Is the ADA/EASD algorithm for the management of type 2 diabetes (January 2009) based on evidence or opinion? A critical analysis (original) (raw)
Abstract
The ADA and the EASD recently published a consensus statement for the medical management of hyperglycaemia in patients with type 2 diabetes. The authors advocate initial treatment with metformin monotherapy and lifestyle modification, followed by addition of basal insulin or a sulfonylurea if glycaemic goals are not met (tier 1 recommendations). All other glucose-lowering therapies are relegated to a secondary (tier 2) status and only recommended for selected clinical settings. In our view, this algorithm does not offer physicians and patients the appropriate selection of options to individualise and optimise care with a view to sustained control of blood glucose and reduction both of diabetes complications and cardiovascular risk. This paper critically assesses the basis of the ADA/EASD algorithm and the resulting tiers of treatment options.
Introduction
In August 2006, the ADA and the EASD published a joint consensus algorithm for the medical management of hyperglycaemia in type 2 diabetes [1]. Recently, an update introduced a two-tier categorisation of ‘well validated’ and ‘less well validated’ therapies [2].
Tier 1 treatments are initial metformin monotherapy and lifestyle modification, followed by addition of basal insulin or a sulfonylurea if glycaemic goals are not met. These interventions are considered to be: ‘the best established and most effective and cost-effective therapeutic strategy for achieving the target glycaemic goals’. Although the authors, Nathan et al., endorse metformin plus insulin as a particularly effective combination, in practice most physicians and patients faced with these second-line options are likely to choose metformin plus a sulfonylurea. Recommended tier 2 approaches for second-line therapy comprise metformin plus either a thiazolidinedione (pioglitazone, since the authors advise against using rosiglitazone) or a glucagon-like peptide-1 (GLP-1) receptor agonist. The tier 2 treatments are recommended for consideration in selected clinical settings only.
The description of this publication as a consensus statement of the ADA and EASD is misleading, as it has not been formally endorsed by the two organisations. Indeed, the ADA states that ‘consensus statements…are not official ADA recommendations’, that they are ‘produced under the auspices of the Association by invited experts’ and that they are ‘not subject to subsequent review or approval’ [3]. In addition, the organisation has declared that the consensus statement represents the authors’ views and not the official opinion of the association [2]. Nevertheless, the recommendations have been published under the auspices of the two societies and are likely to have considerable influence.
We are concerned that the authors of the consensus statement have not consistently employed an evidence-based approach; we also find many of their recommendations questionable. However, we acknowledge that some data were not available at the time of publication of the updated consensus statement. This paper critically assesses the basis of the purported consensus and the resulting tiers of treatment options.
Development process
Evidence-based guidelines have advanced medical practice and supported optimal prescribing for many diseases, and processes for their development are well established [4–6]. At the evidence collation stage, a systematic review of data is performed using a search strategy designed to identify all relevant data. The evidence base typically comprises a complex mix of data of variable quality and relevance, necessitating precise and explicit grading criteria [7]. A systematic review may be followed by a meta-analysis, i.e. a mathematical method of pooling the results of studies that meet predefined criteria. In the absence of a suitable body of evidence, expert/consensus opinion may be used. However, such opinion becomes less influential as the evidence grows. While gaps exist in the management of type 2 diabetes, the evidence base is sufficiently large to allow an evidence-based approach for many aspects. Current ADA standards of care in diabetes therefore classify expert consensus or clinical experience as the lowest forms of evidence [8]. Once collated, a working group discusses the data based on the evidence-based tables and draws conclusions. Guidelines are then developed and graded or weighted according to the strength of the supporting evidence. The draft guidelines should be subjected to peer (and sometimes public) review before being finalised.
The recommendations of Nathan et al. [2] do not appear to meet many of these standards. For example, the strategies used to search for data systematically are not stated and there is no formal grading of evidence. The authors cite the use of ‘clinical judgment, that is, our collective knowledge and clinical experience’ as a principal secondary source of evidence. The panel comprised only seven physicians (five North American, two European). It is therefore questionable whether some recommendations can reflect the available evidence base, as outlined below in terms of the key attributes of glucose-lowering treatments.
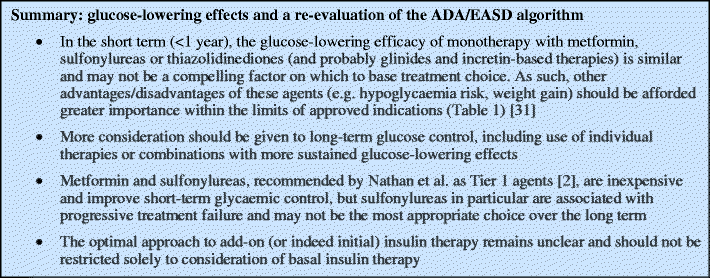
Glucose-lowering effects
The selection of glycaemic targets and glucose-lowering treatments should be individualised on the basis of patient-specific factors (age, stage of diabetes, cardiovascular risk factors, weight, risk associated with hypoglycaemia etc.) and of effects on multiple pathophysiological aspects of type 2 diabetes [9].
According to Nathan et al., glucose-lowering efficacy is the principal factor by which drugs should be differentiated. Their algorithm states that ‘The over-arching principle in selecting a particular intervention will be its ability to achieve and maintain glycaemic goals’ [2]. They tabulate the reductions in HbA1c expected with different classes used as monotherapy, but provide few supporting references. Sulfonylureas and metformin are each said to reduce HbA1c by 1.0% to 2.0%, although the baseline levels, time-scale, patient populations, specific agent and dose are not defined. Thiazolidinediones are said to reduce HbA1c by 0.5% to 1.4%, suggesting lower glucose-lowering efficacy, but this is not supported by evidence from large, randomised head-to-head trials, which found no significant differences vs sulfonylureas or metformin [10, 11] and better long-term efficacy for thiazolidinediones [12]. A systematic evidence-based review also supports the view that these agents produce similar absolute reductions in HbA1c [13]. We agree with Nathan et al. on the importance of maintaining long-term glycaemic control. In this context, the relegation of thiazolidinediones appears puzzling in light of evidence from A Diabetes Outcome Progression Trial (ADOPT), where rosiglitazone was significantly better than glibenclamide or metformin at maintaining glycaemic targets over 4 years [12].
Meta-analysis of randomised controlled trials indicates that GLP-1 receptor agonists (exenatide, liraglutide) also reduce HbA1c by ∼1% and are non-inferior to active comparators [14]. In a head-to-head study, liraglutide was more effective than exenatide, presumably due to its longer half-life [15]. On meta-analysis, the dipeptidyl peptidase-4 (DPP-IV) inhibitors (sitagliptin, vildagliptin) were slightly less effective than other oral glucose-lowering agents [14, 16], but were non-inferior to sulfonylureas over 52 weeks when added to metformin [17, 18].
