A phase II study of afatinib (BIBW 2992), an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumab (original) (raw)
Abstract
Afatinib is an oral, ErbB family blocker, which covalently binds and irreversibly blocks all kinase-competent ErbB family members. This phase II, open-label, single-arm study explored afatinib activity in human epidermal growth factor receptor 2 (HER2)-positive breast cancer patients progressing after trastuzumab treatment. Patients had stage IIIB/IV HER2-positive metastatic breast cancer, with progression following trastuzumab or trastuzumab intolerance and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Patients received 50 mg afatinib once-daily until disease progression. Primary endpoint was objective response rate (Response Evaluation Criteria in Solid Tumors 1.0), with tumor assessments every 8 weeks. Forty-one patients were treated. Patients had received a median of three prior chemotherapy lines (range, 0–15) and 68.3% had received trastuzumab for >1 year. Four patients (10% of 41 treated; 11% of evaluable patients) had partial response. Fifteen patients (37% of 41) had stable disease as best response and 19 (46% of 41) achieved clinical benefit. Median progression-free survival was 15.1 weeks (95% confidence interval [CI]: 8.1–16.7); median overall survival was 61.0 weeks (95% CI: 56.7–not evaluable). Most frequent common terminology criteria for adverse events grade 3 treatment-related adverse events were diarrhea (24.4%) and rash (9.8%). Afatinib monotherapy was associated with promising clinical activity in extensively pretreated HER2-positive breast cancer patients who had progressed following trastuzumab treatment.
Similar content being viewed by others
Introduction
Hyperactivation of the ErbB signaling network has been observed in a variety of malignancies and is associated with tumor cell proliferation and metastasis [[1](/article/10.1007/s10549-012-2003-y#ref-CR1 "Hynes NE, Lane HA (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5(5):341–354. doi: 10.1038/nrc1609
"), [2](/article/10.1007/s10549-012-2003-y#ref-CR2 "Arteaga CL (2002) Epidermal growth factor receptor dependence in human tumors: more than just expression? Oncologist 7(Suppl 4):31–39")\]. The ErbB receptor family consists of four receptor tyrosine kinases: epidermal growth factor receptor (EGFR), also known as human epidermal growth factor receptor (HER)1 or ErbB1, HER2 (_neu_/ErbB2), HER3 (ErbB3), and HER4 (ErbB4) \[[1](/article/10.1007/s10549-012-2003-y#ref-CR1 "Hynes NE, Lane HA (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5(5):341–354. doi:
10.1038/nrc1609
")\]. Increased understanding of the role of the ErbB receptor signaling network in cancer has led to development of various agents designed to specifically target these receptors \[[3](/article/10.1007/s10549-012-2003-y#ref-CR3 "Winer E, Gralow J, Diller L, Karlan B, Loehrer P, Pierce L, Demetri G, Ganz P, Kramer B, Kris M, Markman M, Mayer R, Pfister D, Raghavan D, Ramsey S, Reaman G, Sandler H, Sawaya R, Schuchter L, Sweetenham J, Vahdat L, Schilsky R, Blayney D, Lichter A (2008) Clinical cancer advances 2008: major research advances in cancer treatment, prevention, and screening–a report from the American Society of Clinical Oncology. J Clin Oncol. doi:
10.1200/JCO.2008.21.2134
")\]. Prominent examples include ErbB receptor targeting monoclonal antibodies such as trastuzumab, and small-molecule inhibitors such as gefitinib, erlotinib, and lapatinib.Trastuzumab, a humanized monoclonal antibody directed against the extracellular domain of the HER2 receptor, is indicated for the treatment of HER2-positive breast cancer (BC) in the adjuvant and metastatic setting [[4](/article/10.1007/s10549-012-2003-y#ref-CR4 "Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145. doi: 10.1200/JCO.2006.09.2775
")\]. However, both primary and acquired resistance are significant clinical problems \[[5](/article/10.1007/s10549-012-2003-y#ref-CR5 "Mukohara T (2011) Mechanisms of resistance to anti-human epidermal growth factor receptor 2 agents in breast cancer. Cancer Sci 102(1):1–8. doi:
10.1111/j.1349-7006.2010.01711.xCAS1711
")\]. Resistance to trastuzumab has been described to occur through many different mechanisms \[[6](/article/10.1007/s10549-012-2003-y#ref-CR6 "Nahta R, Esteva FJ (2007) Trastuzumab: triumphs and tribulations. Oncogene 26(25):3637–3643. doi:
10.1038/sj.onc.1210379
")–[8](/article/10.1007/s10549-012-2003-y#ref-CR8 "Bedard PL, Cardoso F, Piccart-Gebhart MJ (2009) Stemming resistance to HER-2 targeted therapy. J Mammary Gland Biol Neoplasia 14(1):55–66. doi:
10.1007/s10911-009-9116-x
")\]. One such mechanism, molecular plasticity of the ErbB pathway axis (HER reprogramming), is commonly observed; some breast tumors switch to alternative HER2 translation resulting in amino-terminally truncated HER2 fragments (611-CTF) \[[9](/article/10.1007/s10549-012-2003-y#ref-CR9 "Anido J, Scaltriti M, Bech Serra JJ, Santiago Josefat B, Todo FR, Baselga J, Arribas J (2006) Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO J 25(13):3234–3244. doi:
10.1038/sj.emboj.7601191
")\] which are no longer recognized by the antibody. In time, trastuzumab may fail to inhibit generation of p95HER2 \[[10](/article/10.1007/s10549-012-2003-y#ref-CR10 "Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J (2001) Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res 61(12):4744–4749")\], a proteolytic fragment which lacks the extracellular trastuzumab-binding domain and, when present, correlates with resistance to trastuzumab \[[11](/article/10.1007/s10549-012-2003-y#ref-CR11 "Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, Di Cosimo S, Matias-Guiu X, Ramon y Cajal S, Arribas J, Baselga J (2007) Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 99(8):628–638. doi:
10.1093/jnci/djk134
")\]. Increased expression of ErbB family members and cognate ligands such as EGFR/HER1 and EGF, TGFa, Hb-EGF, and heregulin has been demonstrated as a mechanism for acquired resistance to trastuzumab \[[12](/article/10.1007/s10549-012-2003-y#ref-CR12 "Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, Arteaga CL (2007) Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res 13(16):4909–4919. doi:
10.1158/1078-0432.CCR-07-0701
")\] and long-term trastuzumab exposure of primary resistant breast cancer cells is associated with HER1 reprogramming \[[13](/article/10.1007/s10549-012-2003-y#ref-CR13 "Narayan M, Wilken JA, Harris LN, Baron AT, Kimbler KD, Maihle NJ (2009) Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer Res 69(6):2191–2194. doi:
10.1158/0008-5472.CAN-08-1056
")\]. Due to the presence of HER reprogramming and other resistance mechanisms in HER2-positive BC, inhibition of more than one member of the ErbB family is expected to improve efficacy in this cancer setting. As such, inhibition of more than one member of the ErbB family may maximize inhibition of ErbB signaling with the potential to improve efficacy of targeted ErbB inhibitors.Afatinib is a novel, potent, small-molecule tyrosine kinase inhibitor (TKI) which irreversibly and selectively targets the ErbB family of receptors: ErbB1 (EGFR/HER1) (IC50 0.5 nM) and ErbB2 (HER2) (IC50 14 nM) [[14](/article/10.1007/s10549-012-2003-y#ref-CR14 "Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, Padera RF, Shapiro GI, Baum A, Himmelsbach F, Rettig WJ, Meyerson M, Solca F, Greulich H, Wong KK (2008) BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 27(34):4702–4711. doi: 10.1038/onc.2008.109
")\]. In vitro studies have shown that afatinib treatment inhibits growth of the trastuzumab-resistant SUM 190 cell line, which over expresses HER2 \[[15](/article/10.1007/s10549-012-2003-y#ref-CR15 "Hickish H, Wheatley D, Lin N, Carey L, Houston S, Mendelson D, Solca F, Uttenreuther-Fischer M, Jones H, Winer E (2009) Use of BIBW 2992, a Novel Irreversible EGFR/HER1 and HER2 tyrosine kinase inhibitor to treat patients with HER2-positive metastatic breast cancer after failure of treatment with trastuzumab. Poster presented at the San Antonio breast cancer symposium, December 9–13, San Antonio, Texas, USA"), [16](/article/10.1007/s10549-012-2003-y#ref-CR16 "Solca F, Adolf GR, Jones H, Uttenreuther-Fischer M (2011) Beyond Trastuzumab: second-generation targeted therapies for HER-2-positive breast cancer. In: Sibilia M (ed) Milestones in drug therapy. Springer, Basel AG, pp 91–117")\] and shows potent antitumor activity in human xenograft models known to depend on ErbB signaling \[[17](/article/10.1007/s10549-012-2003-y#ref-CR17 "Solca F, Baum A, Guth B, Colbatzky F, Blech S, Amelsberg A, Himmelsbach F (2005) BIBW 2992, an irreversible dual EGFR/HER2 receptor tyrosine kinase inhibitor for cancer therapy. In: Proceedings, AACR-NCI-EORTC international conference on molecular targets and cancer therapeutics: 118 (Abstract A244)")\]. Furthermore, afatinib has also shown potent antitumor activity in vivo in SUM 190 xenografts, a HER2-positive but trastuzumab-resistant model known to express large amounts of HER2 and displaying activated EGFR/HER1, HER2, and HER3 \[[16](/article/10.1007/s10549-012-2003-y#ref-CR16 "Solca F, Adolf GR, Jones H, Uttenreuther-Fischer M (2011) Beyond Trastuzumab: second-generation targeted therapies for HER-2-positive breast cancer. In: Sibilia M (ed) Milestones in drug therapy. Springer, Basel AG, pp 91–117")\].Evidence of clinical activity of afatinib monotherapy has also been demonstrated in phase I dose-escalation studies in advanced solid tumors. Stable disease (SD) was achieved in five of 14 BC patients participating in phase I monotherapy trials for up to 12 weeks (three patients) and up to 24 weeks (two patients) [18–20].
This phase II study was conducted to assess the efficacy and safety of afatinib monotherapy in patients with HER2-positive metastatic BC after progression on trastuzumab.
Patients and methods
Study design
This phase II, open-label, single-arm, multicenter trial was conducted in the USA and the UK according to the Declaration of Helsinki and in accordance with the International Conference on Harmonization Harmonized Tripartite Guideline for Good Clinical Practice. Written informed consent was obtained from all participants.
Treatment
All patients received daily oral doses of afatinib (50 mg/day) until disease progression, undue adverse events (AEs) or withdrawal of consent. Dose reduction to 40 mg/day and consecutively 30 mg/day was required for patients experiencing any Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 grade ≥3 drug-related AEs. Patients with CTCAE grade 3 diarrhea or CTCAE grade 2 diarrhea lasting >7 days despite adequate anti-diarrheal treatment, persistent CTCAE grade ≥2 nausea and/or vomiting despite optimal anti-emetic treatment or persistent CTCAE grade ≥3 rash despite optimal supportive care (including systemic antibiotics), were also dose-reduced. Patients with ≥20% decrease in left ventricular ejection fraction (LVEF) from baseline were required to discontinue treatment.
