Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials (original) (raw)
The agreement for study selection between the two reviewers was good (κ coefficient = 0.86). We retrieved 78 RCTs (47 with a low risk of bias), variably reporting post-treatment changes in liver-related, glucose and cardiovascular variables (Table 1; ESM Fig. 1; ESM Tables 1-5).
Table 1 Items related to liver disease, glucose metabolism and cardiovascular risk and the percentage of RCTs assessing their post-treatment changes (total: 78 RCTs included)
Weight loss
Eight RCTs (373 participants, 39% diabetic; six RCTs with a low risk of bias, four RCTs with post-treatment histology) assessed the effect of lifestyle- or drug-induced weight loss in NAFLD [6–13] (ESM Table 1).
Liver disease
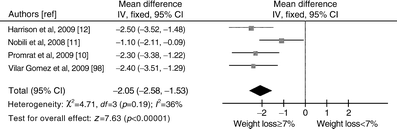
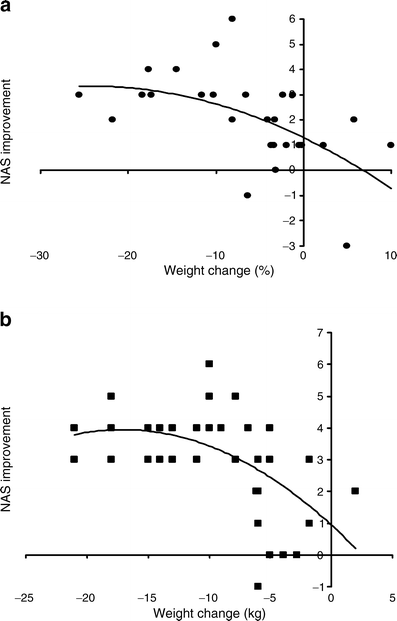
Although a ≥5% weight loss improved hepatic steatosis, a ≥7% weight loss also improved NAS (Fig. 1); fibrosis was unchanged (not shown). The threshold of 7% weight loss was achieved by <50% of patients, even with intensive multidisciplinary lifestyle intervention [8, 10]. Two RCTs suggested no additional NAS improvement with >10% weight loss, but the existence of a lower and an upper threshold weight loss for improving histological disease activity needs further confirmation (Fig. 2).
Fig. 1
Forest plot of RCTs comparing the effect of different degrees of weight loss (%) on histological NAS. Outcome: mean differences in NAS following weight loss ≥7% vs weight loss <7%. IV, inverse variance
Fig. 2
Impact of different degrees of weight loss on histological NAS in two RCTs (adapted from (a) Promrat et al [10] and (b) Vilar Gomez et al [98])
There was no significant publication bias (ESM Fig. 2).
Glucose metabolism and cardiovascular risk
Weight loss substantially improved HOMA, FPG, glucose tolerance and plasma lipids (ESM Table 1). Two RCTs also showed an improvement in plasma adiponectin [8, 12]. Among drugs inducing weight loss, orlistat was safe, well-tolerated with minor adverse gastrointestinal complaints not requiring discontinuation of therapy, but conferred no additional cardio-metabolic or histological benefit over lifestyle intervention alone [7, 12]. There was no significant publication bias for assessed outcomes (not reported).
Long-term durability of achieved benefits and safety of weight loss are unknown.
Physical exercise alone
Reduced aerobic exercise has been linked to the presence and severity of cardio-metabolic and liver disease in NAFLD through several potential mechanisms: reduced hepatic and muscle adenosine monophosphate-activated protein kinase (AMPK)-mediated NEFA oxidation, increased postprandial hepatic lipogenesis, visceral fat-derived NEFA and proinflammatory adipokine overflow to the liver [14–17].
Five RCTs (four RCTs with a low risk of bias) evaluated the effects of 3–6 months of moderate-intensity aerobic exercise alone in NAFLD [13, 18–21] (ESM Table 1).
Liver disease
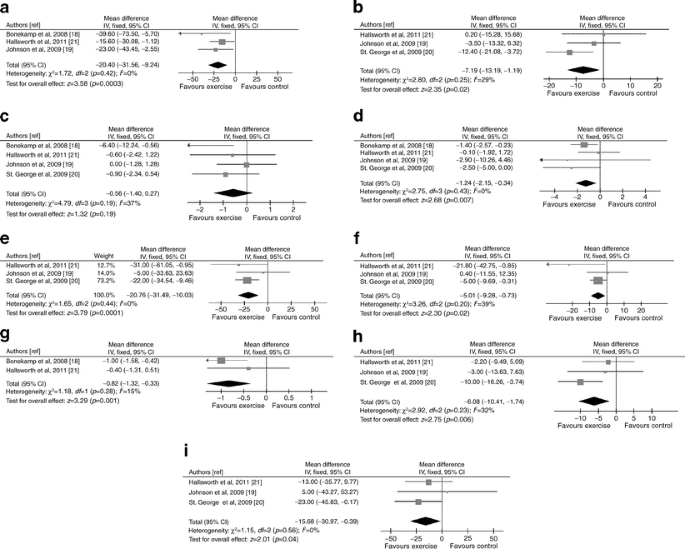
Exercise improved MRS-assessed steatosis and ALT levels (Fig. 3). In the only RCT with post-treatment histology, NAS was unchanged [13]. There was no significant publication bias (ESM Fig. 2)
Fig. 3
Forest plots of RCTs comparing the effect of physical exercise alone on liver disease, glucose metabolism and cardiovascular risk. (a) NMR-assessed liver fat change (%). (b) ALT change (IU/l). (c) Body weight change (%). (d) Waist circumference change (%). (e) HOMA index change (%). (f) FPG change (%). (g) HbAlc change (%). (h) Plasma LDL-cholesterol change (%). (i) Plasma TG change (%).To convert values for HbA1c in % into mmol/mol, subtract 2.15 and multiply by 10.929. IV, inverse variance
Glucose metabolism and cardiovascular risk
Despite no significant body weight changes, exercise improved waist circumference, HOMA, FPG, HbA1c, LDL-cholesterol and triacylglycerol (TG) (Fig. 3). One RCT reported no effect of physical exercise on HDL-cholesterol [20]. No data on inflammatory markers/adipokines are available. There was no significant publication bias for assessed outcomes (not reported).