In type 2 diabetes, insulin is commonly initiated as add-on therapy either as a basal dose of a long-acting analogue (insulin glargine [A21Gly,B31Arg,B32Arg human insulin] or insulin detemir [B29Lys(e-tetradecanoyl),desB30 human insulin]) or prandially using biphasic (premixed) formulations, although the optimal approach and most efficient use of the different long-acting, intermediate-acting (e.g. NPH insulin), rapid-acting and biphasic formulations remains controversial [19]. Nathan et al. recommend the initiation of basal insulin, followed by intensification, if required. However, a recent meta-analysis suggests that greater reductions in HbA1c can be achieved using biphasic or rapid-acting prandial formulations rather than a basal approach [20]. Although the recent 3-year results of the Treating To Target in Type 2 Diabetes study (4-T study) showed that basal (detemir-based) or prandial (insulin aspart [B28Asp human insulin]-based) insulin regimens provided better glycaemic control when added to oral therapy vs adding to a biphasic (aspart-based) regimen, total insulin dose was highest in the basal group (88 U), prandial insulin use was higher in the basal group (51 vs 28 U in the biphasic group) and most patients eventually received more complex insulin regimens irrespective of initial therapy [21]. Glargine appears to offer no benefit in terms of glycaemic control over NPH insulin, while detemir might be slightly less effective than NPH [22].
Clearly, basal insulin has the advantage of greater convenience. Moreover, detemir and glargine are associated with less overall hypoglycaemia than multiple daily injections of rapid-acting analogues and biphasic or NPH insulin [22–27]. However, a systematic review suggests that biphasic insulin is not associated with more nocturnal or more severe hypoglycaemia than basal insulin analogues [27]. In recent head-to-head studies, there was no difference in glycaemic control or hypoglycaemia with glargine vs detemir [28, 29].
Thus, basal insulin has potential advantages over biphasic or prandial insulin regimens in terms of less hypoglycaemia and less weight gain (see below). However, accumulating evidence indicates that control of postprandial hyperglycaemia is also important in achieving HbA1c goals [30]. We suggest that, in some patients, the glycaemic benefits of biphasic or prandial insulin regimens outweigh the risk of hypoglycaemia and these regimens should be positioned as alternatives for initial insulin therapy according to an individualised approach.
Cardiovascular benefit–risk relationships
The effects of glucose-lowering treatments on cardiovascular outcomes are of central importance, as cardiovascular disease is the major cause of death in patients with diabetes. The consensus statement algorithm states: ‘there are insufficient data to support one class (or combination) of glucose-lowering agents over another with regard to their effects on complications’ [2]. Certainly, few prospective studies have assessed cardiovascular outcomes during long-term treatment and the cardiovascular benefit-risk relationship of some agents and combinations remains controversial.
Metformin
In the UK Prospective Diabetes Study (UKPDS), ‘intensive’ treatment starting with metformin in overweight patients reduced the rate of all micro- and macrovascular complications vs less intensive diet-based treatment alone. This reduction was significantly greater than with sulfonylureas or insulin [32]. Metformin also conferred significant reductions in diabetes-related death, all-cause death, myocardial infarction and all macrovascular events combined vs conventional treatment. A significant benefit vs sulfonylureas or insulin was seen for all-cause death [33]. On 10-year post-interventional follow-up, the significant reductions in myocardial infarction, death and any diabetes-related endpoint persisted [33]. Observational analyses have also shown reduced rates of all-cause and cardiovascular mortality with metformin vs sulfonylurea monotherapy [34–36].
Therefore, there is some evidence for a significant beneficial effect of initial metformin monotherapy on cardiovascular outcomes. The UKPDS is often considered to be the most compelling evidence for a macrovascular benefit of any single glucose-lowering medication. However, the sample size was relatively small by current standards. As Nathan et al. note, these findings require confirmation [2].
Sulfonylureas
There are no prospective data clearly supporting an effect of sulfonylureas on macrovascular outcomes. In 1970, the University Group Diabetes Program (UGDP) Study reported a link between tolbutamide and increased cardiovascular risk [37]. In the UKPDS, ‘intensive’ therapy starting with sulfonylureas or insulin reduced microvascular complications (mostly retinopathy) vs diet alone over 11 years, but did not significantly reduce mortality or macrovascular complications (a 16% relative reduction in myocardial infarction had borderline statistical significance) [38]. Individually, neither chlorpropamide nor glibenclamide significantly reduced these endpoints. After 10 years of post-interventional, observational follow-up, significant reductions in myocardial infarction and death were observed in patients initially randomised to sulfonylureas or insulin vs conventional therapy, despite the convergence of glycaemic control and treatments [33]. However, this analysis did not differentiate the relative effect of sulfonylureas or insulin.
Recently, the Action in Diabetes and Vascular Disease: Preterax and Diamicron-MR Controlled Evaluation (ADVANCE) trial showed that intensive therapy based on gliclazide significantly reduced the risk of a combined macrovascular/microvascular endpoint (driven mostly by reduced nephropathy) vs less intensive therapy, but had no significant effect on macrovascular events alone [39].
Observational analyses have shown higher rates of all-cause and cardiovascular mortality with sulfonylurea vs metformin monotherapy [34–37]. Sulfonylurea use was also associated with in-hospital mortality among patients undergoing coronary angioplasty [40].
Metformin plus sulfonylureas
Sulfonylureas are the only oral agents recommended by Nathan et al. for routine addition to metformin monotherapy [2]. No prospective studies have demonstrated a benefit of this combination on diabetes complications. Indeed, concerns about adverse cardiovascular effects of biguanide/sulfonylurea combination therapy were raised by the UGDP study [41]. Subsequently, in the UKPDS, the addition of metformin to sulfonylurea therapy was associated with an increased risk of diabetes-related and all-cause death, although this was not confirmed by an epidemiological analysis [32].
Observational studies have analysed cardiovascular outcomes for metformin/sulfonylurea combination therapy with conflicting results. Some found an increased risk of all-cause and cardiovascular mortality, while others found no association or reduced risk [42]. The difficulty of excluding bias from observational studies is well known and the potential for confounding should be considered. However, a meta-analysis showed an increased risk of the composite of cardiovascular hospitalisation or mortality with sulfonylureas plus metformin vs either metformin monotherapy, sulfonylurea monotherapy or diet [42].
Insulin
Intensive insulin therapy has been shown to protect against long-term macrovascular complications in type 1 diabetes [43] and against microvascular complications in type 1 and type 2 diabetes [38, 44, 45]. However, there is no clear evidence that insulin treatment as such reduces the risk of macrovascular outcomes in type 2 diabetes [46].
