Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies (original) (raw)
Abstract
Aims/hypothesis
Diet is thought to play an important role in the aetiology of type 2 diabetes. Previous studies have found positive associations between meat consumption and the risk of type 2 diabetes, but the results have been inconsistent. We conducted a systematic review and meta-analysis of cohort studies of meat consumption and type 2 diabetes risk.
Methods
We searched several databases for cohort studies on meat consumption and type 2 diabetes risk, up to December 2008. Summary relative risks were estimated by use of a random-effects model.
Results
We identified 12 cohort studies. The estimated summary RR and 95% confidence interval of type 2 diabetes comparing high vs low intake was 1.17 (95% CI 0.92–1.48) for total meat, 1.21 (95% CI 1.07–1.38) for red meat and 1.41 (95% CI 1.25–1.60) for processed meat. There was heterogeneity amongst the studies of total, red and processed meat which, to some degree, was explained by the study characteristics.
Conclusions/interpretation
These results suggest that meat consumption increases the risk of type 2 diabetes. However, the possibility that residual confounding could explain this association cannot be excluded.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.
Introduction
The prevalence of diabetes mellitus is rapidly increasing worldwide. In 2000 an estimated 171 million people had diabetes mellitus worldwide and the number is expected to increase to 366 million by 2030 [1]. Ninety per cent of patients with diabetes have type 2 diabetes mellitus, thus type 2 diabetes accounts for most of the increase in diabetes prevalence. Patients with type 2 diabetes are at increased risk for several complications, including cardiovascular disease, retinopathies, nephropathies, neuropathies, leg ulcers and gangrene [2]. The total costs of diabetes were estimated at US$174 billion in 2007 in the USA [3].
Although overweight, obesity and physical inactivity are established risk factors for type 2 diabetes [4] and may account for much of the increase in rates of type 2 diabetes, evidence suggests that dietary factors may also influence the risk of type 2 diabetes [5]. An ecological study from 1935 [6] and subsequent migration studies [7, 8] suggest that a Western-type diet may be a risk factor for type 2 diabetes. Consistent with this, secular trends in Japan show a positive correlation between the intake of animal fat and protein and the rate of type 2 diabetes among Japanese school children [9]; similar trends have been reported in other Asian populations [10–12]. A Western dietary pattern has been associated with an increased risk of type 2 diabetes in cohort studies [13–15], but it is not clear which component(s) of this dietary pattern may increase the risk of type 2 diabetes.
Snowdon and Phillips [16] first reported an association between meat intake and diabetes in a study of Seventh Day Adventists, a population with a high proportion of vegetarians. Meat intake was associated with: an increased prevalence of diabetes in men and women; and an increased diabetes-associated mortality in men, but not in women. Several subsequent publications have reported an increased risk of diabetes or type 2 diabetes with a high intake of processed meat [17–23], red meat [17–19, 22, 24, 25] and total meat [15, 17, 23], but the results have not been consistent [20, 21, 26, 27]. To clarify this association we conducted a systematic review and meta-analysis of cohort studies of meat intake and type 2 diabetes.
Methods
Search strategy
We searched the PubMed, Medline (OVID), CINAHL and ISI Web of Science (Science Citation Index Expanded and Conference Proceedings Citation Index-Science) from their starting dates to December 2008 for the following search terms: meat, red meat, processed meat, total meat, food or nutrition combined with diabetes, diabetes mellitus or type 2 diabetes, with the searches limited to humans and adults in PubMed. We had no language restrictions in the searches. The reference lists of all the studies that were included in the analysis and of relevant systematic reviews were examined for further studies. We contacted the authors of five studies [15, 17, 20–22] to obtain sufficient detail in order to conduct a dose–response analysis and we received detailed information from all these studies.
Study selection
To be included, the study had to have a cohort design and investigate the relationship between the intake of total meat, red meat and/or processed meat and incidence or mortality of type 2 diabetes. Risk ratios, incidence rate ratios or odds ratios (hereafter referred to as relative risks) had to be available with 95% confidence intervals either in the publication or on request from the authors. For the dose–response analysis, a quantitative measure of intake had to be presented in the publication or be obtainable from the authors. When several publications from the same study were available, only the most recent or most detailed publication was used.
Data extraction
We extracted the following information from each publication into a table: the country where the study was conducted, the sample size and number of cases or deaths, the method for identification of cases and whether the method for assessing diabetes status was validated, the year the study started and ended, the duration of follow-up, the method of dietary assessment (food frequency questionnaire [FFQ], or diet history, only baseline or updated dietary assessment and whether the method had been validated), the type of meat and frequency or quantity of intake, the RRs and 95% CIs, and the factors for which adjustment was made (Table 1).
Table 1 Prospective cohort studies of meat consumption and type 2 diabetes
Statistical methods
We used random effects models, which take into account within- and between-study variation, to calculate summary RRs and 95% CIs for the highest vs the lowest level of consumption [28]. The average of the natural logarithm of the RRs was estimated and the RR from each study was weighted by the inverse of its variance. A two-tailed p < 0.05 was considered statistically significant. The statistical analyses were conducted using Stata, version 9.2 software (StataCorp, College Station, TX, USA).