An analysis of the reasons for dropping out of exercise-based treatments found that NAFLD patients understand the benefits of exercise but lack confidence to perform it, and are afraid of falling, suggesting that these modifiable factors should be targeted to improve compliance to exercise of these patients [22].
Dietary composition manipulation
The optimal nutrient dietary composition for NAFLD is unknown. Three RCTs compared the effect of low-carbohydrate versus low-fat caloric restriction [23–25] (ESM Table 1).
Liver disease
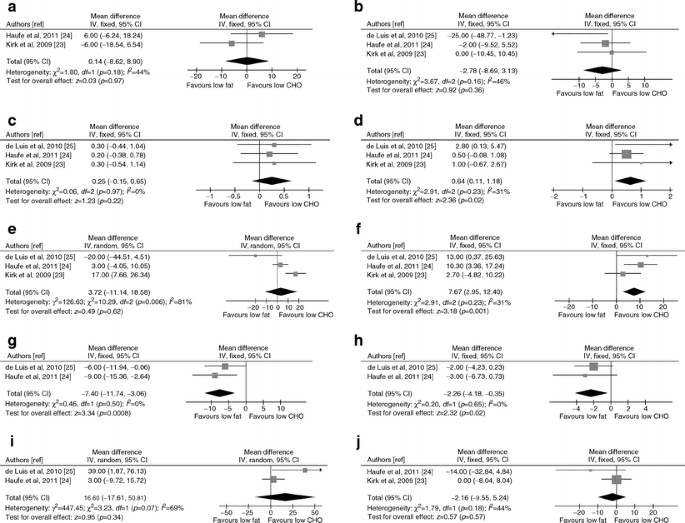
The two regimens yielded similar liver fat and ALT reduction (Fig. 4).
Fig. 4
Forest plots of RCTs comparing the effect of low fat versus low carbohydrate (CHO) dietary caloric restriction on liver disease, glucose metabolism and cardiovascular risk. (a) NMR-assessed liver fat change (%). (b) ALT change (IU/l). (c) Body weight change (%). (d) Waist circumference change (%). (e) HOMA index change (%). (f) FPG change (%). (g) Plasma LDL-cholesterol change (%). (h) Plasma HDL-cholesterol change (%) (i) Plasma TG change (%). (j) Serum adiponectin change (%). IV, inverse variance
Glucose metabolism and cardiovascular risk
The two regimens yielded similar weight loss and improved HOMA, pancreatic beta cell function [24], TG, blood pressure [25], CRP [24] and adiponectin to a similar extent (Fig. 4). For TG and HOMA heterogeneity was high, being explained by the different baseline features of study populations: low-carbohydrate diet significantly improved plasma TG and HOMA index when hypertriacylglycerolaemic [25] or glucose-intolerant [23] NAFLD patients, respectively, were enrolled. Furthermore, in glucose-intolerant NAFLD individuals, low-carbohydrate caloric restriction significantly improved hepatic insulin sensitivity compared with low-fat diet [23].
Low-carbohydrate diet significantly reduced waist circumference and FPG compared with low-fat diet, which in turn improved LDL-C and HDL-C more consistently than the low-carbohydrate diet (Fig. 4).
These studies suggest that caloric restriction is the most important goal for improving hepatic steatosis, but a different nutrient composition may carry additional benefits according to individual patient features.
