The effect of modafinil on counter-regulatory and cognitive responses to hypoglycaemia (original) (raw)
Abstract
Aims/hypothesis
Our hypothesis is that reducing release of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) with modafinil will enhance symptomatic and hormonal responses to hypoglycaemia.
Methods
Nine healthy men received, in random order, two 100-mg doses of modafinil or placebo, followed by an insulin clamp in which plasma glucose was either reduced stepwise to 2.4 mmol/l or was sustained at euglycaemia (four studies). Catecholamines, symptom scores and cognitive function were measured.
Results
Modafinil had no effect on the measured parameters during euglycaemia. During hypoglycaemia, autonomic symptom scores were significantly higher with modafinil (increase at lowest plasma glucose concentration 271.3±118.9 vs 211.2±80.4/40 min, _p_=0.019), and the heart rate response was increased (12,928±184 vs 6773±148 bpm/140 min, _p_=0.016). Deterioration in performance of two cognitive tasks was reduced: Stroop colour–word test (613±204 vs 2375±161/65 min, _p_=0.009) and accuracy of a simple reaction task (11.3±1.8 vs 9.4±3.7, _p_=0.039).
Conclusions/interpretation
We conclude that modafinil improves adrenergic sensitivity and some aspects of cognitive function at hypoglycaemia, possibly by reducing neuronal central GABA concentrations.
Similar content being viewed by others
Introduction
The ventromedial nucleus of the hypothalamus (VMH) is important in the sympathetic and neuroendocrine responses to hypoglycaemia [1, 2, 3]. Its glucose-sensing neurones share characteristics with the pancreatic beta cell, including a specific glucose transporter [4], pancreatic-specific glucokinase [5], intracellular ATP and the K-ATP channel complex [6, 7], and they alter their firing rate in response to changes in glucose concentration [8]. An attractive hypothesis is that brain glucose sensing uses the same cellular mechanisms to control counter-regulation as the beta cell does to control insulin secretion in response to changing plasma glucose concentrations.
Pancreatic beta cells respond to high blood glucose concentration by releasing insulin. We hypothesised that the glucose-sensing neurones of the VMH act in the same way, but releasing a neurotransmitter instead of insulin. The neurotransmitter released must be inhibitory of sympathetic activation, as sympathetic activation occurs in response to low blood glucose. We postulated that the resting state of the VMH was one of tonic inhibition of sympathetic activation, which was de-repressed by hypoglycaemia.
Gamma-aminobutyric acid (GABA) is a major inhibitory neurotransmitter of the brain [9] and is present in pancreatic beta cells and in the VMH [10, 11]. A subgroup of VMH glucose-sensing neurones has recently been shown to receive synaptic input from GABAergic neurones, potentially located outside of the VMH [12, 13]. The relationship between extracellular brain glucose concentrations and the concentration of GABA is controversial and support for this relationship comes mostly from animal studies. Streptozotocin-induced diabetic rats have high GABA levels, low noradrenaline and reduced sympathetic nerve activity within the VMH [14]. Infusion of glucose into rat substantia nigral neurones increases GABA concentrations, whilst both the perfusion of the glucoprivic agent 2-deoxyglucose and systemic hypoglycaemia significantly reduced GABA in some [15] but not all studies [16, 17]. Perfusion of the neurones with K-ATP channel activators lowered GABA levels, and antagonists increased them, in a manner analogous to the insulin secretory responses to these agents by pancreatic beta cells, demonstrating the close relationship between GABA, intracellular ATP levels, the K-ATP channel complex and glucose sensing.
We postulated that pharmacological suppression of brain GABA concentration may enhance counter-regulatory responses to systemic hypoglycaemia. Modafinil, used in the treatment of narcolepsy, lowers brain GABA concentration. It is a wakefulness-promoting agent that improves vigilance and cognitive performance during sleep deprivation in patients with narcolepsy and in healthy volunteers [18, 19, 20]. We examined whether this agent, with acute administration, enhances counter-regulatory responses to hypoglycaemia in human subjects.