We used the method described by Greenland and Longnecker [29] for the dose–response analysis and computed study-specific slopes (linear trends) and 95% CIs from the natural logs of the RRs and CIs across categories of meat intake. The method requires that the distribution of cases and person-years or non-cases and the RRs with the variance estimates for at least three quantitative exposure categories are known. For studies that did not provide the distribution of cases and person-years/non-cases, we estimated the slopes using variance-weighted least squares regression [30]. The median or mean level of meat consumption in each category of intake was assigned to the corresponding relative risk for each study. For studies that reported meat consumption by ranges of intake we estimated the mean intake in each category by calculating the average of the natural logarithm of the upper and lower boundaries, back transformed to non-log scale and rescaled to g/day [31]. When the highest category was open-ended we assumed the open-ended interval length to be the same as the adjacent interval. When the lowest category was open-ended we set the lower boundary to zero. The average serving size was set to 120 g for total meat and red meat and 50 g for processed meat [31], equivalent to a typical quarterpounder hamburger for total and red meat and one hot dog or a small sausage for processed meat.
Heterogeneity between studies was assessed by the Q test and I 2 [32]. I 2 is the amount of total variation that is explained by between study variation. I 2 values of approximately 25%, 50% and 75% are considered to indicate low, moderate and high heterogeneity, respectively. We conducted subgroup analyses by duration of follow-up (<10 years, ≥10 years), number of cases (<500, ≥500, <1,000, ≥1,000), use of updated or baseline and validated or non-validated dietary assessment methods and adjustment for confounding factors such as body mass index, physical activity, energy intake, intake of fibre/cereal fibre or whole grains, magnesium, smoking and alcohol intake. We assessed publication bias with the Egger’s test [33] and the Begg–Mazumdar’s test [34]; the results were considered to indicate publication bias when p < 0.10. To ensure that the results were not simply due to one large study or a study with an extreme result, we did a sensitivity analysis excluding one study at a time to see whether the results were robust. To assess whether the method of assessment of diabetes status influenced the results, we conducted sensitivity analyses according to whether simple self-report or additional measures were used to identify diabetes cases and whether a validation study of the diabetes-assessment method had been conducted. To address the question of temporality (i.e. whether meat intake was a risk factor for diabetes or a result of dietary changes following diabetes diagnosis) we investigated whether the studies excluded prevalent diabetes cases at baseline, and whether the results changed if cases diagnosed during early follow-up were excluded.
Results
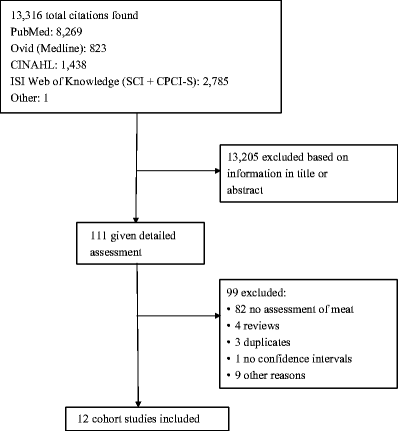
We identified 12 cohort studies in the search that could be included in the analysis (Fig. 1, Table 1). Six of the studies were from the USA, three from Europe, two from Asia and one from Australia.
Fig. 1
Flow chart for meta-analysis
Total meat
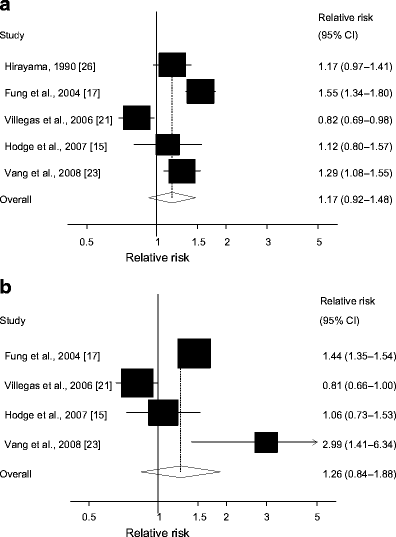
Five cohort studies [15, 17, 21, 23, 26] investigated the association between total meat intake and type 2 diabetes risk and included 6,525 cases among 445,323 participants. The summary RR for all studies was 1.17 (95% CI 0.92–1.48) (Fig. 2a), but there was substantial heterogeneity (I 2 = 86.9%, p < 0.0001). In a sensitivity analysis we excluded the most influential studies: the summary RR ranged from 1.08 (95% CI 0.86–1.35) when an American study [17] was excluded to 1.31 (95% CI 1.12–1.52) when the Chinese study [21] was excluded (results not shown). The heterogeneity was partly explained by the Chinese study [21], and when this study was excluded there was moderate heterogeneity, (I 2 = 57.3% and p = 0.07). There was no indication of publication bias with the Begg–Mazumdar’s test, p = 0.23, or with Egger’s test, p = 0.64, although these tests were based on only a few studies.
