Genome sizes of Eucomis L’Hér. (Hyacinthaceae) and a description of the new species Eucomis grimshawii G.D.Duncan & Zonneveld (original) (raw)
Introduction
Eucomis, a small genus of 12 species in the family Hyacinthaceae, is endemic to the southern African countries, South Africa, Botswana, Lesotho, and Swaziland, as well as Zimbabwe and Malawi in southern Tropical Africa (Duncan 2007). The first described species (now known as E. regia) was illustrated in the early eighteenth century in Hortus Ethamensis (Dillenius 1732). Five of the later species were described by Baker (1878, 1886, 1887, 1895), and the most recently described one was E. schijffii (Reyneke 1976). Eucomis is characterized by the presence of a coma or tuft of leaf-like bracts that develop above the inflorescence. The most recent classification of Eucomis (Reyneke 1972) recognized ten species based on morphological characters, namely E. autumnalis (Mill.) Chitt., E. bicolor Baker., E. comosa (Houtt.) Wehrh., E. humilis Baker, E. montana Compton, E. pallidiflora Baker, E. regia (L.) L’Hér., E. schijffii Reyneke, E. vandermerwei Verd. and E. zambesiaca Baker. In the World Checklist for Eucomis (Govaerts [2006](/article/10.1007/s00606-009-0236-y#ref-CR14 "Govaerts R (2006) World checklist series RBG Kew, UK: genus Eucomis. http://apps.kew.org/wcsp/qsearch.do77
")), 33 names were listed, and Reyneke’s 10 species and 3 subspecies were accepted there. The present study follows Reyneke’s ten recognised species and in addition restores _E. amaryllidifolia_ Baker to species level and describes the new species _E. grimshawii_ G.D.Duncan & Zonn. The species are here divided into two groups: seven mainly small-sized diploids (2_n_ \= 2_x_ \= 30) and five large-sized tetraploids (2_n_ \= 4_x_ \= 60) (Reyneke and Liebenberg [1980](/article/10.1007/s00606-009-0236-y#ref-CR23 "Reyneke WF, Liebenberg H (1980) Karyotype analysis of the genus Eucomis (Liliaceae). J S Afr Bot 46–4:355–360")).Eucomis regia (L.) L’Hér. was the first Eucomis to be cultivated in Britain close to 3 centuries ago, but the species that have featured most prominently in hybridization and selections have been the fully hardy E. comosa (Houtt.) Wehrh. (syn: E. punctata L’Hér.) and E. autumnalis (Mill.) Chitt. (syn: E. undulata Aiton), as well as the less hardy, tall-growing E. pallidiflora Baker.
All the species are summer-flowering with the single exception of E. regia, which hails from the winter rainfall zone (Duncan 2007).
In South Africa the bulbs of several species, particularly E. autumnalis, are used in traditional medicine to treat a number of diseases. Recent investigations have validated this use by finding that extracts of Eucomis contain a high COX-1 inhibitory activity (Taylor and van Staden 2001). Within the bulb trade, the dwarf species have potential as flowering pot plants and the larger members as cut flowers. The only drawback to the appeal of certain species is the foul-smelling flowers (especially E. bicolor Baker, E. humilis Baker, E. schijffii Reyneke and E. vandermerwei Verd.).
The only comprehensive taxonomic treatment of the genus remains that of W.F. Reyneke, in the form of an unpublished M.Sc. thesis produced in South Africa in 1972. J. Compton wrote an excellent account of the genus (1990), and the present article could be regarded as an extension and DNA-based backing to these treatises.
To elucidate the relationships between Eucomis species, the classical taxonomic traits based on morphological characters and geographical distribution are here supplemented with data on nuclear DNA content. These were not investigated earlier in the systematic study of Eucomis. Taxonomy of Eucomis is rather difficult as the flowers are very similar. The main useful characters are fragrance, plant size and leaf color. More than 80 different accessions representing all accepted species were measured in an attempt to understand the relationships within Eucomis better.
Nuclear DNA content can conveniently be measured by flow cytometry using propidium iodide, a stoichiometric DNA stain that intercalates in the double helix. Where many species in a genus have the same chromosome number, differences in DNA 2C value have proven to be very effective in delimiting infrageneric divisions in a number of taxa (Ohri 1998). Greilhuber (1998, 2005) has clearly shown that intraspecific variation of genome size is much less than assumed.