Subjects and methods
Subjects
Nine healthy male volunteers were recruited. They were aged between 21 and 38 years (mean ± SD 27.2±6.2 years) with a mean BMI of 22.4±3.4 kg/m2. None of the subjects had any previous significant medical history or was taking medication. The protocol was approved by the King’s College Hospital Ethics Committee and each subject gave written informed consent before participating. All nine subjects underwent paired hypoglycaemic–hyperinsulinaemic clamp studies in random order, each at least 2 weeks apart, in which they received either modafinil or placebo prior to clamping in a double-blind fashion. Six subjects underwent additional paired euglycaemic clamp studies in random order, receiving either modafinil or placebo in double-blind manner. There was a period of at least 2 weeks between any two studies.
Study design
The evening before the study the subject received either a 100-mg tablet of modafinil or a placebo tablet, depending on randomisation, to take with food. The following morning, subjects were admitted to the Programmed Investigation Unit in King’s College Hospital, having fasted from 22.30 hours on the previous evening. Two intravenous cannulae were placed in the non-dominant arm using aseptic techniques and 1% intradermal lignocaine to anaesthetise the skin. One cannula was inserted into the antecubital vein for infusion of insulin, glucose and potassium; the other was placed retrogradely into a distal wrist or hand vein. The hand was rested in a box of heated air (55–60 °C) to arterialise venous blood [21]. This cannula was used for sampling arterialised blood. Forty-five minutes before starting the study, the subjects took another 100-mg tablet of modafinil or placebo. No less than 30 min after the cannulae were inserted, and after baseline blood samples were taken, a primed continuous infusion of soluble insulin (human Actrapid; Novo Nordisk Pharmaceuticals, Crawley, UK) was started, with a maintenance rate of 1.5 mU·kg body weight−1·min−1. This insulin dose was used in all studies. Arterialised venous plasma glucose was measured at the bedside every 5 min and controlled by adjusting a simultaneous variable infusion of 20% glucose (Clintec Nutrition, Slough, UK). Additional blood samples were taken at pre-determined times for the later measurement of catecholamines, growth hormone, glucagon, cortisol and insulin.
During the hypoglycaemia studies, plasma glucose levels were maintained at 5 mmol/l for 40 min before being sequentially reduced to 4.4, 3.8, 3.4, 2.8 and 2.4 mmol/l. Levels were maintained at 4.4 and 2.4 mmol/l for 20 min only. Each other level was maintained for 40 min, with the target level being reached during the first 10 to 15 min and being maintained until the end of the given time period. After the nadir, the plasma glucose level was restored to 5 mmol/l and maintained at euglycaemia for 20 min. In the euglycaemic studies, arterialised plasma glucose was maintained at 5 mmol/l throughout.
Cognitive function was assessed by four-choice reaction time, simple reaction time, finger tapping and Stroop colour–word interference. Finger tapping, simple reaction time and four-choice reaction time were measured using a laptop computer (Toshiba T1800 calculator). Subjects were trained for all tasks before each study, except for the Stroop colour–word interference test, and each task was performed at least three times until stable results were obtained. Finger tapping and four-choice reaction time were then measured twice at 5 mmol/l and once at each glucose level, or at the equivalent time point in the euglycaemic studies. Simple reaction time was measured twice at 5 mmol/l, once at 2.4 mmol/l and at recovery.