Fig. 2
Total meat and type 2 diabetes. RRs for (a) the highest vs the lowest intake and (b) per 120 g/day of total meat. The RR of each study is represented by a square and the size of the square represents the weight of each study to the overall estimate. 95% CIs are represented by the horizontal lines and the diamond represents the overall estimate and its 95% CI
Dose–response
One study with only two categories of total meat intake was excluded [26], thus four studies [15, 17, 21, 23] were included in the dose–response analysis. The summary RR per 120 g/day increase in total meat intake was 1.26 (95% CI 0.84–1.88, Fig. 2b), but there was substantial heterogeneity (I 2 = 90.6%, p < 0.0001). The heterogeneity was partly explained by the Chinese cohort study [21] and when excluded the three remaining studies yielded a summary RR of 1.46 (95% CI 1.02–2.08) with lower, but still high, heterogeneity (I 2 = 68.1%, p = 0.04).
Red meat
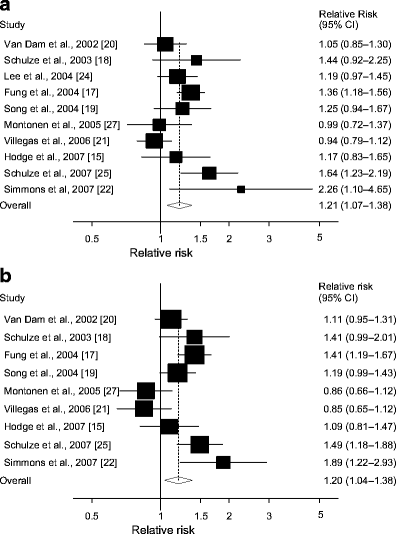
Ten cohort studies [15, 17–22, 24, 25, 27] investigated the association between red meat intake and type 2 diabetes risk; these included 12,226 cases among 433,070 participants. The summary RR for high vs low intake of red meat was 1.21 (95% CI 1.07–1.38) (Fig. 3a). In the sensitivity analysis the summary RR ranged from 1.19 (1.03–1.36) when an American study [17] was excluded to 1.26 (95% CI 1.12–1.41) when the Chinese study [21] was excluded (results not shown). There was moderate heterogeneity (I 2 = 58.5%, p = 0.01, Table 2), but the Chinese study [21] explained most of the heterogeneity and when it was excluded the heterogeneity was reduced (I 2 = 36.7%, p = 0.12; result not shown). There was significant heterogeneity in several, but not all, subgroups (Table 2). However, the heterogeneity in the subgroup analyses was mainly explained by the Chinese study and was reduced when this study was excluded (results not shown). There was no indication of publication bias with Begg–Mazumdar’s test (p = 0.38), or with Egger’s test (p = 0.46).
Fig. 3
Red meat and type 2 diabetes. RRs for (a) the highest vs the lowest intake and (b) per 120 g/day of red meat. The RR of each study is represented by a square and the size of the square represents the weight of each study to the overall estimate. 95% CIs are represented by the horizontal lines and the diamond represents the overall estimate and its 95% CI
Table 2 Subgroup analyses of red meat and processed meat and type 2 diabetesa
Dose–response
One study that did not quantify red meat intake was excluded [24], thus nine studies [15, 17–22, 25, 27] were included in the dose–response analysis. The summary RR per 120 g/day increase in red meat intake was 1.20 (95% CI 1.04–1.38, Fig. 3b), but there was high heterogeneity (I 2 = 68.3%, p = 0.001).
Processed meat
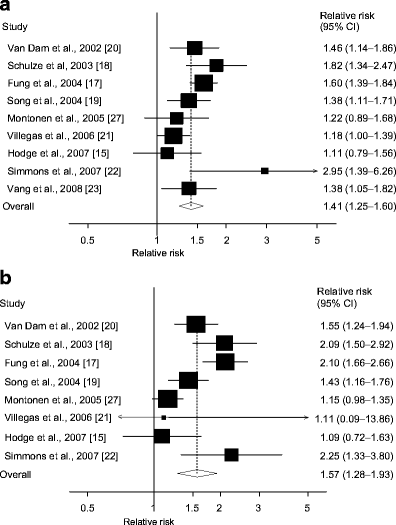
Nine cohort studies [15, 17–23, 27] investigated the association between processed meat intake and type 2 diabetes risk and included 9,999 cases among 380,606 participants. The summary RR for high vs low intake was 1.41 (95% CI 1.25–1.60) (Fig. 4a). In the sensitivity analysis the summary RR ranged from 1.37 (95% CI 1.20–1.56) when an American study [17] was excluded to 1.47 (95% CI 1.30–1.65) when the Chinese study [21] was excluded (results not shown). There was moderate heterogeneity (I 2 = 53.2%, p = 0.03, Table 2), but the latter study [21] explained most of the heterogeneity and when excluded we found the heterogeneity was reduced (I 2 = 36.6%, p = 0.14). Heterogeneity was present in several, but not all, subgroups of studies (Table 2), but when the Chinese cohort study was excluded the heterogeneity in the subgroup analyses was reduced (results not shown). There was no indication of publication bias with Begg–Mazumdar’s test (p = 0.92), or with Egger’s test (p = 0.69).