The evolution of genome size [Cx-value (Greilhuber 1979)] has received increased attention during recent years. Primitive angiosperms are now supposed to have had small genomes; increases up to a factor of 1,000 have occurred independently in various modern taxa (Leitch et al. 1998). Flow cytometry was successfully used to measure the 2C-value for the genera Hosta Tratt., Helleborus L., Clivia Lindl., Nerine Herb., Agapanthus L’Hér., Galanthus L., Narcissus L., Gasteria Duval., Tulipa L., etc., by Zonneveld (2003, 2008, 2009), Zonneveld and Van Iren (2001), Zonneveld and Duncan (2003, 2006), Zonneveld and Van Jaarsveld (2005) and Zonneveld et al. (2003). In this paper it is shown that genome size alone is not sufficient to discriminate all species of Eucomis. Also one subspecies is returned to species status, and a new species is described.
Materials and methods
Plant material
Plant material was obtained mainly from the collections of Kirstenbosch Botanical Garden, J. des Brisay (UK), C. McMaster (RSA) and J. Grimshaw (UK). Where possible, material of known wild origin was used, and care was taken to ensure correct identification of all material. Vouchers of material from the Kirstenbosch and McMaster collections (including all known species) are lodged in the Compton Herbarium at Kirstenbosch, Cape Town, RSA.
Flow cytometric measurement of DNA 2C value
For the isolation of nuclei, about 5 cm of root was chopped together with a piece of Agave americana L. ‘Aureomarginata’ as an internal standard (see below). The chopping was done with a new razor blade in a Petri dish in 0.25 ml nuclei-isolation buffer to which 0.25 mg RNase/ml was added (Zonneveld and Van Iren 2001). After adding 1.75 ml propidium iodide solution (50 mg PI/l in isolation buffer), the suspension with nuclei was filtered through a 30-μm nylon filter. The fluorescence of the nuclei was measured ½ h and 1 h after addition of propidium iodide, using a Partec CA-II flow cytometer. The optical path contained a HBO mercury lamp, filters KG1, BG12, dichroic mirror TK500, filter OG570 and a Leitz 50 × 1 water immersion objective. Data were analyzed by means of DPAC software (Partec GmbH). The 2C DNA content of the sample was calculated as the sample peak mean, divided by the Agave peak mean, and multiplied with the amount of DNA of the Agave standard. At least three different samples, each with at least 5,000 nuclei, were measured twice for each clone. Most histograms revealed a coefficient of variation of less than 5%. The standard deviation was calculated for the DNA content of each species, using all relevant measurements.
Internal standard and absolute DNA content values
When measuring nuclear DNA content by means of flow cytometry, it is necessary to chop tissue from the plant of interest together with an internal standard: this standard must be as close as possible to the plants of interest. In this way, variation in signal intensities due to staining kinetics, to light absorption and quenching by sample components, as well as to instrument and other variables, is reduced to a minimum. Agave americana was chosen as internal standard for Eucomis. Agave americana is available year round, does not mind several weeks without water and, being a large plant, a single specimen can serve a lifetime, thereby further reducing variation in readings. It also has a low background in propidium iodide measurements and shows a single G0 peak, almost lacking G2 arrest.
Fresh male human leucocytes (2C = 7.0 pg; 1 pg = 10−12 g = 0.978 × 109 base pairs; Dolezel et al. 2003) were chosen as primary standard (Tiersch et al. 1989). This yields 2C = 15.9 pg for nuclei of Agave americana L. Based on a published male human genome size of 6.294 × 109 base pairs, the nucleus was calculated as containing 6.436 pg (Dolezel et al. 2003). However, this is based on a human sequence where the size of the very large repeat sequences could not accurately be determined. So in the end, the genome size could be closer to 7 pg than now envisioned.