Cognitive tasks and symptom scoring
For finger tapping, the subject was asked to press a computer key as fast as possible over a 10-second period [22] and the number of taps was counted. This was repeated three times for each test. The mean of the three recordings was used in the analysis. To record four-choice reaction time [22], 500 targets were displayed on the computer screen, one at a time, randomly appearing in one of four quadrants, over a 5-min period. The subject responded to each by pressing the corresponding key on the adapted keyboard. The mean time of reaction and the accuracy of the responses were recorded. For simple reaction time, the screen randomly displayed a series of letters, and the subject was instructed to press the computer spacebar when the letter X appeared, which it did randomly 12 times during the test. Again, the reaction time and the accuracy of the response were measured. In the Stroop colour–word interference test [23, 24] the subject was presented with a sheet of paper on which the names of colours (red, tan, green and blue) were printed in incongruously coloured ink, e.g. the word “red” printed in blue ink. The subject was then asked to name the colour of the ink. The subject could correct himself but there was no prompting. The number of colours read over 45 seconds was recorded, and the number of errors was subtracted from the total score.
Symptoms were assessed using a questionnaire with a linear digital scale, on which subjects graded a standard list of symptoms from 1 (absent) to 7 (severe). Symptom scores were checked four times in the first 60 min and then on two occasions at each plasma glucose level before and after the cognitive test battery. Individual scores were created for all symptoms (total) and individually for both neuroglycopenic symptoms (difficulty speaking, confusion, dizziness, irritability, blurred vision and drowsiness) and autonomic symptoms (sweating, anxiety, tremor, palpitations, feeling hot and tingling) by adding the scores for each relevant symptom at each time point [25]. At the same time points, physiological measurements of heart rate and blood pressure were also recorded.
Measurements
Arterialised plasma glucose was measured at the bedside using a glucose oxidase technique (Yellow Springs glucose analyzer; Yellow Springs Instruments, Yellow Springs, Ohio, USA). Plasma adrenaline and noradrenaline were measured by HPLC with electrochemical detection [26], glucagon [27], cortisol [28], growth hormone [29] and insulin [30] by a radioimmunoassay. Inter- and intra-assay variations were less than 10% for all assays. Studies on one individual were run in the same assay.
Data analysis
Results are expressed as means ± SD unless otherwise stated. The results of the hypoglycaemia studies were compared with each other, and the results of the euglycaemic studies were examined separately. Changes in counter-regulatory hormones and symptom scores were calculated as the AUC using the trapezoidal rule during hypoglycaemia, correcting for the initial 40-min euglycaemic period in hypoglycaemic studies and for the duration of the clamp in euglycaemic studies. Plasma glucose thresholds during the hypoglycaemic studies were defined as the plasma glucose concentration associated with the onset of a significant change in any parameter, a significant change being defined as when any parameter exceeded baseline by more than two standard deviations of the mean throughout the euglycaemic studies, on two or more consecutive occasions. Cognitive function data were analysed by ANOVA with repeated measures design, using data corrected for the initial 40-min euglycaemic period in the hypoglycaemic studies. When there was a statistically significant group–time effect, two-tailed paired t tests were used to localise the effects. Simple reaction time, peak hormone and symptom response were analysed by paired Student’s t test. Results were analysed using SPSS for Windows 10.5 (SPSS, Woking, UK) and differences were regarded as statistically significant if p values were less than 0.05.
Results
Insulin and glucose
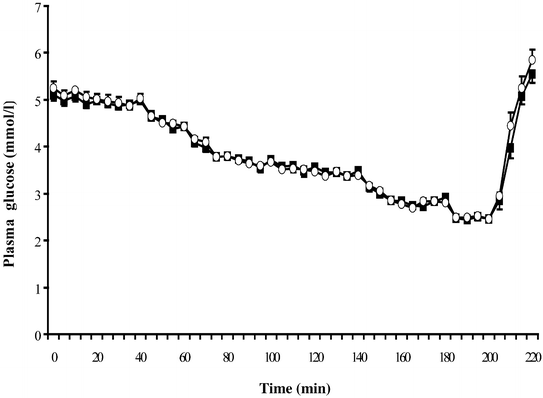
Insulin infusion raised insulin levels in all clamps, with no significant differences in peak insulin levels between the modafinil and placebo groups in either the euglycaemic studies (148.0±35.3 vs 152.7±35.7 mU/l, _p_=0.55) or the hypoglycaemic studies (156.1±40.8 vs 158.9±26.0 mU/l, _p_=0.73). There were no significant differences in plasma glucose concentrations between the modafinil and placebo groups at euglycaemia (5.0±0.1 vs 5.0±0.1, _p_=0.37, CV 1.99% and 2.42% respectively) or at any time point during the hypoglycaemic clamps (_p_=0.43; Fig. 1).