Fig. 4
Processed meat and type 2 diabetes. RRs for (a) the highest vs the lowest intake and (b) per 50 g/day of processed meat. The RR of each study is represented by a square and the size of the square represents the weight of each study to the overall estimate. 95% CIs are represented by the horizontal lines and the diamond represents the overall estimate and its 95% CI
Dose–response
One study with only two categories of intake was excluded [23], thus eight studies [15, 17–22, 27] were included in the dose–response analysis. The summary RR per 50 g/day was 1.57 (95% CI 1.28–1.93) (Fig. 4b). All studies found a positive association, but there was high heterogeneity (I 2 = 74.0%, p < 0.0001).
Subtypes of red and processed meat
Hamburgers, bacon, hot dogs and other processed meats were also associated with an increased risk of type 2 diabetes (Table 2), although these results were based on few studies.
Sensitivity analyses: assessment of type 2 diabetes status, diet instrument and temporality
We excluded two studies from the analyses of total meat and processed meat that did not clearly state that the diabetes cases ascertained were of type 2, but where this could be assumed with a degree of certainty because of the age range of the participants [23, 26]. This did not change the results (summary RRs for high vs low intake were 1.13 [95% CI 0.72–1.77] for total meat and 1.42 [95% CI 1.24–1.63] for processed meat). Furthermore, because the studies varied with respect to how they ascertained type 2 diabetes cases, we assessed whether the results varied according to the method of type 2 diabetes ascertainment (i.e. whether simple self-report was used—defined as answering yes or no to a question of type 2 diabetes diagnosis—or whether additional measures were used, including supplementary questionnaires that assessed symptoms, diagnostic tests and treatment or use of medical records, blood tests and health checks or whether type 2 diabetes status had been validated or not). Restricting the analysis to the seven studies of red meat intake that used additional measures to assess type 2 diabetes status [15, 17–20, 22, 25] or the five studies [17–20, 22] that used a validated method to ascertain type 2 diabetes status, gave slightly higher summary risk estimates (Table 2). The same was found for processed meat where six studies [15, 17–20, 22] used additional measures to assess type 2 diabetes status and five studies used a validated method to ascertain type 2 diabetes status [17–20, 22] (Table 2).
All studies but one used the FFQ to collect dietary intake data. Excluding the study that used a dietary history method [27] from the analyses of red and processed meat did not change the conclusions (the summary RRs were 1.24 [95% CI 1.08–1.42] and 1.43 [95% CI 1.26–1.64], respectively).
To address the question of temporality we investigated whether the cohort studies excluded prevalent diabetes cases at baseline and diabetes cases diagnosed during early follow-up. All cohort studies except one [26] stated that they excluded prevalent diabetes cases reported at baseline. This study was only included in the analysis of total meat and when excluded the summary RR remained unchanged. None of the cohort studies stated that they excluded diabetes cases diagnosed during early follow-up, but the subgroups of studies with longer follow-up (≥10 years)—where early follow-up would have less of an impact on the results—found significant positive associations for all the three main meat groups (Table 2, result not shown for total meat).
Discussion
In this meta-analysis, intake of red meat, processed meat and subgroups of meats (hamburger, bacon, hot dogs and other processed meats) was associated with an increased risk of type 2 diabetes.
The possible limitations of our meta-analysis must be taken into consideration. It is possible that the observed positive association between meat intake and type 2 diabetes could be due to unmeasured or residual confounding or temporal bias. Higher meat intake is often associated with other unhealthy lifestyles including physical inactivity, overweight, smoking and unhealthy dietary patterns. However, most of the studies adjusted for known confounding factors such as age, BMI, family history of type 2 diabetes, smoking, physical activity, total energy intake, alcohol, menopausal status and use of hormone replacement therapy (among women) and for other dietary factors. Studies adjusting for a ‘Western dietary pattern’ also found positive associations, suggesting that confounding from other risk-enhancing foods consumed in this dietary pattern does not explain the adverse effect of meat on the risk of developing type 2 diabetes [17, 18]. Overweight and obesity are major risk factors for type 2 diabetes and a meta-analysis suggested an approximate 20% increase in type 2 diabetes risk for each unit increase in BMI [35]. Even though most of the studies adjusted for BMI, measurement errors due to self-report of BMI could lead to residual confounding that potentially could explain the association between meat intake and type 2 diabetes. However, the studies that relied on self-report of BMI and validated the anthropometric measures found that the correlations between self-reported and measured weight and height were high [23, 36, 37].