Study population
Adult female patients, aged ≥18 years, with a confirmed diagnosis of stage IIIB or IV HER2-positive metastatic BC (HER2 2+ by immunohistochemistry (IHC) and fluorescence in situ hybridization-positive or HER2 3+ by IHC) and a life expectancy of ≥4 months, were included in this study. Patients were required to have experienced disease progression following trastuzumab treatment and/or standard chemotherapy in conjunction with trastuzumab. Patients with an Eastern Cooperative Oncology Group (ECOG) performance score of 0–2 and measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 were included. All patients were required to have a normal hematological profile and adequate liver, kidney, and bone marrow function, and to have recovered from any therapy-related toxicity from previous chemo-, hormone-, immuno- or radiotherapies.
Patients with active infectious disease, gastrointestinal disorders that may interfere with the absorption of the study drug, chronic diarrhea or serious illness or concomitant non-oncological disease considered incompatible with study participation by the investigator, or active/symptomatic brain metastases were excluded. Patients receiving treatment with other investigational drugs, chemo-, hormone-, immuno- or radiotherapy within 4 weeks before the start of the study (2 weeks for trastuzumab) and patients who had received previous treatment with an EGFR/HER1- or HER2-inhibiting agent (except trastuzumab) were also excluded.
Concomitant medications
Concomitant medications or therapy to provide adequate care could be given as clinically necessary, although megestrol acetate and hormonal ablative therapy such as leuprolide were excluded. Proactive management of diarrhea, nausea, vomiting, and rash/acne, was encouraged using appropriate medications in accordance with the recommendations for the treatment of AEs provided in the clinical trial protocol. Treatment with chemo-, hormone-, immuno- or radiotherapy was not permitted, with the exception of bisphosphonates and palliative radiotherapy to non-target lesions.
Efficacy assessments
Tumor assessments were performed after every 8 weeks of treatment throughout the trial. The primary endpoint in this trial was objective response [complete response (CR) and partial response (PR)] as determined by RECIST version 1.0 [21]. Secondary outcome measures included time to progression, progression-free survival (PFS), overall survival (OS), time to objective response, and duration of objective response. In addition, quality of life (QOL) was measured using the European Organization for Research and Treatment-Quality of Life Questionnaire (EORTC QLQ-C30).
Safety and tolerability assessments
The incidence and severity of AEs according to National Cancer Institute CTCAE version 3.0 were used to assess safety. Physical examination, vital signs, laboratory examinations, and cardiac function were regularly monitored. Changes in the selected laboratory parameters were defined as possibly clinically significant in case of a worsening from baseline of ≥2 CTCAE grades.
Pharmacokinetic assessments
For quantification of afatinib plasma concentrations, 5 ml of venous blood was collected on day 1 of course 1 and day 15 of course 2: pre-dose, 1, 2, and 3 h after drug administration. In addition, a voluntary pharmacokinetic (PK) sample could be taken within the time frame of 4–24 h after afatinib administration. Additional pre-dose plasma samples were taken on day 15 of course 1 and on day 1 of course 2 and all subsequent courses. Afatinib drug concentrations were determined by a validated high performance liquid chromatography–mass spectroscopy (HPLC–MS/MS) assay.
Statistical analyses
The analyses in this study were descriptive and exploratory. The efficacy analysis included all patients who received at least one dose of afatinib; in addition, objective response analyses were based on all patients with evaluable tumor measurements. Exact 95% Clopper–Pearson confidence intervals (CIs) were calculated for the proportion of patients showing an objective response to treatment (primary efficacy outcome). Similar point estimates and exact CIs were calculated for CR, PR, and SD. PFS, OS, and time to objective response was estimated from Kaplan–Meier curves. The safety analysis included all patients who received at least one dose of trial medication. Safety findings were summarized using descriptive statistics.
Results
Patient population
This study was conducted at six sites in the USA and six sites in the UK between November 2006 and August 2009. In total 52 patients were screened, and 41 patients (21 from the USA; 20 from the UK) received trial treatment with afatinib. Baseline demographics for treated patients are presented in Table 1. Ninety-three percent of patients had an ECOG performance status of 0–1, and the majority (92.7%) had undergone prior surgery. All patients had received prior trastuzumab (as required by the protocol), with 68.3% of patients receiving this treatment for more than 1 year (range: 23–396 weeks). The median number of prior chemotherapy regimens was three. Patients’ best response to trastuzumab is shown in Table 1. The most common sites of metastatic disease were lymph nodes, liver, bone, and lung.
Table 1 Baseline demographics (treated patients)
For the 41 patients that received at least one dose of afatinib, the mean treatment time on afatinib was 99 days. The majority of patients (73.2%) discontinued due to disease progression; nine (22.0%) discontinued due to AEs and two (4.9%) discontinued for other reasons. Twenty patients (48.8%) required dose reduction to 40 mg, and six patients (14.6%) had a further reduction from 40 to 30 mg.
Antitumor activity
Of the 41 patients treated with afatinib, 35 patients were evaluable for objective response (Table 2). Six patients were not evaluable for response as no baseline or post-baseline imaging measurements were available, but were included in the denominator for response and efficacy assessments. Four patients (10% of 41 patients; 11% of 35 patients evaluable for objective response based on tumor measurement) achieved a PR and no CRs were observed. Three patients had a PR after 8 weeks while one patient had a PR after 16 weeks. The median (range) duration of PR was 12.0 (7.4–56.1) weeks. In one patient, a 30-year old white female with poorly differentiated infiltrating ductal breast carcinoma and lung metastases, PR was maintained for 56 weeks (Table 2) and the duration of overall clinical benefit in this patient was 64 weeks at which time the patient developed a new lesion. An additional 15 patients (37% of 41 patients; 43% of 35 patients) had SD of whom eight patients achieved SD for >4 months and three patients achieved SD for 6–12 months. The maximum duration of SD was 32 weeks.