Fig. 1
Plasma glucose concentrations during hypoglycaemia with (open circles) and without (filled squares) modafinil. Data are expressed as means ± SE
Counter-regulatory hormone responses
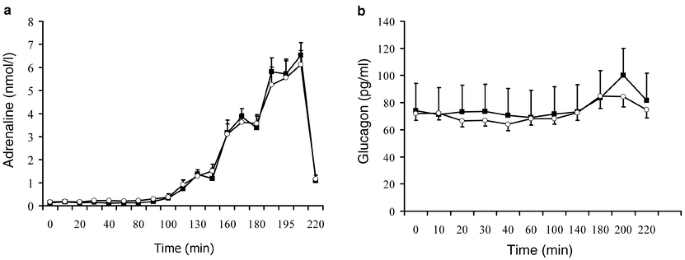
Cortisol and growth hormone levels decreased during euglycaemic clamp, but concentrations of the other hormones remained stable. All five hormones increased with hypoglycaemia, modafinil having no significant effect either at euglycaemia or at hypoglycaemia. Results are shown in Table 1 and Figure 2.
Table 1 Hormone and symptom scores during euglycaemic and hypoglycaemic clamps, with and without modafinil
Fig. 2
Adrenaline (a) and glucagon (b) responses to hypoglycaemia with (open circles) and without (filled squares) modafinil. Data are expressed as means ± SE
Heart rate
Heart rate did not change significantly during euglycaemia in either group. During hypoglycaemia, heart rate increased in both groups, but the increase was significantly greater in the modafinil group, where the change was significantly different from both the euglycaemic studies and the hypoglycaemia with placebo studies (_p_=0.016). Results are shown in Figure 3.
Fig. 3
Change in heart rate during hypoglycaemia with (open circles) and without (filled squares) modafinil. Data are expressed as means ± SE. § p<0.02, comparing AUC (see methods)
Symptom scores
Total and autonomic symptom scores did not change during euglycaemia in either study. There was a small increase in neuroglycopenic scores, mainly due to an increase in drowsiness, during euglycaemia with placebo, such that the final score was higher than at baseline (6.4±1.0 vs 8.7±2.6, _p_=0.03). In the modafinil group there was no difference between baseline and the end of the euglycaemic period (7.2±2.0 vs 7.2±1.5, _p_=0.17).
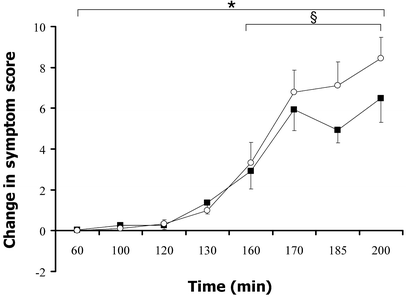
During hypoglycaemia, autonomic and neuroglycopenic symptom scores increased significantly in both groups compared with euglycaemia. With modafinil there was a significantly greater increase in autonomic symptom responses during the study. As shown in Figure 4, the change in autonomic scores was higher with modafinil between 160 and 200 min, i.e. while plasma glucose was lower than 2.8 mmol/l (AUC 271.3±118.9/40 min vs 211.2±80.4/40 min, _p_=0.019). There was no increase in neuroglycopenic symptoms (181.5±258.5 vs 168.6±226.5/140 min, _p_=0.94) at any time.