Insulin-sensitisers: thiazolidinediones
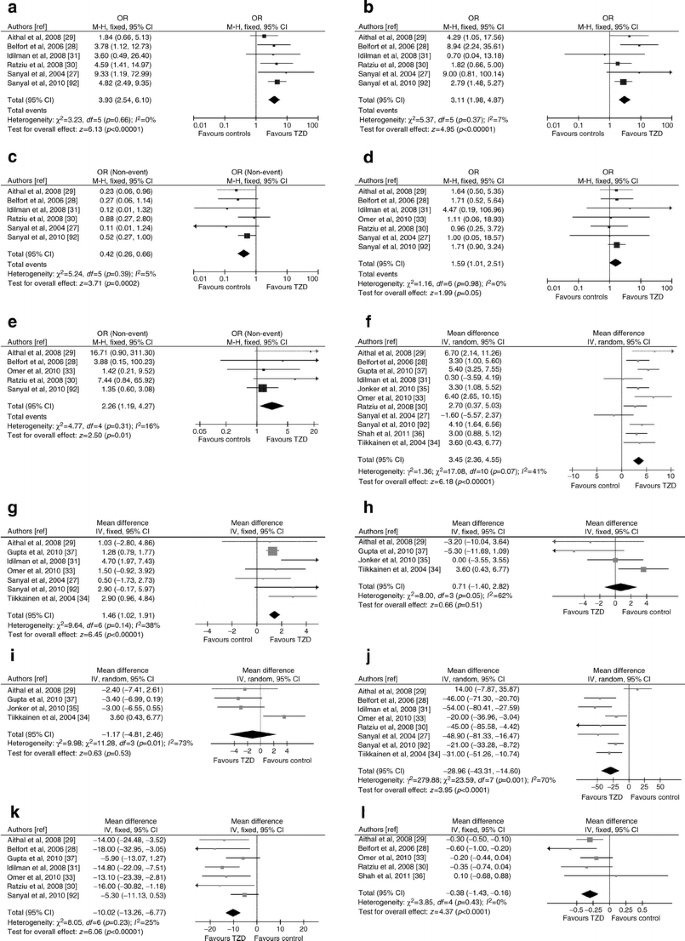
Thiazolidinediones (TZDs) were evaluated in 11 RCTs (862 participants, 38% diabetic; seven RCTs with low risk of bias) [26–37] (ESM Table 2).
Liver disease
Pooled results of seven RCTs with post-treatment histology showed that TZDs improved steatosis, hepatocellular ballooning and inflammation but not fibrosis; however, when considering patients with improved or stable fibrosis stage versus those with worsening fibrosis stage, TZDs significantly reduced the risk of fibrosis progression (Fig. 5). Heterogeneity was low for all assessed outcomes, suggesting a consistent drug effect size across studies. There was no significant publication bias (ESM Fig. 2)
Fig. 5
Forest plots of RCTs comparing the effect of thiazolidinedione on liver disease, glucose metabolism and cardiovascular risk. (a) Improvement in histological steatosis in NASH. (b) Improvement in lobular inflammation in NASH. (c) Improvement in hepatocellular ballooning in NASH. (d) Improvement in fibrosis in NASH. (e) Improvement or stability in fibrosis in NASH. (f) Body weight change (%). (g) Waist circumference change (%). (h) Systolic BP changes (mmHg). (i) Diastolic BP changes (mmHg). (j) HOMA index change (%). (k) FPG change (%). (l) HbAlc change (%). (m) Plasma LDL-cholesterol change (%). (n) Plasma HDL-cholesterol change (%) (o) Plasma TG change (%). (p) Serum C-reactive protein change (mg/l). (q) Serum adiponectin change (%). To convert values for HbA1c in % into mmol/mol, subtract 2.15 and multiply by 10.929. M-H, Mantel–Haenszel
Presence/absence of diabetes, the implementation of lifestyle intervention, different drug, dose or trial duration and risk of bias did not affect outcomes.
Glucose metabolism and cardiovascular risk
TZDs improved HOMA, FPG, HbA1c, HDL-C, TG, CRP and adiponectin, but had no effect on LDL-C and BP (Fig. 5). TZDs improved also hepatic, muscle and adipose tissue insulin resistance [26, 34, 37]. There was no significant publication bias for assessed outcomes (not reported).
For some outcomes heterogeneity was high: for LDL-C, heterogeneity was abated after excluding one RCT [30], showing unexpected LDL-C increase with rosiglitazone (weighed mean difference [WMD] 1.13, 95% CI −2.40, 4.66, p = 0.53, I2 = 34%, n comparisons = 5). For HOMA, heterogeneity was abated after excluding one RCT [29], showing unexpected HOMA increase with pioglitazone (WMD −33%, 95% CI −44%, −22%, p = 0.00001, I2 = 40%, n comparisons = 7).
For BP, after excluding the only RCT using rosiglitazone [34], the remaining trials showed no change in systolic BP (WMD −1.5%, 95% CI −4.4%, −1.2%, p = 0.27, I2 = 12%, n comparisons = 3) or a reduction in diastolic BP (WMD −3.3%, 95% CI −5.5%, −1.0%, p = 0.005, I2 = 0%, n comparisons = 3) with pioglitazone.
For adiponectin, heterogeneity was abated after excluding two RCTs using a lower dose of pioglitazone [29] or did not vigorously implement lifestyle intervention [30] (WMD 118%, 95% CI 82, 155, p = 0.00001, I 2 = 0%, n comparisons = 3).