Table 2 Best response according to RECIST criteria
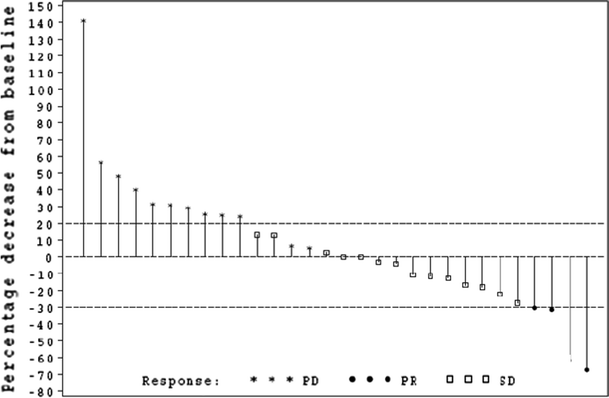
Overall, 19 patients (46% of 41 patients) were classed as having achieved clinical benefit (CR or PR or SD) with a median (range) duration of clinical benefit of 17.1 (7.3–64.0) weeks. A total of 30 patients had available tumor diameter measurements as depicted in the waterfall plot (Fig. 1). Of the 15 evaluable patients with SD, nine patients demonstrated a decrease in tumor size which did not reach the 30% threshold for PR.
Fig. 1
Best RECIST response*. *30 patients had available tumor diameter measurements; five patients had no tumor diameter measurements available (two patients had fewer lesions measured than at baseline, three patients had no post-baseline measurements available, but new lesions documented). RECIST Response Evaluation Criteria in Solid Tumors
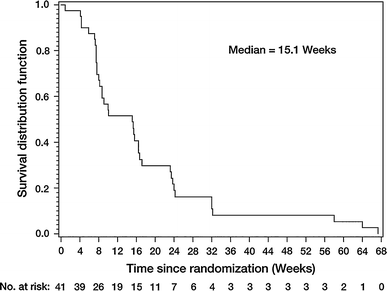
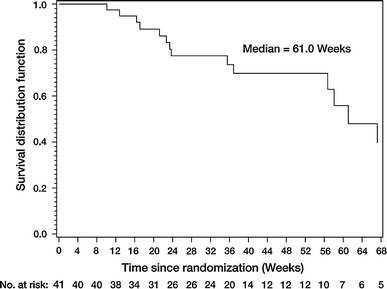
In the total population the median PFS was 15.1 weeks (Fig. 2; 95% CI: 8.1–16.7) and a total of 14 patients were known to have died during, or after, the study. The median OS was 61.0 weeks (95% CI: 56.7–not evaluable) (Fig. 3).
Fig. 2
Progression-free survival (treated set)
Fig. 3
Overall survival (treated set)
Safety and tolerability
Forty patients (97.3%) experienced treatment-related AEs (according to CTCAE version 3.0) during treatment. The most common treatment-related AEs were diarrhea (90.2%), rash (65.9%), and fatigue (41.5%). Most AEs reported were mild to moderate in severity (CTCAE grade 1 or 2). Treatment-related AEs occurring in more than 5% of patients, or with a CTCAE grade 3, are shown in Table 3.
Table 3 Drug-related adverse events according to CTCAE grade (total frequency >5% or grade ≥3), sorted according to frequency
A total of five patients (12%) experienced serious treatment-related AEs: one patient experienced CTCAE grade 3 dehydration and hyponatremia, one patient experienced CTCAE grade 3 dehydration, diarrhea, and nausea, one patient experienced CTCAE grade 3 nausea and vomiting, one patient experienced CTCAE grade 2 vomiting and one patient experienced CTCAE grade 3 rash, a biopsy of which showed features of a leukocytoclastic vasculitis.
No clinically significant changes indicative of an adverse effect of afatinib were observed in LVEF, or electrocardiogram, or for any laboratory parameter assessed including blood chemistry and liver function tests.
Other endpoints
ECOG performance score improved during the course of treatment in 24 (60.0%) patients and remained stable in 15 (37.5%) patients; only one (2.5%) patient had an ECOG status that deteriorated. Thirty-nine of the 41 patients were assessed for QOL. Of these, the overall QOL score improved in 15 (38.5%) patients, remained stable in 18 (46.2%) patients and deteriorated in five (12.8%) patients, based on the best post-baseline response. One patient had only baseline data available and was classified as missing. Notable changes in the QOL functional and symptom scales included improvements in the domains of fatigue, insomnia, and pain, with approximately half of all patients recording an improvement in QOL in these areas; QOL with respect to the occurrence of diarrhea was noted as deteriorating in 12 of 39 (30.8%) patients.
Pharmacokinetics
The starting dose used in this study was 50 mg afatinib; dose reductions to 40 or 30 mg were permitted during treatment and therefore no single-dose PK data were available for these dose levels. Four patients who provided data for PK analysis received a dose of 30 mg afatinib. As such, only PK data from patients receiving 50 or 40 mg afatinib were considered in the PK analysis.
Afatinib plasma concentrations were in steady-state at day 15 (obtained by visual inspection). Steady-state may have been attained earlier, but no PK sampling was performed between days 1 and 15. Trough concentrations were higher in patients receiving afatinib 50 mg compared to those receiving 40 mg afatinib (Supplementary Table S1) and remained stable over the observation period. The overall variability in afatinib trough plasma concentrations was moderate to high (gCV values of 30.7–75.1%; Supplementary Table S1).