Fig. 4
Change in autonomic symptom scores during hypoglycaemia with (open circles) and without (filled squares) modafinil. Data are expressed as means ± SE. * p<0.05, comparing total AUC, 60–200 min, see methods; § _p_=0.019 for AUC, 160–200 min
Cognitive function tests
Modafinil reversed some, but not all, of the deterioration in cognitive performance observed with hypoglycaemia (Table 2). There was no deterioration in performance for any task in modafinil or placebo euglycaemic studies, and modafinil had no significant effect. Performance in all tasks deteriorated during hypoglycaemia with placebo, but performance in two tasks was better sustained in the presence of modafinil during hypoglycaemia (Stroop colour–word test, _p_=0.005 vs placebo at 2.4 mmol/l; and accuracy of simple reaction task, _p_=0.039 vs placebo at 2.4 mmol/l). For the Stroop colour–word test, only two of the nine subjects studied showed significant deterioration with hypoglycaemia and modafinil, while eight of the nine showed deterioration with hypoglycaemia and placebo. In terms of finger tapping, four-choice reaction time and simple reaction time, modafinil had no significant effect on performance during hypoglycaemia (Fig. 5).
Table 2 Effect of hypoglycaemia and modafinil on cognitive responses
Fig. 5
Cognitive function tests during hypoglycaemia with (open bars) and without (closed bars) modafinil. a. Change in number of incorrect scores in Stroop colour-word. b. Deterioration in finger tapping score. c. Change in four-choice reaction time. d. Accuracy of simple reaction time. Data are expressed as means ± SE. * _p_=0.05; # p<0.05; § p<0.02
Discussion
In our study, an acute administration of modafinil (2-[diphenylmethylsulphinyl]acetamide) at a low dose had no effect on the counter-regulatory hormone responses to systemic hypoglycaemia, but it increased adrenergic responses (symptoms and heart rate) and also improved some aspects of cognitive performance at low glucose levels, compared with placebo in healthy volunteers.
Modafinil improves vigilance, logical reasoning, short-term memory and tracking tasks in sleep-deprived healthy volunteers and patients with narcolepsy [18, 19, 20]. Its mechanism of action is not completely understood but is believed to work through its action on brain GABA levels. Modafinil lowers brain GABA concentration indirectly by stimulating the noradrenergic system to activate serotoninergic neurones, which inhibit the release of GABA [31, 32, 33]. Both the noradrenergic and serotoninergic systems are involved in memory processing and cognition [34, 35]. Our finding of reduced drowsiness during the euglycaemic studies are compatible with the known effects of modafinil; and the observed beneficial effects of modafinil on cognitive performance during hypoglycaemia may be related to the activation of both these systems. The observed beneficial effects may also be related to the reduction in GABA concentration or to the activation of another unknown neuronal system [36].
The hypothalamus plays a crucial role in triggering the counter-regulatory response to systemic hypoglycaemia. Hypoglycaemia is associated with an increase in c-fos activity in this region of the brain [37]. A growing body of evidence suggests that the glucose-sensing neurones of the hypothalamus and elsewhere in the brain respond to changes in plasma glucose using mechanisms similar to those used by the pancreatic beta cell [4, 5, 6, 7, 8]. In the latter, a decrease in glucose concentration suppresses insulin secretion. If we postulate that the brain’s response to hypoglycaemia is activation of sympathetic centres, we must also postulate that any neurotransmitter released by glucose-sensing neurones must be inhibitory of the adrenergic responses, since its release by the glucose-sensing neurone will be stimulated by hyperglycaemia and inhibited by hypoglycaemia, the equivalent of insulin release from the pancreatic beta cell. Hypoglycaemia and agents that close the beta cell K-ATP channel both stimulate neuronal GABA, at least in some brain regions, providing indirect evidence for the above hypothesis [15]. Modafinil, like the state of hypoglycaemia, has been shown to enhance c-fos immunoreactivity within the hypothalamus [38]. At the dose used in our study, modafinil had no effect on any of the counter-regulatory hormones measured, but did increase the heart rate and enhance autonomic symptom response to hypoglycaemia. This suggests increased activation of sympathetic neuronal pathways, or possibly enhanced sensitivity of the adrenergic systems during hypoglycaemia, associated with the actions of modafinil. Neither of these was measured in our protocol.