Weight gain (mean 2%, range 0–4.8%) occurred in up to 75% of patients, accompanied by an increased in waist circumference, and was a common cause of dropout, together with ankle oedema (4–25%). Weight gain did not reverse with treatment discontinuation and was not prevented by lifestyle intervention, but was reduced by metformin coadministration [33, [38](/article/10.1007/s00125-011-2446-4#ref-CR38 "Torres D, Jones F, Shaw J, Williams C, Ward J, Harrison S (2011) Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis (NASH): a 12 month-randomized, prospective, open- label trial. Hepatology. doi: 10.1002/hep.24558
")\]. Besides limiting weight gain, the combination of rosiglitazone + metformin offered no significant histological or cardio-metabolic benefit over rosiglitazone alone \[[33](/article/10.1007/s00125-011-2446-4#ref-CR33 "Omer Z, Cetinkalp S, Akyildiz M et al (2010) Efficacy of insulin-sensitizing agents in nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 22:18–23"), [38](/article/10.1007/s00125-011-2446-4#ref-CR38 "Torres D, Jones F, Shaw J, Williams C, Ward J, Harrison S (2011) Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis (NASH): a 12 month-randomized, prospective, open- label trial. Hepatology. doi:
10.1002/hep.24558
")\].NASH and associated cardio-metabolic abnormalities relapsed 1 year after discontinuing TZDs [[38](/article/10.1007/s00125-011-2446-4#ref-CR38 "Torres D, Jones F, Shaw J, Williams C, Ward J, Harrison S (2011) Rosiglitazone versus rosiglitazone and metformin versus rosiglitazone and losartan in the treatment of nonalcoholic steatohepatitis (NASH): a 12 month-randomized, prospective, open- label trial. Hepatology. doi: 10.1002/hep.24558
")\], posing the issue of the required treatment duration and of the benefit/safety of sustained thiazolidinedione treatment. In the Pioglitazone Versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with NASH (PIVENS) and the Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT)-2 trial, liver histology did not improve further despite continued HOMA and transaminase improvement over 2 and 3 years, respectively \[[32](/article/10.1007/s00125-011-2446-4#ref-CR32 "Sanyal AJ, Chalasani N, Kowdley KV et al (2010) Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 362:1675–1685"), [39](/article/10.1007/s00125-011-2446-4#ref-CR39 "Lutchamn G, Modi A, Kleiner DE et al (2007) The effects of discontinuing pioglitazone in patients with non-alcoholic steatohepatitis. Hepatology 46:424–429")\]. These two trials suggest that prolonged treatment with TZDs may offer no additional histological benefit and that metabolic improvement does not necessarily parallel histological improvement.Due to the short trial duration, no cases of congestive heart failure, bone fractures or CVD events were reported. Concern about cardiovascular safety led the European Medicines Agency to recommend withdrawal of rosiglitazone from clinical use.
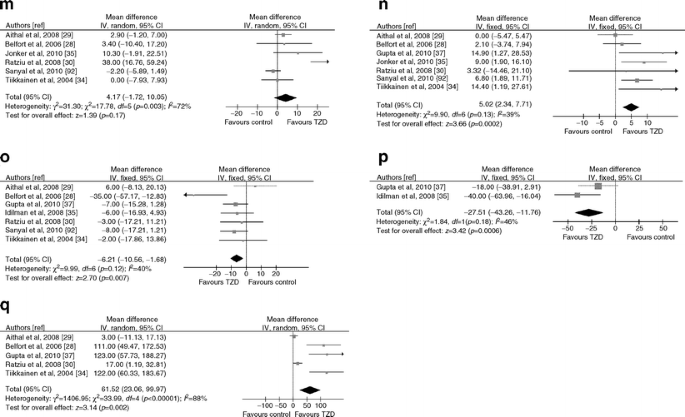
Insulin-sensitisers: metformin
Metformin has anorexigenic and weight-loss properties, decreases gastrointestinal glucose absorption and increases insulin sensitivity by increasing glucose uptake and AMP-kinase-mediated oxidative glucose and lipid metabolism [40].
Eleven RCTs (671 participants, 27% diabetic; six RCTs in NASH with post-treatment histology, three with a low bias risk) evaluated metformin [33, 34, 41–49] (ESM Table 2).
Liver disease
Metformin did not improve liver histology compared with placebo (Fig. 6). Dose, treatment duration (ranging from 6 to 24 months) or diabetic state had no effect on post-treatment histology. There was no significant publication bias (ESM Fig. 2)
Fig. 6
Forest plots of RCTs comparing the effect of metformin on liver disease, glucose metabolism and cardiovascular risk. (a) Improvement in histological steatosis in NASH. (b) Improvement in lobular inflammation in NASH. (c) Improvement in hepatocellular ballooning in NASH. (d) Improvement in fibrosis in NASH. (e) Body weight change (%). (f) Waist circumference change (%). (g) HOMA index change (%). (h) FPG change (%). (i) HbAlc change (%). (j) Plasma LDL-cholesterol change (%). (k) Plasma HDL-cholesterol change (%) (l) Plasma TG change (%). (m) Serum C-reactive protein change (mg/l). (n) Serum adiponectin change (%). To convert values for HbA1c in % into mmol/mol, subtract 2.15 and multiply by 10.929. M-H, Mantel–Haenszel
Glucose metabolism and cardiovascular risk
Metformin significantly reduced body weight, waist circumference, HOMA, FPG, and HbA1c, and increased HDL-C and adiponectin, but had no effect on LDL-C, TG, blood pressure [50] and CRP (Fig. 2). There was no significant publication bias for assessed outcomes (not reported).