Discussion
This phase II study was designed to assess the efficacy and safety of afatinib in patients with HER2-positive metastatic BC after failure of treatment with trastuzumab. Afatinib demonstrated antitumor activity in this patient group with confirmed PRs and durable SD: 19 patients achieved clinical benefit (46% of 41 patients), with four patients (10% of 41 patients) achieving a PR. A total of 15 patients maintained SD with nine of these patients demonstrating a reduction in tumor size. The median duration of clinical benefit was 17.1 weeks. Median PFS was 15.1 weeks and median OS was 61.0 weeks. This was a heavily pretreated population; the median number of prior chemotherapy regimens was three and nearly 70% of patients had received prior trastuzumab therapy for ≥12 months, with 36.6% of these patients reporting a CR or PR on trastuzumab. With the caveat that this study was a single-arm, phase II monotherapy trial with a limited number of patients, these results are interesting when compared to those obtained upon dual HER2-blockade with lapatinib and trastuzumab in a randomized phase II trial in a similar population [[22](/article/10.1007/s10549-012-2003-y#ref-CR22 "Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J (2010) Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437
")\]. Here, the reported PFS was 12.0 weeks and OS was 51.6 weeks for the combination compared to 8.1 and 39.0 weeks for lapatinib alone. No significant difference was observed in overall response rate for the combination arm compared to the monotherapy arm (10.3 vs. 6.9%; _P_ \= 0.46). Data reported here with afatinib confirm preliminary results from ongoing studies showing that resistance to trastuzumab can be circumvented by EGFR/HER1 and HER2 targeted TKI therapy. In addition to the antitumor effects of afatinib, ECOG status and QOL assessments also improved during the study, further supporting the benefits of treatment.Although cross-trial comparisons are limited by methodological differences in study design, patient population and other clinical factors, the results reported in this monotherapy trial are encouraging in relation to effects of other HER2 and EGFR/HER1 inhibitors in similar populations of patients with HER2-positive metastatic BC. Lapatinib, a reversible EGFR/HER1 and HER2 TKI, has shown modest activity in patients with HER2-positive metastatic BC (n = 104) who have received ≥3 lines of prior anticancer therapy and trastuzumab therapy [[23](/article/10.1007/s10549-012-2003-y#ref-CR23 "Burstein HJ, Storniolo AM, Franco S, Forster J, Stein S, Rubin S, Salazar VM, Blackwell KL (2008) A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol 19(6):1068–1074. doi: 10.1093/annonc/mdm601
")\]. Burstein and colleagues reported an overall response rate of 4.3% (95% CI: 1.6–9.1), clinical benefit rate of 5.7% (95% CI: 2.5–10.9), and median PFS of 9.1 weeks (95% CI: 8.0–13.6) with lapatinib monotherapy. With neratinib, an irreversible inhibitor of EGFR/HER1 and HER2, an objective response rate of 24% (95% CI: 14–36) with a median PFS of 22.3 weeks was reported in patients with HER2-positive metastatic BC (_n_ \= 66) who have received prior trastuzumab \[[24](/article/10.1007/s10549-012-2003-y#ref-CR24 "Burstein HJ, Sun Y, Dirix LY, Jiang Z, Paridaens R, Tan AR, Awada A, Ranade A, Jiao S, Schwartz G, Abbas R, Powell C, Turnbull K, Vermette J, Zacharchuk C, Badwe R (2010) Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol 28(8):1301–1307. doi:
10.1200/JCO.2009.25.8707
")\].As noted previously, more recently the effects of dual HER2-blockade have been investigated by Blackwell and colleagues [[22](/article/10.1007/s10549-012-2003-y#ref-CR22 "Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J (2010) Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28(7):1124–1130. doi: 10.1200/JCO.2008.21.4437
")\]. A phase I trial of assessing the safety and preliminary antitumor activity of afatinib in combination with trastuzumab in patients with advanced HER2-positive BC is ongoing.Afatinib showed a manageable side effect profile in this study. Similar to previous studies with afatinib, the most frequently reported AEs were diarrhea and rash [18, 19, [25](/article/10.1007/s10549-012-2003-y#ref-CR25 "Eskens FA, Mom CH, Planting AS, Gietema JA, Amelsberg A, Huisman H, van Doorn L, Burger H, Stopfer P, Verweij J, de Vries EG (2008) A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer 98(1):80–85. doi: 10.1038/sj.bjc.6604108
"), [26](/article/10.1007/s10549-012-2003-y#ref-CR26 "Agus DB, Terlizzi E, Stopfer P, Amelsberg A, Gordon MS (2006) A phase I dose escalation study of BIBW 2992, an irreversible dual EGFR/HER2 receptor tyrosine kinase inhibitor, in a continuous schedule in patients with advanced solid tumours. J Clin Oncol 24(18S):2074")\]. These AEs were generally manageable with appropriate treatment pause, supportive care, and dose reductions. Early and pre-emptive management of diarrhea is crucial to prevent potential complications. Most AEs reported with afatinib were mild to moderate in severity (CTCAE grade 1 or 2); no CTCAE grade 4 treatment-related AEs occurred in this study and no treatment-related deaths were reported. In general, the tolerability profile of afatinib reported here was similar to that of EGFR TKIs and consistent with that expected with this class of agent \[[27](/article/10.1007/s10549-012-2003-y#ref-CR27 "Lynch TJ Jr, Kim ES, Eaby B, Garey J, West DP, Lacouture ME (2007) Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist 12(5):610–621. doi:
10.1634/theoncologist.12-5-610
")\].Cardiotoxicity is a potential issue for patients treated with trastuzumab and it has been suggested to be a class effect for HER2-targeting agents. Therefore, LVEF monitoring is conducted in all afatinib clinical trials. No significant cardiac safety issues were observed in this study.
