tau – NIH Director's Blog (original) (raw)
Changes in Human Microbiome Precede Alzheimer’s Cognitive Declines
Posted on June 27th, 2023 by Lawrence Tabak, D.D.S., Ph.D.
Caption: The human gut teems with bacteria and other microbes. They contribute to our health but also influence our susceptibility to certain diseases, including Alzheimer’s disease. Credit: Donny Bliss, NIH
In people with Alzheimer’s disease, the underlying changes in the brain associated with dementia typically begin many years—or even decades—before a diagnosis. While pinpointing the exact causes of Alzheimer’s remains a major research challenge, they likely involve a combination of genetic, environmental, and lifestyle factors. Now an NIH-funded study elucidates the role of another likely culprit that you may not have considered: the human gut microbiome, the trillions of diverse bacteria and other microbes that live primarily in our intestines [1].
Earlier studies had showed that the gut microbiomes of people with symptomatic Alzheimer’s disease differ from those of healthy people with normal cognition [2]. What this new work advances is that these differences arise early on in people who will develop Alzheimer’s, even before any obvious symptoms appear.
The science still has a ways to go before we’ll know if specific dietary changes can alter the gut microbiome and modify its influence on the brain in the right ways. But what’s exciting about this finding is it raises the possibility that doctors one day could test a patient’s stool sample to determine if what’s present from their gut microbiome correlates with greater early risk for Alzheimer’s dementia. Such a test would help doctors detect Alzheimer’s earlier and intervene sooner to slow or ideally even halt its advance.
The new findings, reported in the journal Science Translational Medicine, come from a research team led by Gautam Dantas and Beau Ances, Washington University School of Medicine, St. Louis. Ances is a clinician who treats and studies people with Alzheimer’s; Dantas is a basic researcher and expert on the gut microbiome.
The pair struck up a conversation one day about the possible connection between the gut microbiome and Alzheimer’s. While they knew about the earlier studies suggesting a link, they were surprised that nobody had looked at the gut microbiomes of people in the earliest, so-called preclinical, stages of the disease. That’s when dementia isn’t detectable, but the brain has formed amyloid-beta plaques, which are associated with Alzheimer’s.
To take a look, they enrolled 164 healthy volunteers, age 68 to 94, who performed normally on standard tests of cognition. They also collected stool samples from each volunteer and thoroughly analyzed them all the microbes from their gut microbiome. Study participants also kept food diaries and underwent extensive testing, including two types of brain scans, to look for signs of amyloid-beta plaques and tau protein accumulation that precede the onset of Alzheimer’s symptoms.
Among the volunteers, about a third (49 individuals) unfortunately had signs of early Alzheimer’s disease. And, as it turned out, their microbiomes showed differences, too.
The researchers found that those with preclinical Alzheimer’s disease had markedly different assemblages of gut bacteria. Their microbiomes differed in many of the bacterial species present. Those species-level differences also point to differences in the way their microbiomes would be expected to function at a metabolic level. These microbiome changes were observed even though the individuals didn’t seem to have any apparent differences in their diets.
The team also found that the microbiome changes correlated with amyloid-beta and tau levels in the brain. But they did not find any relationship to degenerative changes in the brain, which tend to happen later in people with Alzheimer’s.
The team is now conducting a five-year study that will follow volunteers to get a better handle on whether the differences observed in the gut microbiome are a cause or a consequence of the brain changes seen in Alzheimer’s. If it’s a cause, this discovery would raise the tantalizing possibility that specially formulated probiotics or fecal transplants that promote the growth of “good” bacteria over “bad” bacteria in the gut might slow the development of Alzheimer’s and its most devastating symptoms. It’s an exciting area of research and definitely one worth following in the years ahead.
References:
[1] Gut microbiome composition may be an indicator of preclinical Alzheimer’s disease. Ferreiro AL, Choi J, Ryou J, Newcomer EP, Thompson R, Bollinger RM, Hall-Moore C, Ndao IM, Sax L, Benzinger TLS, Stark SL, Holtzman DM, Fagan AM, Schindler SE, Cruchaga C, Butt OH, Morris JC, Tarr PI, Ances BM, Dantas G. Sci Transl Med. 2023 Jun 14;15(700):eabo2984. doi: 10.1126/scitranslmed.abo2984. Epub 2023 Jun 14. PMID: 37315112.
[2] Gut microbiome alterations in Alzheimer’s disease. Vogt NM, Kerby RL, Dill-McFarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, Bendlin BB, Rey FE. Sci Rep. 2017 Oct 19;7(1):13537. doi: 10.1038/s41598-017-13601-y. PMID: 29051531; PMCID: PMC5648830.