In the UKPDS, insulin had no significant effect on any macrovascular outcome [38] and its contribution to the delayed benefit of intensive therapy at follow-up was not investigated [33]. Observational studies have had conflicting results, including increased and decreased risk of cardiovascular events vs other therapies [47–50].
In the Diabetes and Insulin-Glucose infusion in Acute Myocardial Infarction (DIGAMI) study in type 2 diabetes, insulin infusion followed by insulin injections reduced long-term mortality rates by 28% relative to conventional routine glucose-lowering therapy [51]. This contrasted with DIGAMI-2, which reported no difference in total mortality rates and a trend towards more non-fatal recurrent myocardial infarction and stroke in patients receiving acute and chronic insulin therapy vs routine therapy (with or without acute insulin) [52]. A post-hoc analysis from DIGAMI-2 found that the risk of non-fatal myocardial infarction and stroke increased significantly in patients on insulin at discharge (vs no insulin), was unchanged with sulfonylureas and decreased with metformin [53]. The Hyperglycemia and its Effect after Acute Myocardial Infarction on Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus (HEART2D) Study failed to show any benefit of prandial vs basal insulin on cardiovascular outcomes following acute myocardial infarction [54].
Thiazolidinediones
The effect of thiazolidinediones on cardiovascular outcomes has received considerable attention in recent years and these agents are now perhaps the best studied in this respect.
Data from several sources suggest that cardiovascular risk is reduced with pioglitazone [55–58]. In the PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive) trial, participants with type 2 diabetes and macrovascular disease were randomised to pioglitazone vs placebo, alongside guideline-driven therapy [55, 56]. The primary endpoint, a composite of coronary, cerebrovascular and peripheral macrovascular events, showed a trend towards benefit from pioglitazone. The main secondary endpoint (death, myocardial infarction or stroke) showed a significant effect favouring pioglitazone. In subgroup analyses, pioglitazone significantly reduced the risk of recurrent myocardial infarction and recurrent stroke [56]. In subsequent meta-analyses, pioglitazone was associated with reduced rates of all-cause death [57] and of the composite of death, myocardial infarction and stroke [58]. In a UK retrospective cohort study, pioglitazone was associated with a lower risk of all-cause mortality than metformin and a favourable risk profile vs rosiglitazone [36].
Nathan et al. [2] note well publicised meta-analysis data suggesting an increased risk of myocardial infarction with rosiglitazone [59, 60] and advise against its use [2]. However, additional meta-analyses have not all reached the same conclusion [61]. Recently, the Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD) study, which looked at rosiglitazone added to metformin or a sulfonylurea vs metformin/sulfonylurea combination, was inconclusive on possible adverse effects on myocardial infarction, but suggested no impact on overall cardiovascular morbidity or mortality [62]. Large observational analyses have contributed additional real-world evidence with conflicting results [63, 64]. Thus, the cardiovascular benefit–risk profile of rosiglitazone remains controversial.
Incretin-based therapies
Glucagon-like peptide-1 infusion has been shown to confer beneficial cardiovascular effects (using ‘soft’ surrogate endpoints) in patients with or without diabetes [65]. Moreover, animal studies with GLP-1 agonists suggest the potential to reduce infarct size and improve survival after myocardial infarction [65–67]. However, no completed clinical studies have yet examined the effect of GLP-1 agonists or DPP-IV inhibitors on primary ‘hard’ cardiovascular endpoints.

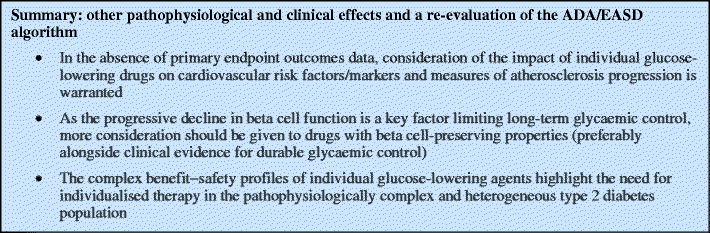
Other important pathophysiological and clinical effects
Nathan et al. acknowledge that drug effects on non-glycaemic cardiovascular risk factors may be important [2]. However, little explicit consideration of the evidence supporting the relative benefit of different agents is provided, and these properties do not appear to have influenced the recommendations. We argue that effects on the pathophysiological abnormalities in type 2 diabetes and in cardiovascular disease warrant greater consideration.
Beta cell protection
The importance of progressive beta cell failure in the pathophysiology of type 2 diabetes is well recognised [9, 68]. Sulfonylureas, in particular, are associated with rapid beta cell decline and treatment failure [9, 12, 32, 38]. Although metformin is associated with beta cell decline, studies suggest that it is not as marked as with sulfonylureas [9, 12, 32].
Accumulating data suggest that thiazolidinediones, GLP-1 agonists and DPP-IV inhibitors may help to maintain beta cell mass and function [9, 68]. For thiazolidinediones, this is consistent with: (1) the maintenance of durable glucose control seen in randomised controlled trials over several years [9, 12]; (2) the delay of treatment failure with rosiglitazone vs either metformin or glibenclamide in ADOPT [12]; and (3) the delayed progression to diabetes seen in prediabetic patients [69, 70].
Analyses of intensive insulin therapy vs oral agents (metformin, gliclazide) in patients with new-onset type 2 diabetes found that recovery and maintenance of beta cell function (HOMA-B) was more favourably affected with insulin [71, 72].
In clinical studies with adjunctive exenatide, short-term reductions in HbA1c have been maintained for over 3 years during open-label extension [9, 15, 73]. Beta cell function was significantly improved with exenatide compared with insulin glargine over 1 year, but returned to pre-treatment values 4 weeks after treatment cessation [74]. Evidence from short-term clinical studies suggests that liraglutide and DPP-IV inhibitors may also benefit beta cell function [9, 15].
Anti-atherogenic effects
Atherogenic risk factors associated with type 2 diabetes include a characteristic dyslipidaemia profile, subclinical inflammation, hypertension and obesity [75]. Different glucose-lowering agents have very distinct patterns of effects on these factors, which may confer antiatherogenic benefits (Table 1). Metformin appears to improve the lipid profile, with decreases in triacylglycerol and LDL-cholesterol levels and (in some studies) increases in HDL-cholesterol [76]. Thiazolidinediones improve diabetic dyslipidaemia, with benefits for pioglitazone over sulfonylureas, metformin and rosiglitazone [77, 78]. A systematic review found that, while thiazolidinediones, sulfonylureas and metformin were equally effective at improving glycaemic control, only metformin improved LDL-cholesterol, only thiazolidinediones improved HDL-cholesterol, and both metformin and thiazolidinediones improved blood pressure [13]. Studies using surrogate clinical measures of atherosclerosis showed that pioglitazone significantly slowed progression of carotid intima–media thickness and prevented progression of coronary atherosclerosis vs glimepiride [79, 80]. Insulin may exert anti-inflammatory actions that could be anti-atherogenic/cardioprotective, although this remains controversial [81]. Insulin may also lower LDL-cholesterol and triacylglycerol levels [82, 83].