Results
General
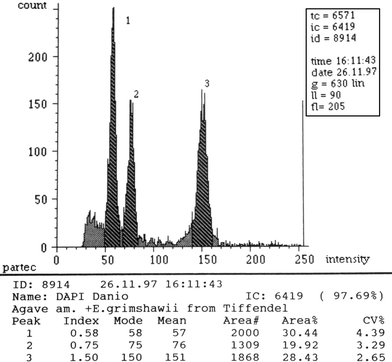
Eucomis leaves, like most members of the Hyacinthaceae, produce a lot of mucus when cut, clogging the flow cytometer. Therefore, nuclei of Eucomis were extracted from the thicker roots. Remarkable was the high content of root nuclei with a doubled DNA content. Usually between 25–50% nuclei are found in roots in most genera investigated (Zonneveld, unpublished) that have doubled their DNA content by endopolyploidy. However, in both diploid and tetraploid Eucomis between 50 and up to 75% of the nuclei are found to have doubled their nuclear DNA content (Fig. 1). Five of the diploid species hardly differ in DNA 2C value, and the same is true for the tetraploid species. The main morphological distinctions between the species are fragrance, the presence or absence of a purple color to the leaf base or flower, the cylindrical or clavate shape of the scape and overall plant size.
Fig. 1
Flow cytometry histogram of fluorescence intensity of more than 6,000 nuclei isolated and stained simultaneously. Peak 1 the nuclei of the standard: Agave americana (15.9 pg). 2 The diploid nuclei of the roots of Eucomis grimshawii (21.2 pg). 3 The 59% nuclei with a tetraploid amount of nuclear DNA of the roots of Eucomis grimshawii (42.1 pg)
The diploid species
E. schijffii Reyneke
A dwarf native of the Drakensberg of KwaZulu-Natal and Lesotho, and the mountains of the southern Eastern Cape, it is the Eucomis species with the highest altitudinal limit, occurring at up to 3,200 m. The rosette has intensely bluish-grey leaves with maroonish undersides. The purplish leaf margins are minutely toothed. The plant grows to 15 cm high, the coma bracts are large, sometimes overhanging the inflorescence, and its purplish-maroon flowers emit a strong, fetid scent. It has clavate, purple scapes, and its DNA 2C value is 22.8 pg.
E. vandermerwei Verd.
This dwarf species reaching up to 24 cm high in flower is restricted to rocky outcrops in the grassland at high altitude in Mpumalanga in northeastern South Africa (Verdoorn 1944). Its brownish-maroon flowers are extremely long lasting, it has cylindrical scapes, and the plant has cryptically marked foliage. It has a fetid smell, and its DNA 2C value is 23.5 pg.
E. zambesiaca Baker
Occurring in the northern parts of South Africa’s Limpopo Province and in the highlands of Malawi and Zimbabwe, this species has creamy-white, sweet-scented racemes that mature to bright green and cylindrical scapes. Reaching 30 cm high, it flowers from mid to late summer. The leaves are always uniformly pale green, and it is a variable species as regards inflorescence width; in some forms these can be as narrow as 15 mm. Its DNA 2C-value is 23.3 pg.
Eucomis grimshawii G.D.Duncan & Zonn., sp. nov.
Haec species habitu E. schijffii similis sed foliis leviter viridibus, margine cartilagineo, floribus albovirentibus, parvioribus, breviviventibus, suaveolentibusque, filamentis triangularibus, antheribus parvis, polline cremeo, fructibus et ovariis valde inflatis differt.
Type: South Africa, Eastern Cape, 3027 (Lady Grey): Hill below Tiffendel Ski Resort north of Rhodes, on shaded moist, grassy, south and southwest facing slopes, under overhanging rocks near a stream (-DD) Dec 2008, J.C. McMaster s.n. (NBG, holo.!) (see Fig. 2).