Another possible limitation is that these findings could be due to a temporal bias. High-protein diets have been promoted for weight loss by certain groups and in the media, and it is possible that overweight type 2 diabetes patients could increase their meat intake to lose weight. If studies included prevalent type 2 diabetes cases, then these findings could be due to a temporal bias. All the studies except one [26] stated that they excluded prevalent cases at baseline, but none of them excluded cases diagnosed during early follow-up. A temporal bias would be likely to have less impact in studies with longer follow-up than in studies with shorter follow-up, because the number of cases diagnosed during early follow-up constitutes a small fraction of the total number of cases. Restricting the analysis to studies with longer follow-up (≥10 years) gave results similar to those obtained in the overall analysis.
The combination of results from several studies increases the statistical power to detect significant associations due to increased sample size, but it also results in heterogeneity. Some heterogeneity is expected as the studies used different dietary assessment methods, took place in different geographic locations and included participants who differed by age, sex and other characteristics. Heterogeneity was sometimes explained by study characteristics. In general there was significant heterogeneity in many subgroups, but because of the limited number of studies in the subgroup analyses, the results are difficult to interpret. For red and processed meat, and to some degree total meat, we found that one study conducted among Chinese women [21] contributed to the heterogeneity; when this study was excluded, both the subgroup and the overall analyses showed much less heterogeneity. The reason for the inconsistent results in this study compared with those of other studies is not clear, but it could be due to a lower absolute meat intake among the participants than in other populations [17, 19, 25].
As with any meta-analysis based on published studies, publication bias is a potential concern. The statistical tests for publication bias did not suggest the presence of publication bias in this meta-analysis, although we may have had limited statistical power in these tests because of the moderate number of studies. The overall results for red and processed meat were robust to the influence of single studies, though the Chinese study most strongly influenced the estimate for total meat, which was based on fewer studies.
Measurement errors in the exposure variable are known to bias effect estimates [38], but none of the studies in this meta-analysis corrected their results for measurement errors. Repeated exposure assessments are important in cohort studies to reduce misclassification due to dietary changes during follow-up. In a cohort study the RR of type 2 diabetes was 1.28 for high vs low haem-iron intake (as a proxy for meat intake) when using cumulative updated averages of intake, but with only the baseline questionnaire the RR was weaker and not significant (RR 1.08) [39]. Four of the twelve included studies used repeated exposure assessments. In the case of type 2 diabetes, measurement errors may not only affect the exposure variable, but also the outcome due to underreporting of diagnosis in asymptomatic individuals. The summary RRs for the association between red and processed meat and type 2 diabetes risk were slightly higher when we restricted the analyses to studies with validated methods for assessing diabetes status. Because we only included prospective cohort studies in this meta-analysis, the measurement errors in both the assessment of exposure and outcome would most likely be non-differential, and thus give attenuated risk estimates [40].
Several mechanisms may both directly and indirectly explain an adverse effect of meat intake on type 2 diabetes risk. Meat is an important source of total and saturated fat and could increase the risk of type 2 diabetes through overweight/obesity [41–44], the metabolic syndrome [45–47] and hyperinsulinaemia and hyperglycaemia [20, 48–50], although some studies found no association [51, 52]. Another possible mechanism may be through the effects of haem-iron derived from meat [19, 24, 39, 53]. Iron can promote oxidative stress by increasing the formation of hydroxyl radicals [54] which can cause damage to tissues, in particular the pancreatic beta cells [54]. Elevated iron status may interfere with glucose metabolism and may reduce pancreatic insulin synthesis and secretion [55] and one study suggested a detrimental effect of red meat intake on glucose metabolism [56]. A number of processed meats contain nitrites and nitrates which can be converted to nitrosamines by interaction with amino compounds either in the stomach or within the food product. Nitrosamines have been found to be toxic to pancreatic beta cells and to increase the risk of type 1 and type 2 diabetes in animal studies [57–59], and of type 1 diabetes in some [60, 61], but not all [62], epidemiological studies. Other possible mechanisms may involve advanced glycation end-products [[63](/article/10.1007/s00125-009-1481-x#ref-CR63 "Peppa M, Goldberg T, Cai W, Rayfield E, Vlassara H (2002) Glycotoxins: a missing link in the "relationship of dietary fat and meat intake in relation to risk of type 2 diabetes in men". Diabetes Care 25:1898–1899")], increased levels of inflammatory mediators [13, 47, 64] and γ-glutamyltransferase [65], and lower levels of adiponectin [66] with high meat intake. Our finding of a positive association between meat intake and type 2 diabetes risk is consistent with the 35–50% lower risk [23] or prevalence [16, 67, 68] of type 2 diabetes among vegetarians compared with omnivores.
In conclusion we found that high intakes of red meat and processed meat are risk factors for type 2 diabetes. We cannot completely rule out the possibility of residual confounding or a temporal bias, but if the association is real, meat could be added to the list of behavioural factors which can be modified to decrease type 2 diabetes risk.