Table 1 Evidence-based clinical advantages and disadvantages of current glucose-lowering therapies in type 2 diabetes
Exenatide and liraglutide may also exert benefits beyond glucose control, such as reduced blood pressure and weight loss [15]. While exenatide had no short-term effect on plasma lipids, significant benefits were observed during 3 years of open-label treatment in responders [73]. DPP-IV inhibitors may affect postprandial lipaemia [84].
Therapeutic effects of glucose-lowering agents on inflammatory mediators, haemostasis markers and other factors such as the anti-inflammatory mediator adiponectin (which is increased by thiazolidinediones) may also have clinical relevance [85, 86].
Effects on body weight
Management of type 2 diabetes should not neglect effects on body weight. Weight gain is an important disadvantage of sulfonylurea and insulin therapy. In the UKPDS, absolute average weight gain was 6.5 kg in the insulin group over 10 years. Relative to dietary therapy, it was 4.0, 2.6 and 1.7 kg with insulin, chlorpropamide and glibenclamide, respectively [38]. Although all insulins increase body weight, prandial (and probably biphasic) regimens generally produce more weight gain than basal regimens [20]. Basal detemir, in particular, consistently shows less weight gain than other formulations, including NPH and glargine [22, 28, 29].
Pioglitazone and rosiglitazone also produce weight gain. In PROactive, the increase was 3.6 kg with pioglitazone over 3 years and in ADOPT it was 4.8 kg with rosiglitazone over 5 years [12, 55]. Despite this, thiazolidinediones ameliorate insulin resistance and the weight gain appears to correlate with improvements in HbA1c [87, 88].
Exenatide, liraglutide and metformin reduce body weight in monotherapy and limit weight gain in combination with sulfonylureas, thiazolidinediones and/or insulin [15, 89]. DPP-IV inhibitors are essentially weight neutral [16].
Consideration of adverse effects
Fluid retention and congestive heart failure
The potential for fluid retention and exacerbation of congestive heart failure (CHF) with thiazolidinediones is well recognised [90]. However, this does not appear to increase cardiovascular mortality rates and appropriate treatment of oedema will prevent CHF [90]. In PROactive, pioglitazone recipients experienced more serious heart failure events than participants on placebo, but without increased heart failure mortality rates [90]. Among patients with serious heart failure events, pioglitazone significantly lowered the risk of the main secondary endpoint vs placebo, with a trend towards lower risk for the primary endpoint and all-cause mortality [90]. A meta-analysis of controlled studies concluded that metformin is the only glucose-lowering agent not associated with measurable harm in patients with diabetes and heart failure, although randomised trials are lacking and warnings concerning lactic acidosis remain [91].
Bone fracture risk
Pioglitazone and rosiglitazone are associated with double the risk of fractures vs other oral agents [92]. Rates are two to three fractures per 100 patient-years, with most occurring in the distal long bones and related to trauma. This risk should be a particular consideration in postmenopausal women.
Gastrointestinal side effects
One of the few limitations of metformin is intolerance to its gastrointestinal side effects in a moderate proportion of patients [31]. This is also the main adverse event associated with exenatide [31].
Acute pancreatitis
Post-marketing cases of acute pancreatitis (including haemorrhagic/necrotising pancreatitis) have been reported with incretin-based therapies, including exenatide and sitagliptin [[93](/article/10.1007/s00125-010-1702-3#ref-CR93 "US Food and Drug Administration (2008) Exenatide (marketed as Byetta) information (18 August 2008 update). Available from www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124713.htm
, accessed 16 January 2010"), [94](/article/10.1007/s00125-010-1702-3#ref-CR94 "U.S. Food and Drug Administration (2008) Sitagliptin (marketed as Januvia and Janumet) information (issued 25 September 2009). Available from
www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm183768.htm
, accessed 16 January 2010")\]. In clinical trials, however, the incidence was 1.79/1,000 person-years for exenatide (seven cases), 2.72 with placebo and 1.35 for comparators \[[95](/article/10.1007/s00125-010-1702-3#ref-CR95 "Bain SC, Stephens JW (2008) Exenatide and pancreatitis: an update. Expert Opin Drug Saf 7:643–644")\]. Recently, data from a large US health insurance database suggested annual acute pancreatitis rates of 0.13% among exenatide users and 0.12% among sitagliptin users \[[96](/article/10.1007/s00125-010-1702-3#ref-CR96 "Dore DD, Seeger JD, Arnold Chan K (2009) Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin 25:1019–1027")\]. This was comparable with the risk from metformin and glibenclamide, making evidence of an association between acute pancreatitis and incretin-based therapies weak at best \[[96](/article/10.1007/s00125-010-1702-3#ref-CR96 "Dore DD, Seeger JD, Arnold Chan K (2009) Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin 25:1019–1027")\].Cancer
Malignancy is an emerging potential safety issue with some glucose-lowering therapies. Observational studies suggest that insulin or insulin secretagogues may be associated with increased risk of pancreatic cancer, whereas metformin may be associated with reduced cancer risk [97–99]. In a recent retrospective cohort study in general practice, patients on insulin or insulin secretagogues were more likely to develop solid cancers vs those on metformin, most of this excess risk being abolished by combination with metformin [99].
Hypoglycaemia
Iatrogenic hypoglycaemia represents a barrier to intensive glucose control, and is a particular issue with insulin and (to some extent) sulfonylureas. Most guidelines recommend HbA1c targets below 7.0% or 6.5% [2, 8, 31, 100], but without reference to specific antidiabetic treatments, diabetes duration or pre-existing cardiovascular disease. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, intensive control was associated with increased all-cause and cardiovascular mortality vs conventional therapy [100]. After 3.5 years, HbA1c was 6.4% with intensive treatment and 7.5% with conventional treatment, and severe hypoglycaemic event rates were 10.5% and 3.5%, respectively. Although the cause of the increased mortality remains unclear, hypoglycaemia represents the most plausible explanation. Recently, alarming results from the statistically powerful UK General Practice Research Database have been published [101]. Among 48,000 patients with type 2 diabetes, the decile with the lowest HbA1c (median 6.4%) had a significantly higher mortality rate (HR 1.52, 95% CI 1.32–1.76) vs the lowest-risk reference decile (median HbA1c 7.5%), and the rate was higher than all other deciles apart from the highest HbA1c (median 10.5%). Major cardiovascular events were also more frequent in this low HbA1c group than any other decile. Within the lowest decile, insulin-treated patients had a greater mortality risk vs the reference decile (HR 1.79, 95% CI 1.45–2.22) than those not treated with insulin (HR 1.30, 95% CI 1.07–1.58), adding support to the hypothesis that premature death might relate to hypoglycaemia. Future controlled intervention studies are needed to clarify whether intensification of glucose control with insulin therapy alone further heightens mortality risk. Accordingly, diabetes guidelines might need revision to define a minimum HbA1c value, especially for patients with long-standing diabetes or established cardiovascular disease.