The effect of modafinil on adrenergic symptoms and heart rate responses during hypoglycaemia in our studies was not a strong effect. However, this was a study carried out in healthy volunteers, with vigorous normal responses to hypoglycaemia in the control study. Our findings now need to be replicated in patients with diabetes and counter-regulatory impairment. In such subjects, there may be greater scope to show an enhancement of responses, as the control responses will be weaker. Absence of subjective awareness of hypoglycaemia is associated with a three-fold increase in the risk of severe hypoglycaemia [39], and fear of such hypoglycaemia limits patients’ attempts to achieve the tight diabetic control that minimises their risk of long-term microvascular complications [40]. An agent that enhances subjective awareness while supporting some aspects of cortical function has the potential to be clinically useful. Modafinil, a medication currently licensed to treat narcolepsy, is worthy of further investigation in this context.
Abbreviations
GABA:
gamma-aminobutyric acid
VMH:
ventromedial hypothalamus
References
- Borg WP, During MJ, Sherwin RS, Borg MA, Brines ML, Shulman GI (1994) Ventromedial hypothalamic lesions in rats suppress counterregulatory responses to hypoglycaemia. J Clin Invest 93:1677–1682
CAS PubMed Google Scholar - Borg WP, Sherwin RS, During MJ, Borg MA, Shulman GI (1995) Local ventromedial hypothalamus glucopenia triggers counterregulatory hormone release. Diabetes 44:180–184
CAS PubMed Google Scholar - Borg MA, Sherwin RS, Borg WP, Tamborlane WV, Shulman GI (1997) Local ventromedial hypothalamus glucose perfusion blocks counterregulation during systemic hypoglycaemia in awake rats. J Clin Invest 99:361–365
CAS PubMed Google Scholar - Leloup C, Arluison M, Lepetit N et al. (1994) Glucose transporter 2 (GLUT 2): Expression in specific brain nuclei. Brain Res 638:221–226
Article CAS PubMed Google Scholar - Lynch RM, Tompkins LS, Brooks HL, Dunn-Meynell AA, Levin BE (2000) Localisation of glucokinase gene expression in the rat brain. Diabetes 49:693–700
CAS PubMed Google Scholar - Lee K, Dixon AK, Richardson PJ, Pinnock RD (1999) Glucose-receptive neurons in the rat ventromedial hypothalamus express KATP channels composed of Kir6.1 and SUR1 subunits. J Physiol 515:439–452
Article CAS PubMed Google Scholar - Ashford MLJ, Boden PR, Treherne MJ (1990) Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch 415:479–483
CAS PubMed Google Scholar - Oomura Y (1983) Glucose as a regulator of neuronal activity. Adv Metab Disord 10:31–65
CAS PubMed Google Scholar - Roberts D, Franckel S (1950) Gamma-amino butyric acid in brain. Fed Proc 9:219
Google Scholar - Malaisse WJ (1992) Glucose sensing by pancreatic beta cell: the mitochondrial part. Int J Biochem 24:693–701
Article CAS PubMed Google Scholar - Beverley JL, Beverley MF, Meguid MM (1995) Alterations in extracellular GABA in the ventromedial hypothalamus of rats in response to acute glucoprivation. Am J Physiol 269:R1174–R1178
PubMed Google Scholar - Song Z, Levin BE, McArdle JJ, Bakhos N, Routh VH (2001) Convergence of pre- and postsynaptic influences on glucosensing neurons in the ventromedial hypothalamic nucleus. Diabetes 50:2673–2681
CAS PubMed Google Scholar - Horvarth TL, Bechmann I, Nafttolin F, Kalra SP, Leranth C (1997) Heterogeneity in the neuropeptide Y-containing neurons of the rat arcuate nucleus: GABAergic and non-GABAergic subpopulations. Brain Res 756:283–286