Fig. 2
Eucomis grimshawii G.D.Duncan & Zonn. sp. nov. as photographed 29 December 2006 at Tiffendell, Eastern Cape, South Africa by C. McMaster
Dwarf geophyte, deciduous, summer-growing, 80–100 mm high. Bulb ovoid, 35–40 × 25 × 30 mm solitary or offset-forming, scales cream, apices obtuse; tunic 1- or 2-layered, membranous, pale brown; cataphyll oblong, 30–40 × 10–12 mm, translucent white, subterranean, adhering to leaf bases, apex obtuse. Leaves 4 or 5, broadly lanceolate, 90–120 × 40–60 mm, spreading to suberect, pale green, longitudinal veins prominent on upper surface, depressed, 5–7 mm apart; midrib prominent on lower surface, yellowish green; margins cartilaginous, flat to weakly undulate, hyaline. Inflorescence few to many flowered, 30–50 mm long, erect, dense; scape clavate, 50–60 × 10–15 mm, erect, pale green, lower half heavily flushed with deep maroonish-magenta, upper half sporadically spotted or blotched with maroonish-magenta; rachis pale green; fertile bracts lanceolate, slightly canaliculate, lengthening towards inflorescence apex, 5–15 × 2–5 mm. Perianth campanulate, weakly sweet-scented, spreading, nectar sweet; lower flowers sessile, pedicels of upper flowers up to 1 mm long; tepals oblong, 3–5 × 3–4 mm, greenish white, curved inwards, soft, short-lived; sterile coma bracts 9–16, broadly lanceolate to ovate, 15–35 × 10–25 mm, pale green, suberect to spreading or weakly deflexed, often obscuring upper flowers; margins cartilaginous, hyaline. Stamens 6, included; filaments narrowly triangular 3–4 × 0.2–1 mm, white, curved inwards; anthers oblong, 1.5 × 0.8 mm, pollen cream. Ovary trilocular, bright green; locules more or less ovoid, 4–5 × 4–5 mm, strongly inflated; style weakly decurved, pale green, 3–4 mm long, stigma penicillate. Capsule flat-topped, membranous, dehiscent, pale brown, ripening rapidly; locules strongly inflated, 7–10 × 10–15 mm. Seed ovoid, 3–3.5 × 2.5 mm, dull blackish brown, 2 or 3 per locule, surface sculpturing reticulate.
Note
It is the smallest species in the genus, and with 21.1 pg it also has the lowest amount of nuclear DNA of them all and might therefore be the most plesiomorphic one. However, we have no further arguments to substantiate this claim. Four accessions of E. grimshawii were measured. They differed consistently about 7% in nuclear DNA content, this despite the fact that they were morphologically indistinguishable. It cannot be excluded that aneuploidy was involved.
Distribution and habitat
Eucomis grimshawii was discovered in 2002 by Dr. J. Grimshaw at Tiffendel Ski Resort, north of Rhodes Hamlet, Southern Drakensberg, just south of Lesotho between 2,720 and 2,740 m altitude. The plant was subsequently found by J.C. McMaster at the same locality and close by at Naude’s Nek Pass to the northeast. They grow in shaded, seasonally wet, south-facing grassy slopes below overhanging rocks, in rich, heavy black soil. They are also encountered in boggy conditions, growing in shade in association with Kniphofia caulescens and K. northiae (Grimshaw, pers. comm.).
The species is most similar to E. schijffii. The latter differs in its intensely glaucous leaves with strongly cartilaginous, minutely crisped purple margins. It is further a taller plant (10–15 cm high) with a purple scape. The tepals are dark purple (instead of greenish white), long-lasting (8–9 days instead of 2–3) and firm (instead of soft), and the flowers emit a strong fetid scent (instead of sweet). Its filaments are purple (instead of white) and broadly triangular with 2-mm-wide bases and have larger anthers (2.5 × 1.5 mm) with bright yellow pollen (instead of cream). Its ovary is much less inflated, has prominent apical grooves, and the capsule with a purple pericarp is also much less inflated, and takes 2 months to ripen (instead of 3–4 weeks). The two species are unusual within the genus in having dull blackish brown seeds with reticulate sculpturing (instead of glossy and smooth).
Eucomis schijffii grows in moist terrain on exposed basalt cliffs and in open rocky, grassy fields, and has a wider distribution along the Lesotho/KwaZulu-Natal border, in northern and southern Lesotho and in the mountains of the southern part of the Eastern Cape (Reyneke 1976). It has a longer flowering period extending from late November to late February.