Links:
Alzheimer’s Disease and Related Dementias (National Institute on Aging/NIH)
Video: How Alzheimer’s Changes the Brain (NIA)
Dantas Lab (Washington University School of Medicine. St. Louis)
Ances Bioimaging Laboratory (Washington University School of Medicine, St. Louis)
NIH Support: National Institute on Aging; National Institute of Diabetes and Digestive and Kidney Diseases
Posted In: News
Tags: aging, Alzheimer’s disease, amyloid plaques, beta amyloid, brain, dementia, diet, fecal transplant, gut, gut bacteria, gut microbiome, microbiome, probiotics, tau
Case Study Unlocks Clues to Rare Resilience to Alzheimer’s Disease
Posted on May 30th, 2023 by Lawrence Tabak, D.D.S., Ph.D.
Caption: Newly discovered Reelin-COLBOS gene variation may delay or prevent Alzheimer’s disease. Credit: Donny Bliss, NIH
Biomedical breakthroughs most often involve slow and steady research in studies involving large numbers of people. But sometimes careful study of even just one truly remarkable person can lead the way to fascinating discoveries with far-reaching implications.
An NIH-funded case study published recently in the journal Nature Medicine falls into this far-reaching category [1]. The report highlights the world’s second person known to have an extreme resilience to a rare genetic form of early onset Alzheimer’s disease. These latest findings in a single man follow a 2019 report of a woman with similar resilience to developing symptoms of Alzheimer’s despite having the same strong genetic predisposition for the disease [2].
The new findings raise important new ideas about the series of steps that may lead to Alzheimer’s and its dementia. They’re also pointing the way to key parts of the brain for cognitive resilience—and potentially new treatment targets—that may one day help to delay or even stop progression of Alzheimer’s.
The man in question is a member of a well-studied extended family from the country of Colombia. This group of related individuals, or kindred, is the largest in the world with a genetic variant called the “Paisa” mutation (or Presenilin-1 E280A). This Paisa variant follows an autosomal dominant pattern of inheritance, meaning that those with a single altered copy of the rare variant passed down from one parent usually develop mild cognitive impairment around the age of 44. They typically advance to full-blown dementia around the age of 50 and rarely live past the age of 60. This contrasts with the most common form of Alzheimer’s, which usually begins after age 65.
The new findings come from a team led by Yakeel Quiroz, Massachusetts General Hospital, Boston; Joseph Arboleda-Velasquez, Massachusetts Eye and Ear, Boston; Diego Sepulveda-Falla, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; and Francisco Lopera, University of Antioquia, Medellín, Colombia. Lopera first identified this family more than 30 years ago and has been studying them ever since.
In the new case report, the researchers identified a Colombian man who’d been married with two children and retired from his job as a mechanic in his early 60s. Despite carrying the Paisa mutation, his first cognitive assessment at age 67 showed he was cognitively intact, having limited difficulties with verbal learning skills or language. It wasn’t until he turned 70 that he was diagnosed with mild cognitive impairment—more than 20 years later than the expected age for this family—showing some decline in short-term memory and verbal fluency.
At age 73, he enrolled in the Colombia-Boston biomarker research study (COLBOS). This study is a collaborative project between the University of Antioquia and Massachusetts General Hospital involving approximately 6,000 individuals from the Paisa kindred. About 1,500 of those in the study carry the mutation that sets them up for early Alzheimer’s. As a member of the COLBOS study, the man underwent thorough neuroimaging tests to look for amyloid plaques and tau tangles, both of which are hallmarks of Alzheimer’s.
While this man died at age 74 with Alzheimer’s, the big question is: how did he stave off dementia for so long despite his poor genetic odds? The COLBOS study earlier identified a woman with a similar resilience to Alzheimer’s, which they traced to two copies of a rare, protective genetic variant called Christchurch. This variant affects a gene called apolipoprotein E (APOE3), which is well known for its influence on Alzheimer’s risk. However, the man didn’t carry this same protective variant.
The researchers still thought they’d find an answer in his genome and kept looking. While they found several variants of possible interest, they zeroed in on a single gene variant that they’ve named Reelin-COLBOS. What helped them to narrow it down to this variant is the man also had a sister with the Paisa mutation who only progressed to advanced dementia at age 72. It turned out, in addition to the Paisa variant, the siblings also shared an altered copy of the newly discovered Reelin-COLBOS variant.
This Reelin-COLBOS gene is known to encode a protein that controls signals to chemically modify tau proteins, which form tangles that build up over time in the Alzheimer’s brain and have been linked to memory loss. Reelin is also functionally related to APOE, the gene that was altered in the woman with extreme Alzheimer’s protection. Reelin and APOE both interact with common protein receptors in neurons. Together, the findings add to evidence that signaling pathways influencing tau play an important role in Alzheimer’s pathology and protection.