Heterogeneity of results for HOMA was abated after excluding trials not effectively implementing lifestyle intervention (as suggested by absence of weight loss in the controls) [43, 46, 49] (WMD −21%, 95% CI −31, −11, p = 0.0001, I 2 = 40%, n comparisons = 7), suggesting that the insulin-sensitising effects of metformin are potentiated when lifestyle intervention is effectively implemented.
Metformin was safe and well-tolerated: gastrointestinal intolerance was the most common adverse effect, not requiring treatment discontinuation.
Lipid-lowering drugs
Statins
The hepatological safety of statins in NAFLD has been recently recognised and their feared potential for worsening glucose tolerance seems largely outweighed by their cardiovascular benefit [50, 51].
Four RCTs (719 participants, three with a low bias risk) assessed statins in NAFLD [52–55] (ESM Table 3).
Liver disease
Statins improved ALT (ESM Fig. 3) and radiological steatosis [53, 54] in hyperlipidaemic NAFLD patients; in the only RCT with post-treatment histology, simvastatin had no effect on liver histology [56]. There was no significant publication bias (ESM Fig. 2)
Glucose metabolism and cardiovascular risk
Statins improve LDL-C, HDL-C and TG without affecting body weight (ESM Fig. 3). One RCT found no effect of statins on waist circumference, BP, FPG and CRP [53]. There was no significant publication bias for assessed outcomes (not reported).
In a post hoc analysis of the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) RCT, stain-treated hyperlipidaemic NAFLD patients experienced a significant (−68%) risk reduction of CVD events compared with both presumed NAFLD patients not on statin and with statin-treated patients with normal transaminases, without significant adverse events, including new-onset diabetes [55]. Importantly, this study demonstrates that statins are safe in NAFLD and that statin-related cardiovascular benefit is greater in patients with NAFLD than in those with normal liver tests.
Ezetimibe
Growing evidence connects non-esterified cholesterol accumulation in mitochondria to hepatocyte apoptosis, liver injury and NASH development [56–61]. On this basis, ezetimibe, a Niemann-Pick C1 like 1 protein inhibitor, was evaluated in two RCTs in NAFLD.
Liver disease
Ezetimibe reduced histological ballooning and fibrosis in one RCT, and MR-assessed liver fat in the other [62, 63] (ESM Table 3).
Glucose metabolism and cardiovascular risk
Ezetimibe improved LDL-C and CRP, without affecting weight, waist and HOMA. In one RCT, ezetimibe treatment was associated with significant HbA1c increase compared with placebo [64].
n-3 polyunsaturated fatty acids
Five RCTs (303 participants, two RCTs with low risk of bias) evaluated polyunsaturated fatty acids (PUFA) [64–68] (ESM Table 3).
Liver disease
PUFA improved ALT (ESM Fig. 4) and steatosis by NMR or ultrasound [65–68]. In the only RCT with post-treatment histology, PUFA ameliorated steatosis without affecting other histological features [68]. There was no significant publication bias (ESM Fig. 2).
Glucose metabolism and cardiovascular risk
PUFA ameliorated HOMA, HDL-C and TG, but had no effect on body weight, BP and LDL-C (ESM Fig. 4). One RCT found no effect of PUFA on waist circumference and CRP [68]. There was no significant publication bias for assessed outcomes (not reported).
Overall, PUFA were well-tolerated, with minor gastrointestinal symptoms.
Probucol
Probucol, a lipophilic lipid-lowering agent with strong antioxidant activity, was evaluated in NASH in one RCT: ALT improved, but post-treatment histology was unavailable [69] (ESM Table 3). Although generally well-tolerated, probucol was associated with a significant fall in HDL-C.
Fibrates
Following consistent anti-steatogenic activity in animal models of NAFLD [70], fibrates were evaluated in five RCTs (315 participants, four RCTs with a low risk of bias) [53, 71–74] (ESM Table 3).
Liver disease
Fibrates had no effect on ALT (ESM Fig. 5), radiological steatosis [75] or liver histology [73]. There was no significant publication bias (ESM Fig. 2).
Glucose metabolism and cardiovascular risk
Fibrates improved HDL-C and TG, had no effect on body weight, waist, HOMA, FPG and LDL-C (ESM Fig. 5). One RCT showed no effect of fibrates on BP and adiponectin [53]. There was no significant publication bias for assessed outcomes (not reported).
Angiotensin receptor blockers
The modulation of insulin sensitivity, systemic inflammation, hepatic lipogenesis and fibrogenesis by the renin-angiotensin system and the frequent coexistence of hypertension prompted evaluation of angiotensin receptor blockers in NAFLD. In a well-designed RCT on hypertensive NASH, telmisartan (an angiotensin receptor blocker with peroxisome proliferator activated receptor [PPAR]-γ-regulating activity) improved steatosis, necroinflammation, fibrosis, HOMA, TG and total cholesterol more consistently than valsartan, despite similar BP reduction, suggesting that the peculiar PPAR-γ-agonist activity may mediate the more consistent metabolic and histological benefits of telmisartan [75] (ESM Table 4).
