing targeted therapies to fight the disease in all its varied forms.
Now, two complementary studies have brought into clearer focus the genomic landscapes of many types of childhood cancer [2, 3]. The studies, which analyzed DNA data representing tumor and normal tissue from more than 2,600 young people with cancer, uncovered thousands of genomic alterations in about 200 different genes that appear to drive childhood cancers. These so-called “driver genes” included many that were different than those found in similar studies of adult cancers, as well as a considerable number of mutations that appear amenable to targeting with precision therapies already available or under development.
Tags: B-cell acute lymphoblastic leukemia, cancer, childhood cancer, childhood leukemia, children, driver genes, driver mutations, environmental factors, gene signature, germline mutations, mutational signatures, oncology, pan-cancer study, precision oncology, TARGET, tumor biology, UV exposure