Abbreviations
FFQ:
Food frequency questionnaire
References
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047–1053
Article PubMed Google Scholar - Anonymous (2006) Standards of medical care in diabetes—2006. Diabetes Care 29(Suppl 1):S4–S42
Google Scholar - Anonymous (2008) Economic costs of diabetes in the U.S. in 2007. Diabetes Care 31:596–615
Google Scholar - Rana JS, Li TY, Manson JE, Hu FB (2007) Adiposity compared with physical inactivity and risk of type 2 diabetes in women. Diabetes Care 30:53–58
Article PubMed Google Scholar - Tuomilehto J, Lindstrom J, Eriksson JG et al (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350
Article CAS PubMed Google Scholar - Himsworth HP (1935) Diet and the incidence of diabetes mellitus. Clin Sci 1:117–148
Google Scholar - Kawate R, Yamakido M, Nishimoto Y, Bennett PH, Hamman RF, Knowler WC (1979) Diabetes mellitus and its vascular complications in Japanese migrants on the Island of Hawaii. Diabetes Care 2:161–170
Article CAS PubMed Google Scholar - Misra A, Ganda OP (2007) Migration and its impact on adiposity and type 2 diabetes. Nutrition 23:696–708
Article PubMed Google Scholar - Kitagawa T, Owada M, Urakami T, Yamauchi K (1998) Increased incidence of non-insulin dependent diabetes mellitus among Japanese schoolchildren correlates with an increased intake of animal protein and fat. Clin Pediatr (Phila) 37:111–115
Article CAS Google Scholar - Wang Y, Mi J, Shan XY, Wang QJ, Ge KY (2007) Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 31:177–188
Article CAS Google Scholar - Cheng Y, Chen KJ, Wang CJ, Chan SH, Chang WC, Chen JH (2005) Secular trends in coronary heart disease mortality, hospitalization rates, and major cardiovascular risk factors in Taiwan, 1971–2001. Int J Cardiol 100:47–52
Article PubMed Google Scholar - Choi YJ, Cho YM, Park CK et al (2006) Rapidly increasing diabetes-related mortality with socio-environmental changes in South Korea during the last two decades. Diabetes Res Clin Pract 74:295–300
Article PubMed Google Scholar - Schulze MB, Hoffmann K, Manson JE et al (2005) Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr 82:675–684
CAS PubMed Google Scholar - van Dam RM, Rimm EB, Willett WC, Stampfer MJ, Hu FB (2002) Dietary patterns and risk for type 2 diabetes mellitus in U.S. men. Ann Intern Med 136:201–209
PubMed Google Scholar - Hodge AM, English DR, O'Dea K, Giles GG (2007) Dietary patterns and diabetes incidence in the Melbourne Collaborative Cohort Study. Am J Epidemiol 165:603–610
Article PubMed Google Scholar - Snowdon DA, Phillips RL (1985) Does a vegetarian diet reduce the occurrence of diabetes? Am J Public Health 75:507–512
Article CAS PubMed Google Scholar - Fung TT, Schulze M, Manson JE, Willett WC, Hu FB (2004) Dietary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med 164:2235–2240
Article PubMed Google Scholar - Schulze MB, Manson JE, Willett WC, Hu FB (2003) Processed meat intake and incidence of Type 2 diabetes in younger and middle-aged women. Diabetologia 46:1465–1473
Article CAS PubMed Google Scholar - Song Y, Manson JE, Buring JE, Liu S (2004) A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: the women's health study. Diabetes Care 27:2108–2115
Article CAS PubMed Google Scholar - van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB (2002) Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 25:417–424
Article PubMed Google Scholar - Villegas R, Shu XO, Gao YT et al (2006) The association of meat intake and the risk of type 2 diabetes may be modified by body weight. Int J Med Sci 3:152–159
CAS PubMed Google Scholar - Simmons RK, Harding AH, Wareham NJ, Griffin SJ (2007) Do simple questions about diet and physical activity help to identify those at risk of type 2 diabetes? Diabet Med 24:830–835
Article CAS PubMed Google Scholar - Vang A, Singh PN, Lee JW, Haddad EH, Brinegar CH (2008) Meats, processed meats, obesity, weight gain and occurrence of diabetes among adults: findings from adventist health studies. Ann Nutr Metab 52:96–104
Article CAS PubMed Google Scholar - Lee DH, Folsom AR, Jacobs DR Jr (2004) Dietary iron intake and Type 2 diabetes incidence in postmenopausal women: the Iowa Women's Health Study. Diabetologia 47:185–194
Article PubMed Google Scholar - Schulze MB, Hoffmann K, Boeing H et al (2007) An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care 30:510–515
Article PubMed Google Scholar - Hirayama T (1990) Life-style and mortality: a large-scale census-based cohort study in Japan. Karger, Basel
Google Scholar - Montonen J, Jarvinen R, Heliovaara M, Reunanen A, Aromaa A, Knekt P (2005) Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr 59:441–448