The PK characteristics of afatinib have previously been evaluated in phase I dose escalation studies, performed in cancer patients and have indicated oral bioavailability and moderately fast absorption [19, [25](/article/10.1007/s10549-012-2003-y#ref-CR25 "Eskens FA, Mom CH, Planting AS, Gietema JA, Amelsberg A, Huisman H, van Doorn L, Burger H, Stopfer P, Verweij J, de Vries EG (2008) A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer 98(1):80–85. doi: 10.1038/sj.bjc.6604108
"), [26](/article/10.1007/s10549-012-2003-y#ref-CR26 "Agus DB, Terlizzi E, Stopfer P, Amelsberg A, Gordon MS (2006) A phase I dose escalation study of BIBW 2992, an irreversible dual EGFR/HER2 receptor tyrosine kinase inhibitor, in a continuous schedule in patients with advanced solid tumours. J Clin Oncol 24(18S):2074"), [28](/article/10.1007/s10549-012-2003-y#ref-CR28 "Spicer J, Calvert H, Vidal L, Azribi F, Perrett R, Shahidi M, Temple G, Futreal A, De Bono J, Plummer R (2007) Activity of BIBW2992, an oral irreversible dual EGFR/HER2 inhibitor, in non-small cell lung cancer (NSCLC) with mutated EGFR. J Thorac Oncol 2(8):S410")–[30](/article/10.1007/s10549-012-2003-y#ref-CR30 "Awada AH, Dumez H, Wolter P, Hendlisz A, Besse-Hammer T, Piccart M, Uttenreuther-Fischer M, Stopfer P, Taton M, Schöffski P (2009) A phase I dose finding study of the 3-day administration of BIBW 2992, an irreversible duall EGFR/HER2 inhibitor, in combination with 3-weekly docetaxel in patients with advanced solid tumors. J Clin Oncol 27:15s")\]. Following oral administration, maximum concentrations of afatinib (_C_ max) are generally observed 1–6 h (_t_ max) post-dose, either after single dose or at steady-state \[[19](/article/10.1007/s10549-012-2003-y#ref-CR19 "Lewis N, Marshall J, Amelsberg A, Cohen RB, Stopfer P, Hwang J, Malik S (2006) A phase I dose escalation study of BIBW 2992, an irreversible dual EGFR/HER2 receptor tyrosine kinase inhibitor, in a 3 week on 1 week off schedule in patients with advanced solid tumours. J Clin Oncol 24(18S):3091"), [25](/article/10.1007/s10549-012-2003-y#ref-CR25 "Eskens FA, Mom CH, Planting AS, Gietema JA, Amelsberg A, Huisman H, van Doorn L, Burger H, Stopfer P, Verweij J, de Vries EG (2008) A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer 98(1):80–85. doi:
10.1038/sj.bjc.6604108
"), [26](/article/10.1007/s10549-012-2003-y#ref-CR26 "Agus DB, Terlizzi E, Stopfer P, Amelsberg A, Gordon MS (2006) A phase I dose escalation study of BIBW 2992, an irreversible dual EGFR/HER2 receptor tyrosine kinase inhibitor, in a continuous schedule in patients with advanced solid tumours. J Clin Oncol 24(18S):2074"), [28](/article/10.1007/s10549-012-2003-y#ref-CR28 "Spicer J, Calvert H, Vidal L, Azribi F, Perrett R, Shahidi M, Temple G, Futreal A, De Bono J, Plummer R (2007) Activity of BIBW2992, an oral irreversible dual EGFR/HER2 inhibitor, in non-small cell lung cancer (NSCLC) with mutated EGFR. J Thorac Oncol 2(8):S410")\]; steady-state is typically reached within 8 days after first administration. The PK findings reported here in patients with advanced metastatic BC appear similar. In this study there was no detectable change (increase or decrease) in afatinib plasma concentrations with long-term treatment.In summary, treatment with afatinib showed promising clinical activity in HER2-positive BC patients who had progressed following treatment with trastuzumab. Afatinib has a manageable AE profile with frequent cutaneous AEs and diarrhea. Further clinical trials of afatinib in this patient population are planned.