Article PubMed Google Scholar - Ohtani N, Ohta M, Sugano T (1997) Microdialysis study of modification of hypothalamic neurotransmitters in streptozotocin-diabetic rats. J Neurochem 69:1622–1628
CAS PubMed Google Scholar - During M, Leone P, Davis KE, Kerr D, Sherwin RS (1995) Glucose modulates rat substantia nigra GABA release in vivo via ATP-sensitive potassium channels. J Clin Invest 95:2403–2408
CAS PubMed Google Scholar - Beverley JL, Vries MG de, Beverly MF, Arseneau LM (2000) Norepinephrine mediates glucoprivic induced increase in GABA in the ventromedial hypothalamus of rats. Am J Physiol 279:R990–R996
Google Scholar - Beverley JL, Vries MG de, Bouman SD, Arseneau LM (2001) Noradrenergic and GABAergic systems in the medial hypothalamus are activated during hypoglycaemia. Am J Physiol 280:R563–R569
Google Scholar - Batejat DM, Lagarde DP (1999) Naps and modafinil as countermeasures for the effects of sleep deprivation on cognitive performance. Aviat Space Environ Med 70:493–498
CAS PubMed Google Scholar - Baranski JV, Pigeau RA (1997) Self-monitoring cognitive performance during sleep deprivation, effects of modafinil, d-amphetamine and placebo. J Sleep Res 6:84–91
CAS PubMed Google Scholar - Pigeau R, Naitoh P, Buguet A et al. (1995) Modafinil D-amphetamine and placebo during 64 hours of sustained mental work. Effects on mood, fatigue, cognitive performance and body temperature. J Sleep Res 4:212–228
PubMed Google Scholar - Liu D, Moberg E, Kollind K, Lins PE, Adamson U, MacDonald IA (1992) Arterial arterialized venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia 35:287–290
CAS PubMed Google Scholar - Heller SR, MacDonald IA (1996) The measurement of cognitive function during acute hypoglycaemia: experimental limitations and their effect on the study of hypoglycaemia unawareness. Diabet Med 13:607–615
Article CAS PubMed Google Scholar - Stroop J (1935) Studies of interference in serial verbal reaction. J Exp Psychol 18:643–652
Google Scholar - Jensen AR, Rohwer WD Jr (1966) The Stroop colour word test—a review. Acta Psychol 25:36–93
Article CAS Google Scholar - Deary IJ, Hepburn DA, MacLeod KM, Frier BM (1993) Partitioning the symptoms of hypoglycaemia using multi-sample confirmatory factor analysis. Diabetologia 36:771–777
CAS PubMed Google Scholar - MacDonald IA, Lake DM (1985) An improved method for extracting catecholamines from body fluids. J Neurosci Meth 13:239–248
Article CAS Google Scholar - Aguilar-Parada E, Eisentraut AM, Unger RH (1969) Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci 257:415–419
CAS PubMed Google Scholar - Cunnah DC, Jessop DS, Besser GM, Rees LH (1987) Measurement of circulating corticotrophin releasing factor in man. J Endocrinol 113:123–131
CAS PubMed Google Scholar - Mazlan M (1989) Development of an immunoradiometric assay for human growth hormone releasing factor and some applications. PhD thesis, University of London, UK
- Morgan CR, Lazaro A (1963) Immunoassay of insulin: two antibody system Diabetes 12:115A
- Tanganelli S, Perez de la Mora, Ferraro L et al. (1995) Modafinil and corticol gamma amino butyric acid outflow. Modulation by 5-hydroxytryptamine neurotoxins. Eur J Pharmacol 273:63–71