Article CAS PubMed Google Scholar - DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Article CAS PubMed Google Scholar - Greenland S, Longnecker MP (1992) Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135:1301–1309
CAS PubMed Google Scholar - Orsini N, Bellocco R, Greenland S (2006) Generalized least squares for trend estimation of summarized dose-response data. Stata Journal 6:40–57
Google Scholar - Norat T, Lukanova A, Ferrari P, Riboli E (2002) Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer 98:241–256
Article CAS PubMed Google Scholar - Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Article PubMed Google Scholar - Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
CAS PubMed Google Scholar - Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–1101
Article CAS PubMed Google Scholar - Hartemink N, Boshuizen HC, Nagelkerke NJ, Jacobs MA, van Houwelingen HC (2006) Combining risk estimates from observational studies with different exposure cutpoints: a meta-analysis on body mass index and diabetes type 2. Am J Epidemiol 163:1042–1052
Article PubMed Google Scholar - Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC (1990) Validity of self-reported waist and hip circumferences in men and women. Epidemiology 1:466–473
Article CAS PubMed Google Scholar - Kushi LH, Kaye SA, Folsom AR, Soler JT, Prineas RJ (1988) Accuracy and reliability of self-measurement of body girths. Am J Epidemiol 128:740–748
CAS PubMed Google Scholar - Kipnis V, Subar AF, Midthune D et al (2003) Structure of dietary measurement error: results of the OPEN biomarker study. Am J Epidemiol 158:14–21
Article PubMed Google Scholar - Rajpathak S, Ma J, Manson J, Willett WC, Hu FB (2006) Iron intake and the risk of type 2 diabetes in women: a prospective cohort study. Diabetes Care 29:1370–1376
Article CAS PubMed Google Scholar - Rothman KJ, Greenland S (1998) Modern epidemiology, 2nd edn. Lippincott Williams & Wilkins, Philadelphia
Google Scholar - French SA, Jeffery RW, Forster JL, McGovern PG, Kelder SH, Baxter JE (1994) Predictors of weight change over two years among a population of working adults: the Healthy Worker Project. Int J Obes Relat Metab Disord 18:145–154
CAS PubMed Google Scholar - Kahn HS, Tatham LM, Heath CW Jr (1997) Contrasting factors associated with abdominal and peripheral weight gain among adult women. Int J Obes Relat Metab Disord 21:903–911
Article CAS PubMed Google Scholar - Schulz M, Kroke A, Liese AD, Hoffmann K, Bergmann MM, Boeing H (2002) Food groups as predictors for short-term weight changes in men and women of the EPIC-Potsdam cohort. J Nutr 132:1335–1340
CAS PubMed Google Scholar - Bes-Rastrollo M, Sanchez-Villegas A, Gomez-Gracia E, Martinez JA, Pajares RM, Martinez-Gonzalez MA (2006) Predictors of weight gain in a Mediterranean cohort: the Seguimiento Universidad de Navarra Study 1. Am J Clin Nutr 83:362–370
CAS PubMed Google Scholar - Damiao R, Castro TG, Cardoso MA, Gimeno SG, Ferreira SR (2006) Dietary intakes associated with metabolic syndrome in a cohort of Japanese ancestry. Br J Nutr 96:532–538
CAS PubMed Google Scholar - Lutsey PL, Steffen LM, Stevens J (2008) Dietary intake and the development of the metabolic syndrome: the atherosclerosis risk in communities study. Circulation 117:754–761
Article PubMed Google Scholar - Azadbakht L, Esmaillzadeh A (2009) Red meat intake is associated with metabolic syndrome and the plasma C-reactive protein concentration in women. J Nutr 139:335–339
CAS PubMed Google Scholar - Feskens EJ, Virtanen SM, Rasanen L et al (1995) Dietary factors determining diabetes and impaired glucose tolerance. A 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care 18:1104–1112
Article CAS PubMed Google Scholar - Mayer-Davis EJ, Monaco JH, Hoen HM et al (1997) Dietary fat and insulin sensitivity in a triethnic population: the role of obesity. The Insulin Resistance Atherosclerosis Study (IRAS). Am J Clin Nutr 65:79–87
CAS PubMed Google Scholar - Mayer EJ, Newman B, Quesenberry CP Jr, Selby JV (1993) Usual dietary fat intake and insulin concentrations in healthy women twins. Diabetes Care 16:1459–1469
Article CAS PubMed Google Scholar - Lundgren H, Bengtsson C, Blohme G et al (1989) Dietary habits and incidence of noninsulin-dependent diabetes mellitus in a population study of women in Gothenburg, Sweden. Am J Clin Nutr 49:708–712
CAS PubMed Google Scholar - Meyer KA, Kushi LH, Jacobs DR Jr, Folsom AR (2001) Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care 24:1528–1535