The neuroimaging exams conducted when the man was age 73 have offered further intriguing clues. They showed that his brain had extensive amyloid plaques. He also had tau tangles in some parts of his brain. But one brain region, called the entorhinal cortex, was notable for having a very minimal amount of those hallmark tau tangles.
The entorhinal cortex is a hub for memory, navigation, and the perception of time. Its degeneration also leads to cognitive impairment and dementia. Studies of the newly identified Reelin-COLBOS variant in Alzheimer’s mouse models also help to confirm that the variant offers its protection by diminishing the pathological modifications of tau.
Overall, the findings in this one individual and his sister highlight the Reelin pathway and brain region as promising targets for future study and development of Alzheimer’s treatments. Quiroz and her colleagues report that they are actively exploring treatment approaches inspired by the Christchurch and Reelin-COLBOS discoveries.
Of course, there’s surely more to discover from continued study of these few individuals and others like them. Other as yet undescribed genetic and environmental factors are likely at play. But the current findings certainly offer some encouraging news for those at risk for Alzheimer’s disease—and a reminder of how much can be learned from careful study of remarkable individuals.
References:
[1] Resilience to autosomal dominant Alzheimer’s disease in a Reelin-COLBOS heterozygous man. Lopera F, Marino C, Chandrahas AS, O’Hare M, Reiman EM, Sepulveda-Falla D, Arboleda-Velasquez JF, Quiroz YT, et al. Nat Med. 2023 May;29(5):1243-1252.
[2] Resistance to autosomal dominant Alzheimer’s disease in an APOE3 Christchurch homozygote: a case report. Arboleda-Velasquez JF, Lopera F, O’Hare M, Delgado-Tirado S, Tariot PN, Johnson KA, Reiman EM, Quiroz YT et al. Nat Med. 2019 Nov;25(11):1680-1683.
Links:
Alzheimer’s Disease & Related Dementias (National Institute on Aging/NIH)
“NIH Support Spurs Alzheimer’s Research in Colombia,” Global Health Matters, January/February 2014, Fogarty International Center/NIS
“COLBOS Study Reveals Mysteries of Alzheimer’s Disease,” NIH Record, August 19, 2022.
Yakeel Quiroz (Massachusetts General Hospital, Harvard Medical School, Boston)
Joseph Arboleda-Velasquez (Massachusetts Eye and Ear, Harvard Medical School, Boston)
Diego Sepulveda-Falla Lab (University Medical Center Hamburg-Eppendorf, Hamburg, Germany)
Francisco Lopera (University of Antioquia, Medellín, Colombia)
NIH Support: National Institute on Aging; National Eye Institute; National Institute of Neurological Disorders and Stroke; Office of the Director
Posted In: News
Tags: Alzheimer’s disease, APOE3, brain, Christchurch variant, cognitive resilience, Colombia, Colombia-Boston biomarker research study, dementia, genetics, genomics, global health, Paisa mutation, Paisa variant, Presinilin-1, Reelin-COLBOS gene variant, tau, tau protein
Getting Closer to a Blood Test for Alzheimer’s Disease?
Posted on March 31st, 2020 by Dr. Francis Collins
iStock/ericsphotography
As research on Alzheimer’s disease (AD) advances, a desperate need remains for an easy blood test to help diagnose the condition as early as possible. Ideally, such a test could also distinguish AD from other forms of dementia that produce similar symptoms. As published recently in Nature Medicine, an NIH-funded research team has designed a simple blood test that is on course to meet these criteria [1].
The latest work builds on a large body of work showing that one secret to predicting a person’s cognitive decline and treatment response in AD lies in a protein called tau. Using the powerful, but expensive, approach of PET scan imaging, we know that tau builds up in the brain as Alzheimer’s disease progresses. We also know that some tau spills from the brain into the bloodstream.
The trouble is that the circulating tau protein breaks down far too quickly for a blood test to offer a reliable measure of what’s happening in a person’s brain. A few years ago, researchers discovered a possible solution: test for blood levels of a slightly different and more stable version of the protein called pTau181 [2]. (The “p” in its name comes from the addition of phosphorus in a particular part of the protein’s structure.)
In the latest study, researchers in the lab of Adam Boxer, University of California, San Francisco, followed up further on this compelling lead. Boxer’s team measured pTau181 levels in blood samples from 362 people between the ages of 58 and 70. Those samples included 56 people with an Alzheimer’s diagnosis, along with 47 people with mild cognitive impairment and 69 healthy controls.
The researchers also included another 190 people diagnosed with frontotemporal lobar degeneration (FTLD). It is a relatively rare form of dementia that leads to a gradual decline in behavior, language, and movement, often in connection with a buildup of tau in the brain.
The study found that levels of pTau181 were roughly 3.5-times higher in the blood of people with AD compared to people without AD. Those with mild cognitive impairment due to underlying AD also showed an intermediate increase in blood levels of pTau181.