Conclusions—implications for treatment guidelines
The algorithm published by Nathan et al. [2] under the auspices of the ADA and EASD has provoked debate on the optimal management of hyperglycaemia in type 2 diabetes [9, 102]. This paper is not designed to propose a specific treatment algorithm, but rather to point out important deficiencies in the algorithm of Nathan et al. and to argue for a re-evaluation of its recommendations. We believe that inconsistencies in the application of accepted evidence-based procedures have resulted in a skewed ranking of agents. In our opinion, the recommended two-tier approach is not evidence based and does not offer the best quality of treatment on the basis of our understanding of the multifactorial pathophysiology of type 2 diabetes or the need for individualised therapy. Methodologically, the ADA–EASD algorithm seems to be based more on an outdated expert opinion model than on the evidence-based approach that represents the current standard for guideline development.
In our opinion, these recommendations do not take full account of the evidence on the appropriate priorities for treatment (in particular, the potential impact on clinically important endpoints such as macrovascular events) or on the benefits of all available classes of glucose-lowering agents. In favouring initial use of metformin monotherapy followed by sulfonylurea, an approach known to fail, this algorithm does not offer physicians and patients the appropriate selection of options to individualise and optimise care with a view to sustained control of blood glucose and reduction of diabetes complications.
Abbreviations
ADOPT:
A Diabetes Outcome Progression Trial
CHF:
Congestive heart failure
DIGAMI:
Diabetes and Insulin-Glucose infusion in Acute Myocardial Infarction
DPP-IV:
Dipeptidyl peptidase-4
GLP-1:
Glucagon-like peptide-1
PROactive:
PROspective pioglitAzone Clinical Trial In macroVascular Events
RECORD:
Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of glycaemia in Diabetes
UKPDS:
UK Prospective Diabetes Study
References
- Nathan DM, Buse JB, Davidson MB et al (2006) Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 49:1711–1721
Article CAS PubMed Google Scholar - Nathan DM, Buse JB, Davidson MB et al (2009) Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 52:17–30
Article CAS PubMed Google Scholar - American Diabetes Association (2008) Clinical practice recommendations 2008. Diabetes Care 31:S1–S2
Article Google Scholar - Schünemann HJ, Fretheim A, Oxman AD, WHO Advisory Committee on Health Research (2006) Improving the use of research evidence in guideline development: 1. Guidelines for guidelines. Health Res Policy Syst 4:13
Article PubMed Google Scholar - Turner T, Misso M, Harris C, Green S (2008) Development of evidence-based clinical practice guidelines (CPGs): comparing approaches. Implement Sci 3:45
Article PubMed Google Scholar - Rosenbrand K, Van Croonenborg J, Wittenberg J (2008) Guideline development. Stud Health Technol Inform 139:3–21
PubMed Google Scholar - Atkins D, Best D, Briss PA et al (2004) Grading quality of evidence and strength of recommendations. BMJ 328:1490
Article PubMed Google Scholar - American Diabetes Association (ADA) (2009) Standards of medicalcare in diabetes—2009. Diabetes Care 32(Suppl 1):S13–S61
Article Google Scholar - DeFronzo RA (2009) From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58:773–795
Article CAS PubMed Google Scholar - Schernthaner G, Matthews DR, Charbonnel B, Hanefeld M, Brunetti P, behalf of the Quartet Study Group (2004) Efficacy and safety of pioglitazone versus metformin in patients with type 2 diabetes mellitus: a double-blind, randomized trial. J Clin Endocrinol Metab 89:6068–6076
Article CAS PubMed Google Scholar - Charbonnel BH, Matthews DR, Schernthaner G, Hanefeld M, Brunetti P, behalf of the QUARTET Study Group (2005) A long-term comparison of pioglitazone and gliclazide in patients with type 2 diabetes mellitus: a randomized, double-blind, parallel-group comparison trial. Diabet Med 22:399–405
Article CAS PubMed Google Scholar - Kahn SE, Haffner SM, Heise MA et al (2006) Glycemic durabilityof rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443
Article CAS PubMed Google Scholar - Bolen S, Wilson L, Vassy J et al (2007) Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 147:386–399
PubMed Google Scholar - Amori RE, Lau J, Pittas AG (2007) Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 298:194–206
Article CAS PubMed Google Scholar - Madsbad S (2009) Exenatide and liraglutide: different approachesto develop GLP-1 receptor agonists (incretin mimetics)—preclinical and clinical results. Best Pract Res Clin Endocrinol Metab 23:463–477
Article CAS PubMed Google Scholar - Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch C (2008) Emerging role of dipeptidyl peptidase-4 inhibitors in the management of type 2 diabetes. Vasc Health Risk Manag 4:753–768
CAS PubMed Google Scholar - Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP, Sitagliptin Study 024 Group (2007) Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 9:194–205
Article CAS PubMed Google Scholar - Ferrannini E, Fonseca V, Zinman B et al (2009) Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab 11:157–166
Article CAS PubMed Google Scholar - Horton ES (2009) Defining the role of basal and prandial insulin for optimal glycemic control. J Am Coll Cardiol 53(5 Suppl):S21–S27
Article CAS PubMed Google Scholar - Lasserson DS, Glasziou P, Perera R, Holman RR, Farmer AJ (2009) Optimal insulin regimens in type 2 diabetes mellitus: systematic review and meta-analyses. Diabetologia 52:1990–2000
Article CAS PubMed Google Scholar - Holman RR, Farmer AJ, Davies MJ, 4-T Study Group et al (2009) Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med 361:1736–1747
Article CAS PubMed Google Scholar - Monami M, Marchionni N, Mannucci E (2008) Long-acting insulin analogues versus NPH human insulin in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract 81:184–189
Article CAS PubMed Google Scholar - Bretzel RG, Nuber U, Landgraf W, Owens DR, Bradley C, Linn T (2008) Once-daily basal insulin glargine versus thrice-daily prandial insulin lispro in people with type 2 diabetes on oral hypoglycaemic agents (APOLLO): an open randomised controlled trial. Lancet 371:1073–1084
Article CAS PubMed Google Scholar - Kann PH, Wascher T, Zackova V et al (2006) Starting insulin therapy in type 2 diabetes: twice-daily biphasic insulin Aspart 30 plus metformin versus once-daily insulin glargine plus glimepiride. Exp Clin Endocrinol Diabetes 114:527–532