Eucomis amaryllidifolia Baker
In the type description (Baker 1878) E. amaryllidifolia has a leaf length of 30–37 cm and a slightly shorter inflorescence. It is a plant from the Eastern Cape and only differs from E. autumnalis in its much narrower leaves of 3 cm. However, it must be kept in mind that this was a plant cultivated in a greenhouse in England. This might explain the seemingly larger size in the type description. One of the authors (G.D.) has never seen a long and narrow leaved plant of this species in nature or in culture. It was later reassessed as a subspecies of E. autumnalis as E. autumnalis subsp. amaryllidifolia (Baker) Reyneke, restricted to the Eastern Cape and Free State in South Africa (Reyneke 1980). In the description of Reyneke (1980), it is a small plant with a leaf length of 13–30 cm, a clavate scape and an inflorescence of only 3–7 cm. The tetraploid chromosome count that was found for a “subsp. _amaryllidifolia_” from Fauresmith, Free State, is most likely a different subspecies (Reyneke and Liebenberg 1980), probably subsp. clavata. In describing the subspecies of E. autumnalis, four taxa are actually mentioned by Reyneke (1980). Going from south to north in RSA, they are (1) a smaller subsp. autumnalis from the south (Eastern Cape), (2) subsp. clavata (widespread in north and northeast of South Africa), (3) subsp. amaryllidifolia (Eastern Cape) and (4) a larger subsp. autumnalis from the north (KwaZulu-Natal and Limpopo provinces in South Africa, and Zimbabwe, but not Malawi).
Eucomis autumnalis subsp. autumnalis and subsp. clavata have a tetraploid amount of nuclear DNA (Tables 1, 2). All plants measured here as subsp. amaryllidifolia were small, diploid plants with on average 23.4 pg and came from the south, i.e., Eastern Cape. Reyneke and Liebenberg (1980) concluded also that all tetraploids were allotetraploid, based on the absence of identical chromosome pairs for the large chromosomes. However, if a diploid is considered as incongruent as a subspecies of an allotetraploid, then Eucomis amaryllidifolia is a good species. Moreover, as a diploid it is separated reproductively from the allotetraploid E. autumnalis. Therefore, it seems best to consider the large tetraploid form as subsp. autumnalis with or without a narrow leaf and restore the small diploid form as E. amaryllidifolia.
Table 1 Eucomis accessions with their 2C amount of DNA per nucleus, average, standard deviation (SD), locality and origin
Table 2 Eucomis accessions with their avarage 2C amount of DNA per nucleus, number of different clones measured and 12 morphological characters
Eucomis bicolor Baker
The well-known and very hardy E. bicolor frequents various high-altitude habitats from the northern part of the Eastern Cape to KwaZulu-Natal, Lesotho and southern Mpumalanga. The name E. reichenbachii that has crept into some nursery lists is a mistake and is in fact E. bicolor. E. bicolor is the only diploid that has a large size. It has a fetid smell, cylindrical scapes and often a purple base to the leaves. Its amount of nuclear DNA of 25.7 pg deviates from that of the other diploids.
Eucomis regia (L.) L’Hér.
Eucomis regia is native to the winter rainfall zone of the Northern and Western Cape. This rather variable plant is confined to heavy clay soil in open aspects or between large rocks or low bushy cover. The bulbs are usually solitary, and the uniformly green leaves lie flat on the ground or spread over rocks. The inflorescence reaches up to 20 cm high. In some specimens the leafy bracts of the coma are disproportionately large, almost completely obscuring the green, unpleasant-smelling flowers. It has clavate scapes and is the only Eucomis that flowers from late winter to late spring. It has a rather deviating amount of nuclear DNA with 31.3 pg. It is unclear if there is any relationship between the differences in flowering time and amount of DNA for this species.
The tetraploid species
Eucomis autumnalis (Mill.) Chitt.
By far the most widespread Eucomis is the greenish-cream flowering E. autumnalis. Eucomis autumnalis leaves have undulate edges (Chittenden 1951) and according to Reyneke (1980) it comprises three subspecies: subsp. autumnalis, subsp. clavata and subsp. amaryllidifolia. Eucomis amaryllidifolia Baker from the Eastern Cape is here considered as a separate, diploid species. However, the diploid is apart from its overall size very similar to the tetraploid, ‘southern’ forms of subsp. autumnalis, the flowers differing only in their shorter tepals (6–8 mm long). Reyneke and Liebenberg (1980) consider all tetraploids as likely allotetraploids based on the fact that none has identical chromosome pairs. If that is accepted, an allotetraploid E. autumnalis cannot be derived solely from a doubling of the diploid E. amaryllidifolia.