Importantly, people with FLTD had normal blood levels of pTau181. As a result, the blood test could reliably distinguish between a person with AD and a person with FLTD. That’s important because, while FLTD is a relatively rare condition, its prevalence is similar to AD in people under the age of 65. But both conditions have similar symptoms, making it often challenging to distinguish them.
The findings add to evidence that the new blood test can help in diagnosing AD and in distinguishing it from other neurodegenerative conditions. In fact, it does so with an accuracy that often rivals more expensive PET scans and more invasive cerebrospinal fluid tests, which are now the only reliable ways to measure tau.
There’s still plenty of work to do before this blood test is ready for a doctor’s office. But these initial findings are very promising in helping to simplify the diagnosis of this devastating condition that now affects an estimated 5.5 million Americans [3].
References:
[1] Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, Bourakova V, Cobigo Y, Heuer H, Spina S, VandeVrede L, Chai X, Proctor NK, Airey DC, Shcherbinin S, Duggan Evans C, Sims JR, Zetterberg H, Blennow K, Karydas AM, Teunissen CE, Kramer JH, Grinberg LT, Seeley WW, Rosen H, Boeve BF, Miller BL, Rabinovici GD, Dage JL, Rojas JC, Boxer AL; Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) investigators. Nat Med. 2020 Mar 2.
[2] Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, Airey DC, Knopman DS, Roberts RO, Machulda MM, Jack CR Jr, Petersen RC, Dage JL. Alzheimers Dement. 2018 Aug;14(8):989-997.
[3] Alzheimer’s Disease Fact Sheet. National Institute on Aging, May 22, 2019.
Links:
Alzheimer’s Disease & Related Dementias (National Institute on Aging/NIH)
What Are Frontotemporal Disorders? (NIA)
Accelerating Medicines Partnership: Alzheimer’s Disease (NIH)
Adam Boxer (University of California, San Francisco)
NIH Support: National Institute on Aging; National Institute of Neurological Disorders and Stroke; National Center for Advancing Translational Sciences
Posted In: News
Tags: aging, Alzheimer's blood test, Alzheimer’s disease, blood test, brain, cognition, cognitive decline, dementia, diagnostics, Frontotemporal disorders, frontotemporal lobar degeneration, FTLD, neurology, PET scans, pTau191, senior health, tau, tauopathy
New Study Points to Targetable Protective Factor in Alzheimer’s Disease
Posted on September 10th, 2019 by Dr. Francis Collins

Credit: gettyimages/Creatista
If you’ve spent time with individuals affected with Alzheimer’s disease (AD), you might have noticed that some people lose their memory and other cognitive skills more slowly than others. Why is that? New findings indicate that at least part of the answer may lie in differences in their immune responses.
Researchers have now found that slower loss of cognitive skills in people with AD correlates with higher levels of a protein that helps immune cells clear plaque-like cellular debris from the brain [1]. The efficiency of this clean-up process in the brain can be measured via fragments of the protein that shed into the cerebrospinal fluid (CSF). This suggests that the protein, called TREM2, and the immune system as a whole, may be promising targets to help fight Alzheimer’s disease.
The findings come from an international research team led by Michael Ewers, Institute for Stroke and Dementia Research, Ludwig-Maximilians-Universität München, Germany, and Christian Haass, Ludwig-Maximilians-Universität München, Germany and German Center for Neurodegenerative Diseases. The researchers got interested in TREM2 following the discovery several years ago that people carrying rare genetic variants for the protein were two to three times more likely to develop AD late in life.
Not much was previously known about TREM2, so this finding from a genome wide association study (GWAS) was a surprise. In the brain, it turns out that TREM2 proteins are primarily made by microglia. These scavenging immune cells help to keep the brain healthy, acting as a clean-up crew that clears cellular debris, including the plaque-like amyloid-beta that is a hallmark of AD.
In subsequent studies, Haass and colleagues showed in mouse models of AD that TREM2 helps to shift microglia into high gear for clearing amyloid plaques [2]. This animal work and that of others helped to strengthen the case that TREM2 may play an important role in AD. But what did these data mean for people with this devastating condition?
There had been some hints of a connection between TREM2 and the progression of AD in humans. In the study published in Science Translational Medicine, the researchers took a deeper look by taking advantage of the NIH-funded Alzheimer’s Disease Neuroimaging Initiative (ADNI).
ADNI began more than a decade ago to develop methods for early AD detection, intervention, and treatment. The initiative makes all its data freely available to AD researchers all around the world. That allowed Ewers, Haass, and colleagues to focus their attention on 385 older ADNI participants, both with and without AD, who had been followed for an average of four years.
Their primary hypothesis was that individuals with AD and evidence of higher TREM2 levels at the outset of the study would show over the years less change in their cognitive abilities and in the volume of their hippocampus, a portion of the brain important for learning and memory. And, indeed, that’s exactly what they found.