Article CAS PubMed Google Scholar - Raskin P, Allen E, Hollander P et al (2005) Initiating insulin therapy in type 2 diabetes: a comparison of biphasic and basal insulin analogs. Diabetes Care 28:260–265
Article CAS PubMed Google Scholar - Robbins DC, Beisswenger PJ, Ceriello A et al (2007) Mealtime 50/50 basal + prandial insulin analogue mixture with a basal insulin analogue, both plus metformin, in the achievement of target HbA1c and pre- and postprandial blood glucose levels in patients with type 2 diabetes: a multinational, 24-week, randomized, open-label, parallel-group comparison. Clin Ther 29:2349–2364
Article CAS PubMed Google Scholar - Ilag LL, Kerr L, Malone JK, Tan MH (2007) Prandial premixed insulin analogue regimens versus basal insulin analogue regimens in the management of type 2 diabetes: an evidence-based comparison. Clin Ther 29 (Spec No):1254–1270
- Hollander P, Cooper J, Bregnhøj J, Pedersen CB (2008) A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clin Ther 30:1976–1987
Article CAS PubMed Google Scholar - Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G (2008) A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia 51:408–416
Article CAS PubMed Google Scholar - Woo V, Shestakova MV, Ørskov C, Ceriello A (2008) Targets andtactics: the relative importance of HbA1c, fasting and postprandial plasma glucose levels to glycaemic control in type 2 diabetes. Int J Clin Pract 62:1935–1942
Article CAS PubMed Google Scholar - Matthaei S, Bierwirth R, Fritsche A et al (2009) Medical antihyperglycaemic treatment of type 2 diabetes mellitus: update of the evidence-based guideline of the German Diabetes Association. Exp Clin Endocrinol Diabetes 117:522–557
Article CAS PubMed Google Scholar - UK Prospective Diabetes Study (UKPDS) Group (1998) Effect ofintensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352:854–865
Article Google Scholar - Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA (2008)10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359:1577–1589
Article CAS PubMed Google Scholar - Johnson JA, Majundar SR, Simpson SH, Toth EL (2002) Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care 25:2244–2248
Article CAS PubMed Google Scholar - Evans JM, Ogston SA, Emslie-Smith A, Morris AD (2006) Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia 49:930–936
Article CAS PubMed Google Scholar - Tzoulaki I, Molokhia M, Curcin V et al (2009) Risk of cardiovascular disease and all cuase mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 339:b4731
Article PubMed Google Scholar - Meinert CL, Knatterud GL, Prout TE, Klimt CR (1970) A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetesm. II. Mortality results. Diabetes 19(Suppl):789–830
PubMed Google Scholar - UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
Article Google Scholar - The ADVANCE Collaborative Group (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572
Article Google Scholar - Garratt KN, Brady PA, Hassinger NL, Grill DE, Terzic A, Holmes DR Jr (1999) Sulfonylurea drugs increase early mortality in patients with diabetes mellitus after direct angioplasty for acute myocardial infarction. J Am Coll Cardiol 33:119–124
Article CAS PubMed Google Scholar - The University Group Diabetes Program (1975) A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. V. Evaluation of phenformin therapy. Diabetes 24(Suppl 1):65–184
Google Scholar - Rao AD, Kuhadiya N, Reynolds K, Fonseca VA (2008) Is the combination of sulfonylureas and metformin associated with an increased risk of cardiovascular disease or all-cause mortality? A meta-analysis of observational studies. Diabetes Care 31:1672–1678
Article PubMed Google Scholar - Nathan DM, Cleary PA, Backlund JY et al (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353:2643–2653
Article PubMed Google Scholar - Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
Article Google Scholar - Ohkubo Y, Kishikawa H, Araki E et al (1995) Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 28:103–117
Article CAS PubMed Google Scholar - Muis MJ, Bots ML, Grobbee DE, Stolk RP (2005) Insulin treatment and cardiovascular disease; friend or foe? A point of view. Diabet Med 22:118–126
Article CAS PubMed Google Scholar - Engel-Nitz NM, Martin S, Sun P, Buesching D, Fonseca V (2008) Cardiovascular events and insulin therapy: a retrospective cohort analysis. Diabetes Res Clin Pract 81:97–104
Article CAS PubMed Google Scholar - Kumar R, Lee TT, Jeremias A et al (2007) Comparison of outcomes using sirolimus-eluting stenting in diabetic versus nondiabetic patients with comparison of insulin versus non-insulin therapy in the diabetic patients. Am J Cardiol 100:1187–1191
Article CAS PubMed Google Scholar - Margolis DJ, Hoffstad O, Strom BL (2008) Association between serious ischemic cardiac outcomes and medications used to treat diabetes. Pharmacoepidemiol Drug Saf 17:753–759
Article PubMed Google Scholar - Anselmino M, Ohrvik J, Malmberg K, Standl E, Rydén L, Euro Heart Survey Investigators (2008) Glucose lowering treatment in patients with coronary artery disease is prognostically important not only in established but also in newly detected diabetes mellitus: a report from the Euro Heart Survey on Diabetes and the Heart. Eur Heart J 29:177–184
Article PubMed Google Scholar - Malmberg K, Norhammar A, Wedel H, Rydén L (1999) Glycometabolic state at admission: important risk marker of mortality in conventionally treated patients with diabetes mellitus and acute myocardial infarction. Long-term results from the Diabetes and Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) Study. Circulation 99:2626–2632
CAS PubMed Google Scholar - Malmberg K, Rydén L, Wedel H et al (2005) Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): effects on mortality and morbidity. Eur Heart J 26:650–651
Article CAS PubMed Google Scholar - Mellbin LG, Malmberg K, Norhammer A, Wedel H, Ryden L, for the DIGAMI 2 Investigators (2008) The impact of glucose lowering treatment on long-term prognosis in patients with type 2 diabetes and myocardial infarction: a report from the DIGAMI 2 trial. Eur Heart J 29:166–176
Article CAS PubMed Google Scholar - Raz I, Wilson PW, Strojek K et al (2009) Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 32:381–386
Article CAS PubMed Google Scholar - Dormandy JA, Charbonnel B, Eckland DJ et al (2005) Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366:1279–1289