Article CAS PubMed Google Scholar - Jiang R, Ma J, Ascherio A, Stampfer MJ, Willett WC, Hu FB (2004) Dietary iron intake and blood donations in relation to risk of type 2 diabetes in men: a prospective cohort study. Am J Clin Nutr 79:70–75
CAS PubMed Google Scholar - Wolff SP (1993) Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br Med Bull 49:642–652
CAS PubMed Google Scholar - Rajpathak SN, Crandall JP, Wylie-Rosett J, Kabat GC, Rohan TE, Hu FB (2009) The role of iron in type 2 diabetes in humans. Biochim Biophys Acta 1790:671–681
CAS PubMed Google Scholar - Papakonstantinou E, Panagiotakos DB, Pitsavos C et al (2005) Food group consumption and glycemic control in people with and without type 2 diabetes: the ATTICA study. Diabetes Care 28:2539–2540
Article PubMed Google Scholar - Helgason T, Ewen SW, Ross IS, Stowers JM (1982) Diabetes produced in mice by smoked/cured mutton. Lancet 2:1017–1022
Article CAS PubMed Google Scholar - Portha B, Giroix MH, Cros JC, Picon L (1980) Diabetogenic effect of N-nitrosomethylurea and N-nitrosomethylurethane in the adult rat. Ann Nutr Aliment 34:1143–1151
CAS PubMed Google Scholar - Ito M, Kondo Y, Nakatani A, Naruse A (1999) New model of progressive non-insulin-dependent diabetes mellitus in mice induced by streptozotocin. Biol Pharm Bull 22:988–989
CAS PubMed Google Scholar - Dahlquist GG, Blom LG, Persson LA, Sandstrom AI, Wall SG (1990) Dietary factors and the risk of developing insulin dependent diabetes in childhood. BMJ 300:1302–1306
Article CAS PubMed Google Scholar - Sipetic SB, Vlajinac HD, Kocev NI, Marinkovic JM, Radmanovic SZ, Bjekic MD (2005) The Belgrade childhood diabetes study: a multivariate analysis of risk determinants for diabetes. Eur J Public Health 15:117–122
Article PubMed Google Scholar - Siemiatycki J, Colle E, Campbell S, Dewar RA, Belmonte MM (1989) Case-control study of IDDM. Diabetes Care 12:209–216
Article CAS PubMed Google Scholar - Peppa M, Goldberg T, Cai W, Rayfield E, Vlassara H (2002) Glycotoxins: a missing link in the "relationship of dietary fat and meat intake in relation to risk of type 2 diabetes in men". Diabetes Care 25:1898–1899
Article PubMed Google Scholar - Aeberli I, Molinari L, Spinas G, Lehmann R, l'Allemand D, Zimmermann MB (2006) Dietary intakes of fat and antioxidant vitamins are predictors of subclinical inflammation in overweight Swiss children. Am J Clin Nutr 84:748–755
CAS PubMed Google Scholar - Lee DH, Steffen LM, Jacobs DR Jr (2004) Association between serum gamma-glutamyltransferase and dietary factors: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr 79:600–605
CAS PubMed Google Scholar - Zyriax BC, Algenstaedt P, Hess UF et al (2008) Factors contributing to the risk of cardiovascular disease reflected by plasma adiponectin: data from the coronary risk factors for atherosclerosis in women (CORA) study. Atherosclerosis 200:403–409
Article CAS PubMed Google Scholar - Fraser GE (1999) Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am J Clin Nutr 70:532S–538S
CAS PubMed Google Scholar - Tonstad S, Butler T, Yan R, Fraser GE (2009) Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 32:791–796
Article PubMed Google Scholar
Acknowledgements
This project has received funding from the Throne Holst Foundation and Statistics for Innovation (SFI)2. We thank: A. Hodge for supplementary data from the Melbourne Collaborative Cohort Study; T. Fung for supplementary data from the Nurses Health Study 2; R. van Dam for supplementary data from the Health Professionals’ Follow-up Study; R. Villegas for supplementary data from the Shanghai Women’s Health Study; and R. Simmons for supplementary data from the EPIC-Norfolk study.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
- Department of Biostatistics, Institute of Basic Medical Sciences, University of Oslo, P.O. Box 1122, 0317, Oslo, Norway
D. Aune & M. B. Veierød - Department of Nutrition, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway
D. Aune, G. Ursin & M. B. Veierød - Department of Preventive Medicine, Kennet J. Norris Comprehensive Cancer Center, University of Southern California Keck School of Medicine, Los Angeles, CA, USA
G. Ursin
Authors
- D. Aune
- G. Ursin
- M. B. Veierød
Corresponding author
Correspondence toD. Aune.
Rights and permissions
About this article
Cite this article
Aune, D., Ursin, G. & Veierød, M.B. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies.Diabetologia 52, 2277–2287 (2009). https://doi.org/10.1007/s00125-009-1481-x
- Received: 05 May 2009
- Accepted: 02 July 2009
- Published: 07 August 2009
- Issue Date: November 2009
- DOI: https://doi.org/10.1007/s00125-009-1481-x