The subsp. autumnalis has a cylindrical scape and semi-erect leaves, occurring in open grassland in the Eastern Cape, KwaZulu-Natal and Limpopo provinces of South Africa, and in Zimbabwe. It includes naturally occurring forms with striking burgundy-pink blooms whose leaves are purplish-burgundy when grown in full sun (Duncan 2007). Its DNA 2C value is 47.6 pg.
The subsp. clavata (Baker) Reyneke has, as its name suggests, clavate scapes, although not always (Reyeneke 1980). The description calls for a large leaved plant with a comparable rather short inflorescence of 7–13 cm. Further, it has hard, double-layered pericarps contrary to the thin pericarp of ssp. autumnalis. It is widespread in the north and northeast of South Africa, and its DNA 2C value is 46.9 pg.
Eucomis comosa (Houtt.) Wehrh.
The sweet-scented E. comosa (syn: E. punctata), is found from the Eastern Cape to Limpopo in northern South Africa. The typical variety occurs in a range of dry to moist habitats, while the var. striata (Donn) Wild. is confined to swampy conditions, having distinctive stripes instead of spots on the outer leaf bases. It has cylindrical scapes, and the DNA 2Cvalue of E. comosa var. comosa is 48.6 pg. Surprisingly, one of the three accessions received as var. striata turned out to be a pentaploid with 61.0 pg.
Eucomis pallidiflora Baker
Giant within the group, the plain green E. pallidiflora subsp. pallidiflora is native to wetland marshes in the Eastern Cape, Lesotho and KwaZulu-Natal. It has sweet-smelling and greenish-cream flowers, and cylindrical scapes. Its DNA 2C value is 47.7 pg. Eucomis pallidiflora subsp. pole-evansii, previously E. pole-evansii (Brown 1918), occurs further north in Mpumalanga and Swaziland and is even more robust than subsp. pallidiflora, its racemes reaching up to 2 m high or more. Its DNA 2C-value is 46.4 pg, but this difference falls within the standard deviation (Table 1). Both subspecies are further characterized by their long pedicels of 15–50 mm.
Eucomis humilis Baker
Eucomis humilis has greenish-cream, foul-smelling flowers on a cylindrical or clavate scape. We found E. humilis, despite its often small size, to be tetraploid with a DNA 2C value of 47.8 pg.
A perusal of W.F. Reyneke’s thesis on Eucomis and examination of herbarium material annotated by him shows that E. humilis is an extremely variable species, which has given rise to some confusion among gardeners. Eucomis humilis grows to 40 cm high and occurs in small groups at high altitude in moist grassland below rocky overhangs in northeastern Lesotho and western KwaZulu-Natal. It comprises several distinct forms, including a short form with pale green flowers arranged tightly together on a short, compact inflorescence that is shorter than the length of the leaves, and a very different-looking, robust form with larger, cream-coloured flowers arranged more loosely on a much longer inflorescence, a portion of which overtops the leaves. The flowers of the green form have pinkish-maroon filaments, but in the cream form they are broader and deep purple. The leaves of both forms are broadly lance-shaped with crisped or undulate margins, and are usually heavily spotted with maroon or purple on the undersides, as well as on the scape. The leaves of the short form are bright green, while those of the robust form are much larger and dark green with a purple tinge, with deep purple margins. According to Reyneke (1972), these two forms occur in association in the wild, and at certain localities a number of intermediate forms occur between the two extremes, making it difficult to assign taxonomic rank to any particular form. In addition to these, a dwarf form exists, having rather short green leaves with purple spotting on the undersides, and short creamy-green inflorescences that appear above the leaves.
It has become clear that a robust, cream-flowering form of E. humilis has for years been incorrectly listed and illustrated by nurseries in the Northern Hemisphere as E. montana Compton. According to the literature and herbarium material, the true E. montana has a more northerly distribution, with green flowers, purple ovaries and broader leaves (see the description below).