Article CAS PubMed Google Scholar - Lins JS, Roussel B, Akaoka H, Fort P, Debilly G, Jouvet M (1992) Role of catecholamines in the modafinil and amphetamine induced wakefulness, a comparative pharmacological study in the cat. Brain Res 591:319–326
Article PubMed Google Scholar - Tanganelli S, Fuxe K, Ferraro L, Janson AM, Bianchi C (1992) Inhibitory effects of the psychoactive drug modafinil on gamma-aminobutyric acid outflow from the cerebral cortex of the awake freely moving guinea pig. Naunyn-Schmiedebergs Arch Pharmacol 345:461–465
Google Scholar - Buhot MC (1997) Serotonin receptors in cognitive behaviors. Curr Opin Neurobiol 7:243–254
Article CAS PubMed Google Scholar - Coull JT, Frith CD, Dolan RJ, Frackowiak RS, Grasby PM (1997) The neural correlates of the noradrenergic modulation of human attention, arousal and learning. Eur J Neurosci 9:589–598
CAS PubMed Google Scholar - Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM (2001) Dopaminergic role in stimulant-induced wakefulness. J Neurosci 21:1787–1794
CAS PubMed Google Scholar - Levin BE, Govek EK, Dunn-Meynell AA (1998) Reduced glucose-induced neuronal activation in the hypothalamus of diet-induced obese rats. Brain Res 808:317–319
Article CAS PubMed Google Scholar - Engber TM, Koury EJ, Dennis SA, Miller MS, Contreras PC, Bhat RV (1998) Differential patterns of regional c-Fos induction in the rat brain by amphetamine and the novel wakefulness-promoting agent modafinil. Neurosci Lett 241:95–98
Article CAS PubMed Google Scholar - Gold AE, MacLeod KM, Frier BM (1994) Frequency of severe hypoglycaemia in patients with type 1 diabetes with impaired awareness of hypoglycaemia. Diabetes Care 17:697–703
CAS PubMed Google Scholar - Pramming S, Thorsteinsson B, Bendston I, Binder C (1991) Symptomatic hypoglycaemia in 411 type 1 diabetic patients. Diabet Med 8:217–222
CAS PubMed Google Scholar
Acknowledgements
This study was supported by a research grant from the Novo Nordisk UK Research Foundation. D. Smith was supported by the King’s College Charitable Trust, and we also acknowledge the generosity of B. Cuddigan. In addition, we thank J. Jones for the insulin, growth hormone and cortisol assays.
Author information
Authors and Affiliations
- Department of Diabetes, Guy’s, King’s and St Thomas’, King’s College School of Medicine, King’s Denmark Hill Campus, Bessemer Rd, London, SE5 9PJ, UK
D. Smith, A. Pernet, J. M. Rosenthal, E. M. Bingham, H. Reid & S. A. Amiel - Department of Physiology and Pharmacology, Queens Medical Centre, University of Nottingham, UK
I. A. Macdonald
Authors
- D. Smith
You can also search for this author inPubMed Google Scholar - A. Pernet
You can also search for this author inPubMed Google Scholar - J. M. Rosenthal
You can also search for this author inPubMed Google Scholar - E. M. Bingham
You can also search for this author inPubMed Google Scholar - H. Reid
You can also search for this author inPubMed Google Scholar - I. A. Macdonald
You can also search for this author inPubMed Google Scholar - S. A. Amiel
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toS. A. Amiel.
Rights and permissions
About this article
Cite this article
Smith, D., Pernet, A., Rosenthal, J.M. et al. The effect of modafinil on counter-regulatory and cognitive responses to hypoglycaemia.Diabetologia 47, 1704–1711 (2004). https://doi.org/10.1007/s00125-004-1513-5
- Received: 05 March 2004
- Accepted: 01 June 2004
- Published: 23 October 2004
- Issue Date: October 2004
- DOI: https://doi.org/10.1007/s00125-004-1513-5