References
- Hynes NE, Lane HA (2005) ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer 5(5):341–354. doi:10.1038/nrc1609
Article PubMed CAS Google Scholar - Arteaga CL (2002) Epidermal growth factor receptor dependence in human tumors: more than just expression? Oncologist 7(Suppl 4):31–39
Article PubMed CAS Google Scholar - Winer E, Gralow J, Diller L, Karlan B, Loehrer P, Pierce L, Demetri G, Ganz P, Kramer B, Kris M, Markman M, Mayer R, Pfister D, Raghavan D, Ramsey S, Reaman G, Sandler H, Sawaya R, Schuchter L, Sweetenham J, Vahdat L, Schilsky R, Blayney D, Lichter A (2008) Clinical cancer advances 2008: major research advances in cancer treatment, prevention, and screening–a report from the American Society of Clinical Oncology. J Clin Oncol. doi:10.1200/JCO.2008.21.2134
- Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145. doi:10.1200/JCO.2006.09.2775
Article PubMed CAS Google Scholar - Mukohara T (2011) Mechanisms of resistance to anti-human epidermal growth factor receptor 2 agents in breast cancer. Cancer Sci 102(1):1–8. doi:10.1111/j.1349-7006.2010.01711.xCAS1711
Google Scholar - Nahta R, Esteva FJ (2007) Trastuzumab: triumphs and tribulations. Oncogene 26(25):3637–3643. doi:10.1038/sj.onc.1210379
Article PubMed CAS Google Scholar - Browne BC, O’Brien N, Duffy MJ, Crown J, O’Donovan N (2009) HER-2 signaling and inhibition in breast cancer. Curr Cancer Drug Targets 9(3):419–438
Article PubMed CAS Google Scholar - Bedard PL, Cardoso F, Piccart-Gebhart MJ (2009) Stemming resistance to HER-2 targeted therapy. J Mammary Gland Biol Neoplasia 14(1):55–66. doi:10.1007/s10911-009-9116-x
Article PubMed Google Scholar - Anido J, Scaltriti M, Bech Serra JJ, Santiago Josefat B, Todo FR, Baselga J, Arribas J (2006) Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO J 25(13):3234–3244. doi:10.1038/sj.emboj.7601191
Article PubMed CAS Google Scholar - Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J (2001) Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res 61(12):4744–4749
PubMed CAS Google Scholar - Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, Di Cosimo S, Matias-Guiu X, Ramon y Cajal S, Arribas J, Baselga J (2007) Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst 99(8):628–638. doi:10.1093/jnci/djk134
Google Scholar - Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, Arteaga CL (2007) Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res 13(16):4909–4919. doi:10.1158/1078-0432.CCR-07-0701
Article PubMed CAS Google Scholar - Narayan M, Wilken JA, Harris LN, Baron AT, Kimbler KD, Maihle NJ (2009) Trastuzumab-induced HER reprogramming in “resistant” breast carcinoma cells. Cancer Res 69(6):2191–2194. doi:10.1158/0008-5472.CAN-08-1056
Article PubMed CAS Google Scholar - Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, Padera RF, Shapiro GI, Baum A, Himmelsbach F, Rettig WJ, Meyerson M, Solca F, Greulich H, Wong KK (2008) BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 27(34):4702–4711. doi:10.1038/onc.2008.109
Article PubMed CAS Google Scholar - Hickish H, Wheatley D, Lin N, Carey L, Houston S, Mendelson D, Solca F, Uttenreuther-Fischer M, Jones H, Winer E (2009) Use of BIBW 2992, a Novel Irreversible EGFR/HER1 and HER2 tyrosine kinase inhibitor to treat patients with HER2-positive metastatic breast cancer after failure of treatment with trastuzumab. Poster presented at the San Antonio breast cancer symposium, December 9–13, San Antonio, Texas, USA
- Solca F, Adolf GR, Jones H, Uttenreuther-Fischer M (2011) Beyond Trastuzumab: second-generation targeted therapies for HER-2-positive breast cancer. In: Sibilia M (ed) Milestones in drug therapy. Springer, Basel AG, pp 91–117
- Solca F, Baum A, Guth B, Colbatzky F, Blech S, Amelsberg A, Himmelsbach F (2005) BIBW 2992, an irreversible dual EGFR/HER2 receptor tyrosine kinase inhibitor for cancer therapy. In: Proceedings, AACR-NCI-EORTC international conference on molecular targets and cancer therapeutics: 118 (Abstract A244)
- Shaw H, Plummer R, Vidal L, Perrett R, Pilkington M, Temple G, Fong P, Amelsberg A, Calvert H, de Bono J (2006) A phase I dose escalation study of BIBW 2992, an irreversible dual EGFR/HER2 receptor tyrosine kinase inhibitor, in patients with advanced solid tumours. J Clin Oncol 24(18S):3027
Google Scholar - Lewis N, Marshall J, Amelsberg A, Cohen RB, Stopfer P, Hwang J, Malik S (2006) A phase I dose escalation study of BIBW 2992, an irreversible dual EGFR/HER2 receptor tyrosine kinase inhibitor, in a 3 week on 1 week off schedule in patients with advanced solid tumours. J Clin Oncol 24(18S):3091
Google Scholar - Plummer R, Vidal L, Perrett R, Spicer J, Stopfer P, Shahidi M, Temple G, Futreal A, Calvert H, de Bono J (2007) A Phase I and pharmacokinetic (PK) study of BIBW 2992, an oral irreversible dual EGFR/HER2 inhibitor. Eur J Cancer Suppl 5(4):108
Article Google Scholar - Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Article PubMed CAS Google Scholar - Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J (2010) Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 28(7):1124–1130. doi:10.1200/JCO.2008.21.4437
Article PubMed CAS Google Scholar - Burstein HJ, Storniolo AM, Franco S, Forster J, Stein S, Rubin S, Salazar VM, Blackwell KL (2008) A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol 19(6):1068–1074. doi:10.1093/annonc/mdm601
Article PubMed CAS Google Scholar - Burstein HJ, Sun Y, Dirix LY, Jiang Z, Paridaens R, Tan AR, Awada A, Ranade A, Jiao S, Schwartz G, Abbas R, Powell C, Turnbull K, Vermette J, Zacharchuk C, Badwe R (2010) Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol 28(8):1301–1307. doi:10.1200/JCO.2009.25.8707
Article PubMed CAS Google Scholar - Eskens FA, Mom CH, Planting AS, Gietema JA, Amelsberg A, Huisman H, van Doorn L, Burger H, Stopfer P, Verweij J, de Vries EG (2008) A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer 98(1):80–85. doi:10.1038/sj.bjc.6604108
Article PubMed CAS Google Scholar - Agus DB, Terlizzi E, Stopfer P, Amelsberg A, Gordon MS (2006) A phase I dose escalation study of BIBW 2992, an irreversible dual EGFR/HER2 receptor tyrosine kinase inhibitor, in a continuous schedule in patients with advanced solid tumours. J Clin Oncol 24(18S):2074
Google Scholar - Lynch TJ Jr, Kim ES, Eaby B, Garey J, West DP, Lacouture ME (2007) Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist 12(5):610–621. doi:10.1634/theoncologist.12-5-610
Article PubMed CAS Google Scholar - Spicer J, Calvert H, Vidal L, Azribi F, Perrett R, Shahidi M, Temple G, Futreal A, De Bono J, Plummer R (2007) Activity of BIBW2992, an oral irreversible dual EGFR/HER2 inhibitor, in non-small cell lung cancer (NSCLC) with mutated EGFR. J Thorac Oncol 2(8):S410
Article Google Scholar - Marshall J, Shapiro GI, Terlizzi E, Stopfer P, Amelsberg A, Gordon M (2008) A Phase I dose escalation trial of BIBW 2992, an irreversible EGFR/HER2 kinase inhibitor, for 20 and 13 days in combination with docetaxel every 21 days. Ann Oncol 19(Supp 8):474
Google Scholar - Awada AH, Dumez H, Wolter P, Hendlisz A, Besse-Hammer T, Piccart M, Uttenreuther-Fischer M, Stopfer P, Taton M, Schöffski P (2009) A phase I dose finding study of the 3-day administration of BIBW 2992, an irreversible duall EGFR/HER2 inhibitor, in combination with 3-weekly docetaxel in patients with advanced solid tumors. J Clin Oncol 27:15s
Article Google Scholar
Acknowledgments
This study was supported by Boehringer Ingelheim. The authors would like to acknowledge the editorial assistance of Ogilvy Healthworld Medical Education. Boehringer Ingelheim provided financial support for this assistance.