E. montana Compton
A native of Swaziland, the northeastern Free State and eastern Mpumalanga in South Africa, E. montana is a robust plant up to 50 cm high with very large, semi-erect, ovate leaves that are spotted on the underside towards the base, and usually have flat margins (Compton 1967). It has clavate scapes and green flowers with purple/dark-brown filaments, and the rather short inflorescence is produced well below the tips of the leaves. It has a DNA 2C value of 48.7 pg. Eucomis montana grows in large groups at high altitude on rocky mountain slopes in partial shade of boulders, flowering in midsummer. We are aware of only one authentic illustration, a watercolor painting (Fabian 1982), and the species is very rare in cultivation. It is very similar to large forms of E. humilis, including its fetid flowers. The only difference seems to be the green tepals and the purple ovary.
Discussion
Nuclear DNA content was measured in 85 accessions of Eucomis. The ploidy results are generally in agreement with the results of Reyneke (see below for the exceptions). It cannot be excluded that, as has been stated by Reyneke and Liebenberg (1980), the fact that Eucomis could have 30/32 or 60/64 chromosomes might indicate that aneuploidy may play a role. Four dwarf species with 22.6, 23.3, 23.4 and 23.5 pg had very close values. The same is true for all the tetraploid plants varying only from 46.4–48.6 pg, well within the range of variation. Therefore, it was often not possible based on the DNA 2C value alone to subscribe an unknown accession to a certain species. Exceptions are E. bicolor with 25.7 pg and E. regia with 31.3 pg and the new species E. grimshawii with 21.1 pg.
Moreover, very few morphological characters are useful in Eucomis. Those that have been investigated are compared in Table 2. Genome size as investigated here (Tables 1, 2) complements the work based mainly on morphological characters of (Reyneke 1972).
The evolutionary origin of the tetraploid species
Ploidy seems to play a bigger role than envisioned in the speciation of Eucomis. Earlier cytological investigation of Eucomis has shown that about half the species are diploid (Reyneke and Liebenberg 1980), and this is confirmed here (Table 1). Only in E. comosa was a pentaploid found.
Polyploid tulips are concentrated in the middle and upper mountains, whereas the diploids are mainly found in the deserts and lower mountains (Botschantzeva 1962). The opposite seems true for Eucomis with all the diploid species (except certain forms of E. regia) occurring high up in the mountains. In most cases polyploidy is not an argument (any longer) to assign a taxon specific status (Woods and Bamford 1937). The origin of the tetraploid species of Eucomis is unknown so far. Reyneke and Liebenberg (1980) state that all tetraploids are allotetraploids. These allotetraploids must be derived from two different diploid parents. Moreover, it seems likely that plants with sweet smells have pollinators different from those with fetid smells. Besides, sweet-smelling E. autumnalis, E. pallidiflora and E. comosa have morphologically most in common with sweet-smelling E. amaryllidifolia. So if these sweet tetraploids have two different sweet diploid parents, they are likely to be: E. amaryllidifolia and E. zambesiaca. The very large coma bracts and lower amount of nuclear DNA of the sweet-scented E. grimshawii do not seem to fit in here. Different forms of each diploid species and local adaptations might have resulted in the end in “different” tetraploid species. The two fetid tetraploids E. humilis and E. montana are morphologically most similar to E. bicolor. Therefore, their parents could be the fetid E. bicolor and E. schijffii. The heavily maroon spotted upper leaf surfaces of E. vandermerwei seem to exclude it as a parent. Just doubling E. bicolor (→51.4 pg) would give too high a value, unless genome downsizing has taken place, as often happens in tetraploids. The above hypotheses are not in contradiction with the amounts of nuclear DNA as measured here.
Conclusions
Flow cytometry can be considered as a quick and useful method to produce a systematic data source. Moreover, it can be used to investigate imported bulbs, precluding the need to grow them to maturity for identification purposes. The difference between the highest and lowest DNA contents for the diploids is about 10 pg. This 50% increase in DNA content without changing the number of chromosomes must be the result of a vast number of genomic changes. Depending on the size of the total genome, 1 pg amounts to several thousand genes. Therefore, flow cytometry is not a one-character-based taxonomy as the largest genome contains roughly 1010 more base pairs than the smallest and has chromosomes that are on average 50% longer. The data presented here for DNA content agree in most respects with the most recent classification of Eucomis. Flow cytometry as a taxonomic and diagnostic tool is applicable even in the case of dormant bulbs or sterile plants, and therefore has applications for conservation monitoring.