In another RCT on hypertensive NAFLD patients, losartan plus simvastatin significantly improved ultrasonographic steatosis, visceral adiposity, HOMA and CRP compared with amlodipine plus simvastatin, despite similar BP reduction [76] (ESM Table 4).
Endocannabinoid receptor antagonists
The cannabinoid type 1 receptor (CB1) receptor antagonist rimonabant experimentally antagonised appetite, caloric intake, hepatic lipogenesis and fibrogenesis and was evaluated in abdominally obese, dyslipidaemic NAFLD patients from the ADAGIO-Lipids trial [[77](/article/10.1007/s00125-011-2446-4#ref-CR77 "Fogari R, Mugellini A, Zoppi A et al (2011) Losartan alone or in combination with simvastatin improved visceral adipose tissue and inflammation in hypertensive normocholesterolemic patients with nonalcoholic hepatic steatosis. Eur J Gastroenterol Hepatol. doi: 10.1097/MEG.0b013e32834ba188
")\]: rimonabant reversed CT-assessed steatosis in 48% of patients versus 19% on placebo (_p_ \= 0.03) and extensively improved all cardio-metabolic variables (ESM Table [4](/article/10.1007/s00125-011-2446-4#MOESM5)). Depressive and anxiety disorders were more common with rimonabant (≅2.0% vs 1% with placebo). Concern about psychiatric adverse effects led to withdrawal of rimonabant, but the development of peripherally acting CB1 antagonists is an area of intense research.Anti-TNF-α agents (pentoxifylline)
The anti-TNF-α agent pentoxifylline has been evaluated in four RCTs in NASH [78–81] (three with low risk of bias, two assessing post-treatment histology) (ESM Table 4).
Liver disease
Pooled data analysis showed that pentoxifylline improved histological steatosis and lobular inflammation (ESM Fig. 6). There was no significant publication bias (ESM Fig. 2).
Glucose metabolism and cardiovascular risk
Pentoxifylline had no effect on body weight and HOMA (ESM Fig. 6). One RCT found no impact on plasma LDL-C, HDL-C and TG [80]. There was no significant publication bias for assessed outcomes (not reported).
Overall, pentoxifylline was safe and well-tolerated with minor gastrointestinal symptoms.
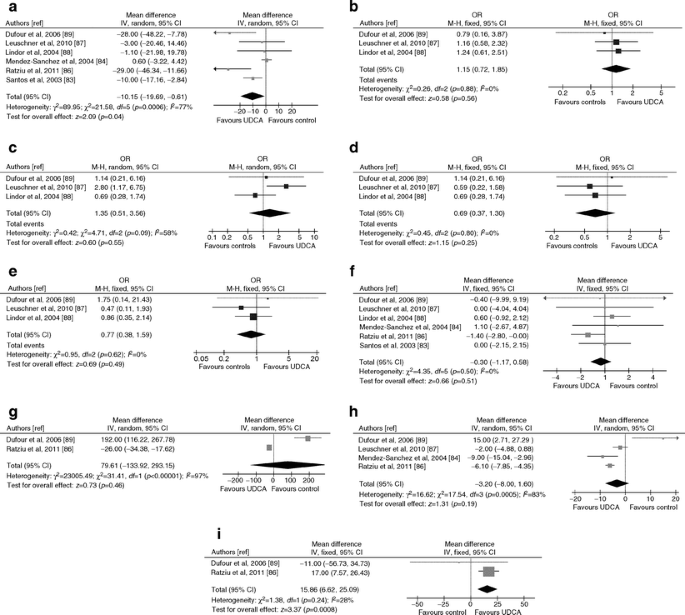
Ursodeoxycholic acid (UDCA)
Seven RCTs (639 participants, 21% diabetic; three RCTs with post-treatment histology, five RCTs with a low risk of bias) evaluated UDCA in NAFLD (ESM Table 5) [[82](/article/10.1007/s00125-011-2446-4#ref-CR82 "Zein CO, Yerian LM, Gogate P et al (2011) Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. doi: 10.1002/hep.24544
")–[88](/article/10.1007/s00125-011-2446-4#ref-CR88 "Lindor KD, Kowdley KV, Heathcote EJ et al (2004) Ursodeoxycholic acid for the treatment of non-alcoholic steatohepatitis: results of a randomized trial. Hepatology 39:770–778")\].Liver disease
Overall, UDCA improved ALT but not liver histology (Fig. 7). For ALT and for lobular inflammation, heterogeneity was high and was abated when considering RCT evaluating high-dose (twofold higher than the conventional dose) UDCA or UDCA + vitamin E, showing a modest benefit: for ALT WMD −20 IU/l, 95% CI −37, −3, p = 0.02, I2 = 40%, n comparisons = 3; for lobular inflammation OR 2.3; 95% CI 1.1, 5.0; p = 0.03, I 2 = 0%, n comparisons = 2). There was no significant publication bias (ESM Fig. 2).