Conflicts of interest
Nancy Lin: currently conducting research supported by Genentech, GlaxoSmithKline and has conducted research supported by Infinity; David Mendelson: research funding from Boehringer Ingelheim; Martina Uttenreuther-Fischer: employed by Boehringer Ingelheim GmBH & Co Germany, Senior Clinical Research Physician; Hilary Jones: employed by Boehringer Ingelheim, UK, Senior Clinical Scientist; Sven Wind: employed by Boehringer Ingelheim GmBH & Co, Germany, Project Pharmacokineticist; Richard Vinisko: employed by Boehringer Ingelheim, Principal Biostatistician; Tamas Hickish: consultant/advisor for Boehringer Ingelheim, honoraria from Boehringer Ingelheim, research funding from Boehringer Ingelheim, other remuneration from Boehringer Ingelheim; Tamas Hickish: is the Principal Investigator of this study; Eric Winer, Duncan Wheatley, Lisa Carey, Stephen Houston, Pamela Munster, Laurie Frakes, Steve Kelly, Agustin Garcia, Susan Cleator: no disclosures.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
- Dana-Farber Cancer Institute, 450 Brookline Avenue, Boston, MA, 02215, USA
Nancy U. Lin & Eric P. Winer - Royal Cornwall Hospital, Truro, UK
Duncan Wheatley - University of North Carolina, Chapel Hill, NC, USA
Lisa A. Carey - Royal Surrey County Hospital, Guildford, UK
Stephen Houston - Pinnacle Oncology Hematology, Scottsdale, AZ, USA
David Mendelson - UCSF, Helen Diller Family Comprehensive Cancer Center, San Francisco, CA, USA
Pamela Munster - San Diego Pacific Oncology and Hematology Associates Inc., Encinitas, CA, USA
Laurie Frakes - Derriford Hospital, Plymouth, UK
Steve Kelly - USC Norris Comprehensive Cancer Center and Hospital, Los Angeles, CA, USA
Agustin A. Garcia - Mary’s Hospital, London, UK
Susan Cleator - Boehringer Ingelheim Pharma GmbH & Co KG, Biberach, Germany
Martina Uttenreuther-Fischer - Boehringer Ingelheim Ltd, Bracknell, UK
Hilary Jones - Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, NJ, USA
Sven Wind - Dorset Cancer Centre and Bournemouth Hospital, Bournemouth, UK
Tamas Hickish - Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT, USA
Richard Vinisko
Authors
- Nancy U. Lin
You can also search for this author inPubMed Google Scholar - Eric P. Winer
You can also search for this author inPubMed Google Scholar - Duncan Wheatley
You can also search for this author inPubMed Google Scholar - Lisa A. Carey
You can also search for this author inPubMed Google Scholar - Stephen Houston
You can also search for this author inPubMed Google Scholar - David Mendelson
You can also search for this author inPubMed Google Scholar - Pamela Munster
You can also search for this author inPubMed Google Scholar - Laurie Frakes
You can also search for this author inPubMed Google Scholar - Steve Kelly
You can also search for this author inPubMed Google Scholar - Agustin A. Garcia
You can also search for this author inPubMed Google Scholar - Susan Cleator
You can also search for this author inPubMed Google Scholar - Martina Uttenreuther-Fischer
You can also search for this author inPubMed Google Scholar - Hilary Jones
You can also search for this author inPubMed Google Scholar - Sven Wind
You can also search for this author inPubMed Google Scholar - Richard Vinisko
You can also search for this author inPubMed Google Scholar - Tamas Hickish
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toNancy U. Lin.
Electronic supplementary material
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Lin, N.U., Winer, E.P., Wheatley, D. et al. A phase II study of afatinib (BIBW 2992), an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumab.Breast Cancer Res Treat 133, 1057–1065 (2012). https://doi.org/10.1007/s10549-012-2003-y
- Received: 15 February 2012
- Accepted: 16 February 2012
- Published: 15 March 2012
- Issue Date: June 2012
- DOI: https://doi.org/10.1007/s10549-012-2003-y