Article CAS PubMed Google Scholar - Betteridge DJ, DeFronzo RA, Chilton RJ (2008) PROactive: time for a critical appraisal. Eur Heart J 29:969–983
Article PubMed Google Scholar - Mannucci E, Monami M, Lamanna C, Gensini GF, Marchionni N (2008) Pioglitazone and cardiovascular risk. A comprehensive meta-analysis of randomized clinical trials. Diabetes Obes Metab 10:1221–1238
CAS PubMed Google Scholar - Lincoff AM, Wolski K, Nicholls SJ, Nissen SE (2007) Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 298:1180–1188
Article CAS PubMed Google Scholar - Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471
Article CAS PubMed Google Scholar - Singh S, Loke YK, Furberg CD (2007) Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 298:1189–1195
Article CAS PubMed Google Scholar - Diamond GA, Bax L, Kaul S (2007) Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Ann Intern Med 147:578–581
PubMed Google Scholar - Home PD, Pocock SJ, Beck-Nielsen H et al (2009) Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373:2125–2135
Article CAS PubMed Google Scholar - Gerrits CM, Bhattacharya M, Manthena S, Baran R, Perez A, Kupfer S (2007) A comparison of pioglitazone and rosiglitazone for hospitalization for acute myocardial infarction in type 2 diabetes. Pharmacoepidemiol Drug Saf 16:1065–1071
Article PubMed Google Scholar - Walker AM, Koro CE, Landon J (2008) Coronary heart disease outcomes in patients receiving antidiabetic agents in the PharMetrics database 2000–2007. Pharmacoepidemiol Drug Saf 17:760–768
Article PubMed Google Scholar - Hausenloy DJ, Yellon DM (2008) GLP-1 therapy: beyond glucose control. Circ Heart Fail 1:147–149
Article PubMed Google Scholar - Timmers L, Henriques JP, de Kleijn DP et al (2009) Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol 53:501–510
Article CAS PubMed Google Scholar - Noyan-Ashraf MH, Momen MA, Ban K et al (2009) The GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes following experimental myocardial infarction in mice. Diabetes 58:975–983
Article CAS PubMed Google Scholar - Wajchenberg BL (2007) Beta-cell failure in diabetes and preservation by clinical treatment. Endocr Rev 28:187–218
Article CAS PubMed Google Scholar - The Dream (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) Trial Investigators (2006) Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomized controlled trial. Lancet 368:1096–1105
Article Google Scholar - Defronzo RA, Banerji M, Bray GA et al (2009) Actos Now for the prevention of diabetes (ACT NOW) study. BMC Endocr Disord 29:9–17
Google Scholar - Chen H-S, Wu T-E, Jap T-S, Hsiao L-C, Lee S-H, Lin H-D (2008) Beneficial effects of insulin on glycemic control and β-cell function in newly diagnosed type 2 diabetes with severe hyperglycemia after short-term intensive insulin therapy. Diabetes Care 31:1927–1932
Article CAS PubMed Google Scholar - Weng J, Li Y, Xu W et al (2008) Effect of intensive insulin therapy on β-cell function and glycemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet 371:1753–1760
Article CAS PubMed Google Scholar - Klonoff DC, Buse JB, Nielsen LL et al (2008) Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin 24:275–286
CAS PubMed Google Scholar - Bunck MC, Diamant M, Corner A et al (2009) One-year treatment with exenatide improves beta-cell function, compared to insulin glargine, in metformin treated type 2 diabetes patients: a randomized, controlled trial. Diabetes Care 32:762–768
Article CAS PubMed Google Scholar - Rydén L, Standl E, Bartnik M et al (2007) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J 28:88–136
Article PubMed Google Scholar - Buse JB, Tan MH, Prince MJ, Erickson PP (2004) The effects of oral anti-hyperglycemic medications on serum lipid profiles in patients with type 2 diabetes. Diabetes Obes Metab 6:133–156
Article CAS PubMed Google Scholar - Chiquette E, Ramirez G, DeFronzo R (2004) A meta-analysis comparing the effect of thiazolidinediones on cardiovascular risk factors. Arch Intern Med 164:2097–2104
Article CAS PubMed Google Scholar - Betteridge DJ (2007) Effects of pioglitazone on lipid and lipoprotein metabolism. Diabetes Obes Metab 9:640–647
Article CAS PubMed Google Scholar - Mazzone T, Meyer PM, Feinstein SB et al (2006) Effect of pioglitazone compared with glimepiride on carotid intima–media thickness in type 2 diabetes: a randomized trial. JAMA 296:2572–2581
Article CAS PubMed Google Scholar - Nissen SE, Nicholls SJ, Wolski K et al (2008) Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA 299:1561–1573
Article CAS PubMed Google Scholar - Dandona P, Chaudhuri A, Ghanim H, Mohanty P (2008) Use of insulin to improve glycemic control in diabetes mellitus. Cardiovasc Drugs Ther 22:241–251
Article CAS PubMed Google Scholar - Rosenstock J, Sugimoto D, Strange P, Stewart JA, Soltes-Rak E, Dailey G (2006) Triple therapy in type 2 diabetes: insulin glargine or rosiglitazone added to combination therapy of sulfonylurea plus metformin in insulin-naive patients. Diabetes Care 29:554–559
Article CAS PubMed Google Scholar - Triplitt C, Glass L, Miyazaki Y et al (2006) Comparison of glargine insulin versus rosiglitazone addition in poorly controlled type 2 diabetic patients on metformin plus sulfonylurea. Diabetes Care 29:2371–2377
Article CAS PubMed Google Scholar - Matikainen N, Mänttäri S, Schweizer A et al (2006) Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia 49:2049–2057
Article CAS PubMed Google Scholar - Schernthaner G (2009) Pleiotropic effects of thiazolidinediones on traditional and non-traditional atherosclerotic risk factors. Int J Clin Pract 63:912–929
Article CAS PubMed Google Scholar - Bailey CJ (2008) Metformin: effects on micro and macrovascular complications in type 2 diabetes. Cardiovasc Drugs Ther 22:215–224
Article CAS PubMed Google Scholar - Lebovitz HE, Dole JF, Patwardhan R, Rappaport EB, Freed MI, The Rosiglitazone Clinical Trials Study Group (2001) Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab 86:280–288
Article CAS PubMed Google Scholar - Miyazaki Y, Mahankali A, Matsuda M et al (2002) Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab 87:2784–2791
Article CAS PubMed Google Scholar - Hermann LS, Schersten B, Bitzen PO, Kjellström T, Lindgärde F, Melander A (1994) Therapeutic comparison of metformin and sulfonylurea, alone and in various combinations. A double-blind controlled study. Diabetes Care 17:1100–1109
Article CAS PubMed Google Scholar - Erdmann E, Wilcox RG (2008) Weighing up the cardiovascular benefits of thiazolidinedione therapy: the impact of increased risk of heart failure. Eur Heart J 29:12–20