In individuals with comparable AD, whether mild cognitive impairment or dementia, those having higher levels of a TREM2 fragment in their CSF showed a slower decline in memory. Those with evidence of a higher ratio of TREM2 relative to the tau protein in their CSF also progressed more slowly from normal cognition to early signs of AD or from mild cognitive impairment to full-blown dementia.
While it’s important to note that correlation isn’t causation, the findings suggest that treatments designed to boost TREM2 and the activation of microglia in the brain might hold promise for slowing the progression of AD in people. The challenge will be to determine when and how to target TREM2, and a great deal of research is now underway to make these discoveries.
Since its launch more than a decade ago, ADNI has made many important contributions to AD research. This new study is yet another fine example that should come as encouraging news to people with AD and their families.
References:
[1] Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer’s disease. Ewers M, Franzmeier N, Suárez-Calvet M, Morenas-Rodriguez E, Caballero MAA, Kleinberger G, Piccio L, Cruchaga C, Deming Y, Dichgans M, Trojanowski JQ, Shaw LM, Weiner MW, Haass C; Alzheimer’s Disease Neuroimaging Initiative. Sci Transl Med. 2019 Aug 28;11(507).
[2] Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Parhizkar S, Arzberger T, Brendel M, Kleinberger G, Deussing M, Focke C, Nuscher B, Xiong M, Ghasemigharagoz A, Katzmarski N, Krasemann S, Lichtenthaler SF, Müller SA, Colombo A, Monasor LS, Tahirovic S, Herms J, Willem M, Pettkus N, Butovsky O, Bartenstein P, Edbauer D, Rominger A, Ertürk A, Grathwohl SA, Neher JJ, Holtzman DM, Meyer-Luehmann M, Haass C. Nat Neurosci. 2019 Feb;22(2):191-204.
Links:
Alzheimer’s Disease and Related Dementias (National Institute on Aging/NIH)
Alzheimer’s Disease Neuroimaging Initiative (University of Southern California, Los Angeles)
Ewers Lab (University Hospital Munich, Germany)
Haass Lab (Ludwig-Maximilians-Universität München, Germany)
German Center for Neurodegenerative Diseases (Bonn)
Institute for Stroke and Dementia Research (Munich, Germany)
NIH Support: National Institute on Aging
Posted In: News
Tags: ADNI, aging, Alzheimer’s disease, Alzheimer’s Disease Neuroimaging Initiative, amyloid plaques, biomarker, brain, cognition, dementia, geriatrics, GWAS, immune system, late-onset Alzheimer's disease, memory, microglia, neurobiology, neurodegenerative disorders, tau, TREM2
Largest-Ever Alzheimer’s Gene Study Brings New Answers
Posted on March 12th, 2019 by Dr. Francis Collins
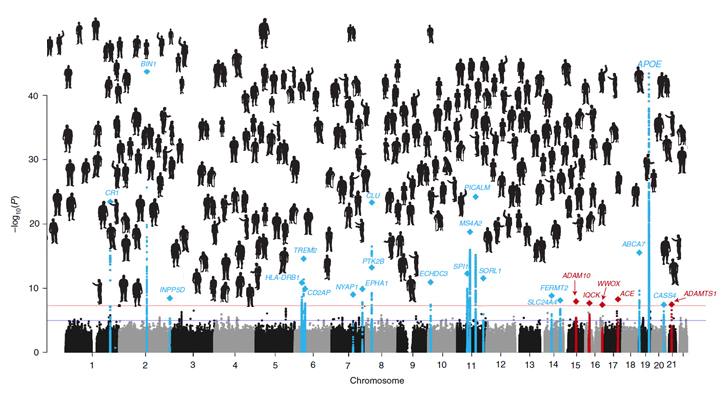
Predicting whether someone will get Alzheimer’s disease (AD) late in life, and how to use that information for prevention, has been an intense focus of biomedical research. The goal of this work is to learn not only about the genes involved in AD, but how they work together and with other complex biological, environmental, and lifestyle factors to drive this devastating neurological disease.
It’s good news to be able to report that an international team of researchers, partly funded by NIH, has made more progress in explaining the genetic component of AD. Their analysis, involving data from more than 35,000 individuals with late-onset AD, has identified variants in five new genes that put people at greater risk of AD [1]. It also points to molecular pathways involved in AD as possible avenues for prevention, and offers further confirmation of 20 other genes that had been implicated previously in AD.
The results of this largest-ever genomic study of AD suggests key roles for genes involved in the processing of beta-amyloid peptides, which form plaques in the brain recognized as an important early indicator of AD. They also offer the first evidence for a genetic link to proteins that bind tau, the protein responsible for telltale tangles in the AD brain that track closely with a person’s cognitive decline.