Fig. 7
Forest plots of RCTs comparing the effect of UDCA on liver disease, glucose metabolism and cardiovascular risk. (a) Improvement in serum ALT (IU/l). (b) Improvement in histological steatosis in NASH. (c) Improvement in lobular inflammation in NASH. (d) Improvement in hepatocellular ballooning in NASH. (e) Improvement in fibrosis in NASH. (f) Body weight change (%). (g) HOMA index change (%). (h) FPG change (%). (i) Serum adiponectin change (%). M-H, Mantel–Haenszel
Glucose metabolism and cardiovascular risk
UDCA improved adiponectin (Fig. 7). For HOMA and FPG heterogeneity was abated after excluding one RCT evaluating the combination of UDCA + vitamin E, the latter potentially worsening HOMA (see below), showing a consistent benefit with UDCA on both HOMA and FPG (for FPG: WMD −6%, 95% CI −9, −2, p = 0.0005, I 2 = 40%, n comparisons = 3).
One RCT reported significant improvement in HbA1c and HDL-C with high-dose UDCA [85]. There was no significant publication bias for assessed outcomes (not reported).
Minor gastrointestinal effects occurred in >40% of patients on high-dose UDCA.
Semi-synthetic bile acids
Besides their role in nutrient absorption, bile acids are signalling molecules that exert genomic and non-genomic effects by activating TGR5 and farnesoid X receptor (FXR).
TGR5 is a G-protein-coupled receptor (expressed in brown adipose tissue muscle and gut), activation of which by bile acids increases energy expenditure and glucagon-like peptide-1 (GLP-1) secretion and attenuates diet-induced obesity [89, 90].
FXR is a nuclear hormone receptor expressed in the liver, intestine and kidney. In the liver, FXR controls lipogenesis, very-LDL metabolism, gluconeogenesis, glycogen synthesis and insulin sensitivity, and also has also anti-inflammatory and anti-fibrotic properties [90].
On this basis, a novel class of semi-synthetic bile acid agonists of TGR5/FXR is being evaluated for the treatment of obesity-related disorders, including NAFLD.
In the first RCT, Int-747, a semi-synthetic derivative of the human bile acid CDCA, administered for 6 weeks to diabetic NAFLD patients, was well-tolerated, ameliorated markers of liver fibrosis, insulin resistance and induced weight loss compared with placebo (ESM Table 4) [91]. The ongoing FXR Ligand NASH Treatment (FLINT) double blind, placebo controlled, multicentre trial is evaluating the effects of obeticholic acid in NASH.
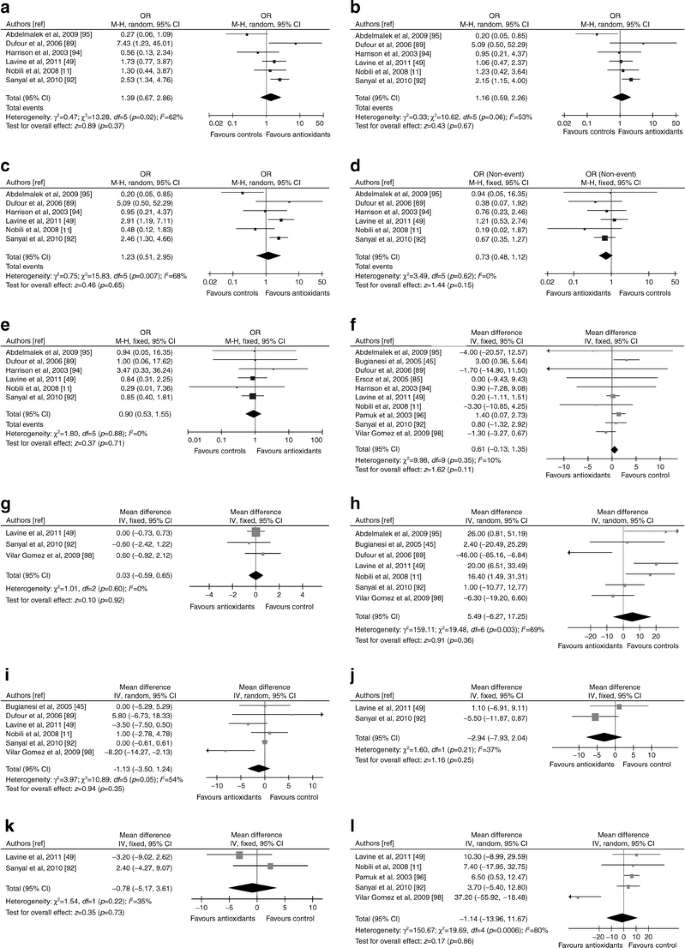
Antioxidants
Fifteen RCTs (1,141 participants, 9% diabetic, seven RCTs with low risk of bias) evaluated antioxidants in NAFLD: overall, heterogeneity in study population, duration, type and dose of drug, lifestyle intervention implementation, was considerable [6, 32, 49, 92–99] (ESM Table 5).
Liver disease
Pooled results of the seven RCTs (685 patients, 4% diabetic) with post-treatment histology showed no histological improvement and high heterogeneity (Fig. 8). Heterogeneity was reduced when considering only the five RCTs with vitamin E, showing modest improvement in steatosis (OR 1.83; 95% CI 1.03, 3.25; I 2 = 35%, p = 0.04) and lobular inflammation (OR 1.57; 95% CI 1.03, 2.39; I 2 = 0%, p = 0.04). One RCT reported also an improvement in NAS score with Viusid [98]. Antioxidants as a group or vitamin E did not slow fibrosis progression (Fig. 8).