Article CAS PubMed Google Scholar - Eurich DT, McAlister FA, Blackburn DF et al (2007) Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: systematic review. BMJ 335:497–501
Article CAS PubMed Google Scholar - Bodmer M, Meier C, Kraenzlin ME, Meier CR (2009) Risk of fractures with glitazones: a critical review of the evidence to date. Drug Saf 32:539–547
Article CAS PubMed Google Scholar - US Food and Drug Administration (2008) Exenatide (marketed as Byetta) information (18 August 2008 update). Available from www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124713.htm, accessed 16 January 2010
- U.S. Food and Drug Administration (2008) Sitagliptin (marketed as Januvia and Janumet) information (issued 25 September 2009). Available from www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm183768.htm, accessed 16 January 2010
- Bain SC, Stephens JW (2008) Exenatide and pancreatitis: an update. Expert Opin Drug Saf 7:643–644
Article PubMed Google Scholar - Dore DD, Seeger JD, Arnold Chan K (2009) Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin 25:1019–1027
Article CAS PubMed Google Scholar - Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL (2009) Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 137:482–488
Article PubMed Google Scholar - Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM (2009) New users of metformin are at low risk of incident cancer: a cohort study among people with type 2 diabetes. Diabetes Care 32:1620–1625
Article CAS PubMed Google Scholar - Currie CJ, Poole CD, Gale EA (2009) The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 52:1766–1777
Article CAS PubMed Google Scholar - The Action to Control Cardiovascular Risk in Diabetes Study Group (2008) Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559
Article Google Scholar - Currie CJ, Peters JR, Tynan A et al (2010) Survival as a function of HbA1c in people with type 2 diabetes: a retrospective cohort study. Lancet 375:481–489
Article CAS PubMed Google Scholar - Woo V (2009) Important differences: Canadian Diabetes Association 2008 clinical practice guidelines and the consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 52:552–553
Article CAS PubMed Google Scholar
Duality of interest
G. Schernthaner has received lecture fees from AstraZeneca/BMS, Eli Lilly, GSK, Merck, NovoNordisk, sanofi-aventis, Servier and Takeda. A. H. Barnett has received lecture fees from AstraZeneca/BMS, Eli Lilly, MSD, Novartis, NovoNordisk, sanofi-aventis and Servier. D. J. Betteridge has received lecture fees and honoraria for advisory boards from AstraZeneca, Eli Lilly, GSK Merck, NovoNordisk, Pfizer, Boehringer Ingelheim and Takeda. B. Charbonnel has received lecture fees from AstraZeneca/BMS, Boehringer Ingelheim, GSK, Merck, NovoNordisk, Roche, sanofi-aventis and Takeda. M. Hanefeld has received lecture from BACER-AG, sanofi-aventis, GlaxoSmithKline, Novartis, Takeda and MSD. M. T. Malecki has received lecture fees from Berlin-Chemie, Bioton, Eli Lilly, NovoNordisk, Roche and Servier, and grant support from Eli Lilly. R. Nesto has received lecture fees from GSK and sanofi-aventis. A. Scheen has received lecture fees from AstraZeneca/BMS, Eli Lilly, GlaxoSmithKline, Merck Sharp & Dohme, NovoNordisk, sanofi-aventis and Takeda. J. Seufert has received lecture fees from AstraZeneca/BMS, Bayer, Berlin Chemie, Eli Lilly, GlaxoSmithKline, Lifescan, Merck Sharp & Dohme, Novartis, NovoNordisk, Pfizer, sanofi-aventis and Takeda. R. DeFronzo has received lecture fees from Amylin, BMS, Eli Lilly, ISIS, Merck, Novartis and Takeda. The remaining authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
- Department of Medicine I, Rudolfstiftung Hospital-Vienna, Juchgasse 25, 1030, Vienna, Austria
G. Schernthaner - University of Birmingham and Heart of England NHS Trust, Birmingham, UK
A. H. Barnett - University College London Hospitals, London, UK
D. J. Betteridge - University of Valencia, Valencia, Spain
R. Carmena - Department of Endocrinology, University of Udine, Udine, Italy
A. Ceriello - University Hospital, Nantes, France
B. Charbonnel - Center for Clinical Studies, GWT Dresden, Dresden, Germany
M. Hanefeld - University Hospital Zurich, Zurich, Switzerland
R. Lehmann - Jagiellonian University, Krakow, Poland
M. T. Malecki - Lahey Clinic Medical Center, Burlington, MA, USA
R. Nesto - University of Latvia, Riga, Latvia
V. Pirags - CHU Sart Tilman, University of Liège, Liège, Belgium
A. Scheen - University Hospital of Freiburg, Freiburg, Germany
J. Seufert - Karolinska Institutet, Stockholm, Sweden
A. Sjohölm - University of Ioannina, Ioannina, Greece
A. Tsatsoulis - University of Texas Health Science Center, San Antonio, TX, USA
R. DeFronzo
Authors
- G. Schernthaner
You can also search for this author inPubMed Google Scholar - A. H. Barnett
You can also search for this author inPubMed Google Scholar - D. J. Betteridge
You can also search for this author inPubMed Google Scholar - R. Carmena
You can also search for this author inPubMed Google Scholar - A. Ceriello
You can also search for this author inPubMed Google Scholar - B. Charbonnel
You can also search for this author inPubMed Google Scholar - M. Hanefeld
You can also search for this author inPubMed Google Scholar - R. Lehmann
You can also search for this author inPubMed Google Scholar - M. T. Malecki
You can also search for this author inPubMed Google Scholar - R. Nesto
You can also search for this author inPubMed Google Scholar - V. Pirags
You can also search for this author inPubMed Google Scholar - A. Scheen
You can also search for this author inPubMed Google Scholar - J. Seufert
You can also search for this author inPubMed Google Scholar - A. Sjohölm
You can also search for this author inPubMed Google Scholar - A. Tsatsoulis
You can also search for this author inPubMed Google Scholar - R. DeFronzo
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toG. Schernthaner.
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00125-010-1797-6
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 2.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by-nc/2.0/.
About this article
Cite this article
Schernthaner, G., Barnett, A.H., Betteridge, D.J. et al. Is the ADA/EASD algorithm for the management of type 2 diabetes (January 2009) based on evidence or opinion? A critical analysis.Diabetologia 53, 1258–1269 (2010). https://doi.org/10.1007/s00125-010-1702-3
- Received: 02 September 2009
- Accepted: 05 January 2010
- Published: 31 March 2010
- Issue Date: July 2010
- DOI: https://doi.org/10.1007/s00125-010-1702-3