The new findings are the latest from the International Genomics of Alzheimer’s Project (IGAP) consortium, led by a large, collaborative team including Brian Kunkle and Margaret Pericak-Vance, University of Miami Miller School of Medicine, Miami, FL. The effort, spanning four consortia focused on AD in the United States and Europe, was launched in 2011 with the aim of discovering and mapping all the genes that contribute to AD.
An earlier IGAP study including about 25,500 people with late-onset AD identified 20 common gene variants that influence a person’s risk for developing AD late in life [2]. While that was terrific progress to be sure, the analysis also showed that those gene variants could explain only a third of the genetic component of AD. It was clear more genes with ties to AD were yet to be found.
So, in the study reported in Nature Genetics, the researchers expanded the search. While so-called genome-wide association studies (GWAS) are generally useful in identifying gene variants that turn up often in association with particular diseases or other traits, the ones that arise more rarely require much larger sample sizes to find.
To increase their odds of finding additional variants, the researchers analyzed genomic data for more than 94,000 individuals, including more than 35,000 with a diagnosis of late-onset AD and another 60,000 older people without AD. Their search led them to variants in five additional genes, named IQCK, ACE, ADAM10, ADAMTS1, and WWOX, associated with late-onset AD that hadn’t turned up in the previous study.
Further analysis of those genes supports a view of AD in which groups of genes work together to influence risk and disease progression. In addition to some genes influencing the processing of beta-amyloid peptides and accumulation of tau proteins, others appear to contribute to AD via certain aspects of the immune system and lipid metabolism.
Each of these newly discovered variants contributes only a small amount of increased risk, and therefore probably have limited value in predicting an average person’s risk of developing AD later in life. But they are invaluable when it comes to advancing our understanding of AD’s biological underpinnings and pointing the way to potentially new treatment approaches. For instance, these new data highlight intriguing similarities between early-onset and late-onset AD, suggesting that treatments developed for people with the early-onset form also might prove beneficial for people with the more common late-onset disease.
It’s worth noting that the new findings continue to suggest that the search is not yet over—many more as-yet undiscovered rare variants likely play a role in AD. The search for answers to AD and so many other complex health conditions—assisted through collaborative data sharing efforts such as this one—continues at an accelerating pace.
References:
[1] Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Kunkle BW, Grenier-Boley B, Sims R, Bis JC, et. al. Nat Genet. 2019 Mar;51(3):414-430.
[2] Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, et al. Nat Genet. 2013 Dec;45(12):1452-8.
Links:
Alzheimer’s Disease Genetics Fact Sheet (National Institute on Aging/NIH)
Genome-Wide Association Studies (NIH)
Margaret Pericak-Vance (University of Miami Health System, FL)
NIH Support: National Institute on Aging; National Heart, Lung, and Blood Institute; National Human Genome Research Institute; National Institute of Allergy and Infectious Diseases; Eunice Kennedy Shriver National Institute of Child Health and Human Development; National Institute of Diabetes and Digestive and Kidney Disease; National Institute of Neurological Disorders and Stroke
Posted In: News
Tags: AD, aging, aging brain, Alzheimer’s disease, amyloid plaques, beta amyloid, brain, dementia, early-onset Alzheimer's disease, gene variants, genes, genetics, genome-wide association studies, genomics, GWAS, IGAP, International Genomics of Alzheimer's Project, late-onset Alzheimer's disease, older people, tau
Sleep Loss Encourages Spread of Toxic Alzheimer’s Protein
Posted on February 5th, 2019 by Dr. Francis Collins

Credit: iStock/bowdenimages
In addition to memory loss and confusion, many people with Alzheimer’s disease have trouble sleeping. Now an NIH-funded team of researchers has evidence that the reverse is also true: a chronic lack of sleep may worsen the disease and its associated memory loss.
The new findings center on a protein called tau, which accumulates in abnormal tangles in the brains of people with Alzheimer’s disease. In the healthy brain, active neurons naturally release some tau during waking hours, but it normally gets cleared away during sleep. Essentially, your brain has a system for taking the garbage out while you’re off in dreamland.
The latest findings in studies of mice and people further suggest that sleep deprivation upsets this balance, allowing more tau to be released, accumulate, and spread in toxic tangles within brain areas important for memory. While more study is needed, the findings suggest that regular and substantial sleep may play an unexpectedly important role in helping to delay or slow down Alzheimer’s disease.
It’s long been recognized that Alzheimer’s disease is associated with the gradual accumulation of beta-amyloid peptides and tau proteins, which form plaques and tangles that are considered hallmarks of the disease. It has only more recently become clear that, while beta-amyloid is an early sign of the disease, tau deposits track more closely with disease progression and a person’s cognitive decline.