Fig. 8
Forest plots of RCTs comparing the effect of antioxidants on liver disease, glucose metabolism and cardiovascular risk. (a) Improvement in histological steatosis in NASH. (b) Improvement in lobular inflammation in NASH. (c) Improvement in hepatocellular ballooning in NASH. (d) Improvement in fibrosis in NASH. (e) Improvement or stability in fibrosis in NASH. (f) Body weight change (%). (g) Waist circumference change (%). (h) HOMA index change (%). (i) FPG change (%). (j) Plasma LDL-cholesterol change (%). (k) Plasma HDL-cholesterol change (%). (l) Plasma TG change (%).M-H, Mantel–Haenszel
Predictors of histological response to antioxidants are unclear: weight loss, circulating oxidative stress markers or vitamin E deficiency do not predict histological response [49, 95, 96, 100]. There was no significant publication bias (ESM Fig. 1)
Glucose metabolism and cardiovascular risk
Antioxidants had no effect on body weight, waist circumference, LDL-C and HDL-C. For HOMA, FPG and TG heterogeneity was high (Fig. 8): when considering only the RCTs with vitamin E, active treatment had no significant effect on FPG (WMD −0.04, 95% CI −0.66, 0.57, p = 0.89, I 2 = 0%, n comparisons = 5), but was associated with a modestly higher risk of increasing HOMA (WMD 10.5, 95% CI 0.3, 20.6, p = 0.04, I2 = 45%, n comparisons = 4) and plasma TG (WMD 6.00, 95% CI 1.26, 10.75, p = 0.01, I 2 = 0%, n comparisons = 4) compared with controls. One RCT showed an improvement in plasma adiponectin with the combination of UDCA + vitamin E compared with placebo [88]. There was no significant publication bias for assessed outcomes (not reported).
Other drugs: l-carnitine, probiotics, incretin analogues, caspase inhibitors
l-carnitine is a precursor of carnitine-palmitoyltransferase, the rate-limiting enzyme in mitochondrial fatty acid transport and oxidation. When added to lifestyle intervention for 6 months, it improved steatosis, inflammation, fibrosis, HOMA, FPG, plasma lipids and C-reactive protein (ESM Table 5) [101].
Gut bacteria may promote liver injury and systemic inflammation through endotoxin-mediated toll-like receptor-4 axis activation and other mechanisms, predisposing to NASH, diabetes and atherosclerosis [[102](/article/10.1007/s00125-011-2446-4#ref-CR102 "Malaguarnera M, Gargante MP, Russo C et al (2010) l-Carnitine supplementation to diet: a new tool in treatment of nonalcoholic steatohepatitis. A randomized and controlled clinical trial. Am J Gastroenterol. doi: 10.1038/ajg.2009.719
")\]. Three RCTs assessed probiotics in NAFLD: the first, evaluating VSL3, was prematurely terminated for an increase in hepatic steatosis \[[103](/article/10.1007/s00125-011-2446-4#ref-CR103 "Musso G, Gambino R, Cassader M (2011) Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Ann Rev Med 62:361–380")\]; the others, assessing a mixture of _Lactobacillus_ spp. plus either _Bifidobacterium bifidum_ or _Streptococcus thermophilus_, found a significant improvement in MRS-assessed hepatic fat and aminotransferases, respectively \[[104](/article/10.1007/s00125-011-2446-4#ref-CR104 "Solga SF, Buckley G, Clark JM, Horska A, Diehl AM (2008) The effect of a probiotic on hepatic steatosis. J Clin Gastroenterol 42:1117–1119"), [105](/article/10.1007/s00125-011-2446-4#ref-CR105 "Wong VWS, Wong GLH, Wong CH, Chan HLY (2011) Treatment of non-alcoholic steatohepatitis with probiotics—a proof-of-concept study with serial gut microbiota analysis by ultra-deep sequencing. J Hepatol 54:349")\].The effect of probiotics in NAFLD is being evaluated in clinical trials (trial registration clinicaltrials.gov NCT00099723, NCT00808990, NCT00870012).
Incretin GLP-1 analogues improved insulin secretion by stimulating pancreatic beta cell growth and insulin release, and also improved hepatic steatosis and insulin resistance in mouse models [106]. Exenatide significantly improved transaminases in three RCTs enrolling diabetic patients [107], and its effects on liver histology in NASH are being tested in clinicaltrials.gov NCT00529204 and NCT00650546. In the Liraglutide Effect and Action in Diabetes (LEAD)-2 RCT, 2 years of liraglutide significantly reduced liver enzymes, CT-assessed hepatic steatosis, body fat and blood pressure and improved glycaemic control in diabetic patients with NAFLD (ESM Table 5) [108].
In NASH, hepatocyte apoptosis correlates with disease severity, and reducing hepatocyte apoptosis may has a potential for altering the course of the liver disease. In a phase 2 RCT, 124 patients with biopsy-proven NASH were randomised to once-daily placebo or 1, 5, 10 or 40 mg of the selective caspase inhibitor GS-9450 for 4 weeks: at EOT, NASH patients treated with 5–40 mg/day of GS-9450 significantly improved ALT levels, but not other metabolic variables, without significant side effects [109].