Such findings have raised hopes among researchers including David Holtzman, Washington University School of Medicine, St. Louis, that tau-targeting treatments might slow this devastating disease. Though much of the hope has focused on developing the right drugs, some has also focused on sleep and its nightly ability to reset the brain’s metabolic harmony.
In the new study published in Science, Holtzman’s team set out to explore whether tau levels in the brain naturally are tied to the sleep-wake cycle [1]. Earlier studies had shown that tau is released in small amounts by active neurons. But when neurons are chronically activated, more tau gets released. So, do tau levels rise when we’re awake and fall during slumber?
The Holtzman team found that they do. The researchers measured tau levels in brain fluid collected from mice during their normal waking and sleeping hours. (Since mice are nocturnal, they sleep primarily during the day.) The researchers found that tau levels in brain fluid nearly double when the animals are awake. They also found that sleep deprivation caused tau levels in brain fluid to double yet again.
These findings were especially interesting because Holtzman’s team had already made a related finding in people. The team found that healthy adults forced to pull an all-nighter had a 30 percent increase on average in levels of unhealthy beta-amyloid in their cerebrospinal fluid (CSF).
The researchers went back and reanalyzed those same human samples for tau. Sure enough, the tau levels were elevated on average by about 50 percent.
Once tau begins to accumulate in brain tissue, the protein can spread from one brain area to the next along neural connections. So, Holtzman’s team wondered whether a lack of sleep over longer periods also might encourage tau to spread.
To find out, mice engineered to produce human tau fibrils in their brains were made to stay up longer than usual and get less quality sleep over several weeks. Those studies showed that, while less sleep didn’t change the original deposition of tau in the brain, it did lead to a significant increase in tau’s spread. Intriguingly, tau tangles in the animals appeared in the same brain areas affected in people with Alzheimer’s disease.
Another report by Holtzman’s team appearing early last month in Science Translational Medicine found yet another link between tau and poor sleep. That study showed that older people who had more tau tangles in their brains by PET scanning had less slow-wave, deep sleep [2].
Together, these new findings suggest that Alzheimer’s disease and sleep loss are even more intimately intertwined than had been realized. The findings suggest that good sleep habits and/or treatments designed to encourage plenty of high quality Zzzz’s might play an important role in slowing Alzheimer’s disease. On the other hand, poor sleep also might worsen the condition and serve as an early warning sign of Alzheimer’s.
For now, the findings come as an important reminder that all of us should do our best to get a good night’s rest on a regular basis. Sleep deprivation really isn’t a good way to deal with overly busy lives (I’m talking to myself here). It isn’t yet clear if better sleep habits will prevent or delay Alzheimer’s disease, but it surely can’t hurt.
References:
[1] The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M, Geerling JC, Fuller PM, Lucey BP, Holtzman DM. Science. 2019 Jan 24.
[2] Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Lucey BP, McCullough A, Landsness EC, Toedebusch CD, McLeland JS, Zaza AM, Fagan AM, McCue L, Xiong C, Morris JC, Benzinger TLS, Holtzman DM. Sci Transl Med. 2019 Jan 9;11(474).
Links:
Alzheimer’s Disease and Related Dementias (National Institute on Aging/NIH)
Accelerating Medicines Partnership: Alzheimer’s Disease (NIH)
Holtzman Lab (Washington University School of Medicine, St. Louis)
NIH Support: National Institute on Aging; National Institute of Neurological Disorders and Stroke; National Center for Advancing Translational Sciences; National Cancer Institute; National Institute of Biomedical Imaging and Bioengineering
Posted In: News
Tags: aging, Alzheimer's, Alzheimer’s disease, beta amyloid, brain, cognition, dementia, memory, sleep, sleep loss, sleep-wake cycle, tau, tau protein
Antibody Makes Alzheimer’s Protein Detectable in Blood
Posted on May 2nd, 2017 by Dr. Francis Collins
Caption: The protein tau (green) aggregates abnormally in a brain cell (blue). Tau spills out of the cell and enters the bloodstream (red). Research shows that antibodies (blue) can capture tau in the blood that reflect its levels in the brain.
Credit: Sara Moser
Age can bring moments of forgetfulness. It can also bring concern that the forgetfulness might be a sign of early Alzheimer’s disease. For those who decide to have it checked out, doctors are likely to administer brief memory exams to assess the situation, and medical tests to search for causes of memory loss. Brain imaging and spinal taps can also help to look for signs of the disease. But an absolutely definitive diagnosis of Alzheimer’s disease is only possible today by examining a person’s brain postmortem. A need exists for a simple, less-invasive test to diagnose Alzheimer’s disease and similar neurodegenerative conditions in living people, perhaps even before memory loss becomes obvious.
One answer may lie in a protein called tau, which accumulates in abnormal tangles in the brains of people with Alzheimer’s disease and other “tauopathy” disorders. In recent years, researchers have been busy designing an antibody to target tau in hopes that this immunotherapy approach might slow or even reverse Alzheimer’s devastating symptoms, with promising early results in mice [1, 2]. Now, an NIH-funded research team that developed one such antibody have found it might also open the door to a simple blood test [3].
Tags: Accelerating Medicines Partnership, aging brain, Alzheimer's, Alzheimer's blood test, Alzheimer’s disease, AMP-AD, AMP-AD Biomarkers Project, antibody, blood test, brain, chronic traumatic encephalopathy, CTE, immunotherapy, neurodegenerative disorders, progressive supranuclear palsy, tau, tau imaging, tau protein, tau tangle, tauopathy
Alzheimer’s Disease: Tau Protein Predicts Early Memory Loss
Posted on May 24th, 2016 by Dr. Francis Collins
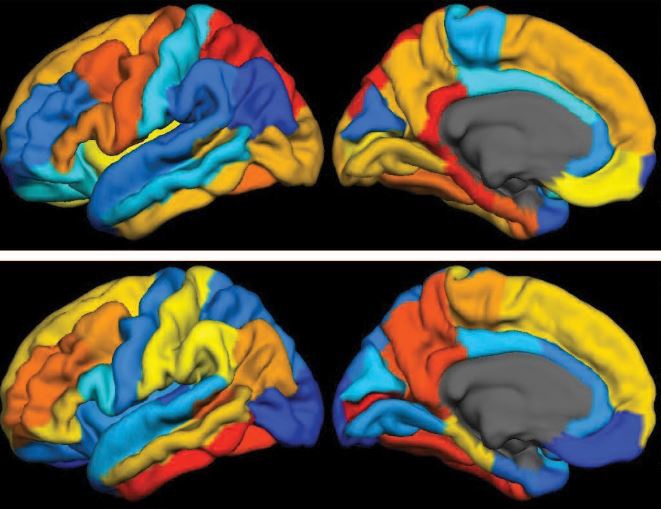
Caption: PET scan images show distribution of tau (top panel) and beta-amyloid (bottom panel) across a brain with early Alzheimer’s disease. Red indicates highest levels of protein binding, dark blue the lowest, yellows and oranges indicate moderate binding.
Credit: Brier et al., Sci Transl Med
In people with Alzheimer’s disease, changes in the brain begin many years before the first sign of memory problems. Those changes include the gradual accumulation of beta-amyloid peptides and tau proteins, which form plaques and tangles that are considered hallmarks of the disease. While amyloid plaques have received much attention as an early indicator of disease, until very recently there hadn’t been any way during life to measure the buildup of tau protein in the brain. As a result, much less is known about the timing and distribution of tau tangles and its relationship to memory loss.
Now, in a study published in Science Translational Medicine, an NIH-supported research team has produced some of the first maps showing where tau proteins build up in the brains of people with early Alzheimer’s disease [1]. The new findings suggest that while beta-amyloid remains a reliable early sign of Alzheimer’s disease, tau may be a more informative predictor of a person’s cognitive decline and potential response to treatment.
Tags: Accelerating Medicines Partnership, age-related memory loss, aging, Alzheimer’s disease, AMP, AMP-AD, ß-amyloid, beta amyloid, brain, brain scan, cerebral cortex, cognitive decline, dementia, early Alzheimer's disease, frontal lobe, imaging, memory, memory loss, neurological disease, neurology, parietal lobe, PET scans, tau, tau protein, temporal lobe, translational medicine
Creative Minds: Of Arsenic and Misfolded Proteins
Posted on October 29th, 2015 by Dr. Francis Collins
John Hanna
Taking out the trash is a must in every household. Inside our cells, it’s also essential because if defective proteins are not properly disposed of, they can accumulate and make a mess of the cell’s inner workings, leading to health problems.
John Hanna, a physician-scientist at Brigham and Women’s Hospital, Boston, is on a quest to study the cell’s trash disposal system in greater detail. In particular, this 2014 NIH Director’s Early Independence awardee wants to learn more about how cells identify proteins that need to be discarded, how such proteins are steered towards the molecular garbage can, and how, when the process breaks down, neurodegenerative conditions, cancers, and other diseases can arise.
That’s a complex challenge, so Hanna will start by zeroing in on one particular component of cellular waste management—the component that clears out proteins damaged by arsenic. Although arsenic is notorious for being the poison of choice in countless true crime shows and mystery novels, this semi-metallic element is found naturally in soil, water, air, and some foods.
Tags: Alzheimer’s disease, arsenic, arsenic poisoning, basic research, biotech, Cuz1, Early Independence Award, metalloid, misfolded proteins, neurodegenerative disorders, proteasome, protein degradation, protein folding, Saccharomyces cerevisiae, tau, Tmc1, toxins, ubiquitin, yeast


