A polymorphism in the gene encoding carnosinase (CNDP1) as a predictor of mortality and progression from nephropathy to end-stage renal disease in type 1 diabetes mellitus (original) (raw)
Abstract
Aims/hypothesis
Homozygosity for a five leucine repeat (5L–5L) in the carnosinase gene (CNDP1) has been found to be cross-sectionally associated with a low frequency of diabetic nephropathy (DN), mainly in type 2 diabetes. We prospectively investigated in patients with type 1 diabetes whether: (1) 5L–5L is associated with mortality; (2) there is an interaction of 5L–5L with DN or sex for prediction of mortality; and (3) 5L–5L is associated with progression to end-stage renal disease (ESRD).
Methods
In this prospective study in white European patients with type 1 diabetes, individuals with DN were defined by persistent albuminuria ≥300 mg/24 h. Controls without nephropathy were defined by persistent (>15 years) normoalbuminuria <30 mg/24 h. Leucine repeats were assessed with a fluorescent DNA analysis system. Onset of ESRD was defined by need to start chronic dialysis or kidney transplantation.
Results
The study involved 916 patients with DN and 1,170 controls. During follow-up for 8.8 years, 107 patients (14%) with 5L–5L died compared with 182 patients (13.8%) with other genotypes (p = 0.99). There was no significant interaction of 5L–5L with DN for prediction of mortality (p = 0.57), but a trend towards interaction with sex (p = 0.08). In patients with DN, HR for ESRD in 5L–5L vs other genotypes was not constant over time, with increased risk for 5L–5L beyond 8 years of follow-up (p = 0.03).
Conclusions/interpretation
CNDP1 polymorphism was not associated with mortality, and nor was there an interaction of this polymorphism with DN for prediction of mortality in patients with type 1 diabetes. CNDP1 polymorphism predicts progression to ESRD in patients with DN, but only late after baseline measurements.
Similar content being viewed by others
Introduction
Diabetes is the leading cause of end-stage renal disease (ESRD) in the Western world. In the last decade, diabetic patients in the USA and Europe accounted for 40% and 26% of the populations receiving dialysis, respectively [1–3]. Poor glycaemic control and high blood pressure are acknowledged contributors to the pathophysiology of diabetic nephropathy (DN) [4], but epidemiological and familial studies have suggested genetic predisposition to be involved as well [5–7]. Analyses of family-based studies in Turkish, Pima Indian and African-American patients with type 2 diabetes have mapped a major susceptibility locus to chromosome 18 [8, 9]. In a cross-sectional study in patients with type 1 and type 2 diabetes, this locus was identified as the carnosinase gene 1 (CNDP1); homozygosity for five copies of a trinucleotide repeat encoding leucine (5L–5L) in this gene was found to be more common in patients without DN than in those with DN [10]. This finding was confirmed by findings from other cross-sectional studies in patients with type 2 diabetes [11, 12] and has been suggested to be consistent with a protective role of the 5L–5L genotype against DN, particularly in women [13]. However, these findings from cross-sectional studies could be subject to selection bias. They could, for instance, be explained by a possible interaction of the 5L–5L genotype with DN, sex or both for prediction of mortality. Survival (dis)advantages in subgroups may induce false associations in cross-sectional studies [14].
In this observational study in a white European cohort of patients with type 1 diabetes, we therefore aimed to investigate prospectively: (1) the association of CNDP1 gene polymorphism with mortality; (2) a potential interaction of this gene polymorphism with DN or sex for prediction of mortality; and (3) an association of this gene polymorphism with development of ESRD.
Methods
Patients
The present study included patients from three European coordinating centres in Denmark, Finland and France. All the individuals included were of European descent. In Denmark between 1993 and 2000, all Danish patients with type 1 diabetes attending the outpatient clinic at Steno Diabetes Center were invited to participate in a study of genetic risk factors for micro- and macrovascular complications of diabetes [15]. In France and Belgium, 17 diabetes clinics participated in a study of the genetic risk factors for diabetes complications between 1994 and 2001, which included eligible patients with type 1 diabetes [16, 17]. In Finland, between 1994 and 2002, patients with type 1 diabetes were recruited from 56 referral centres to participate in the prospective Finnish Diabetic Nephropathy Study (FinnDiane).
Type 1 diabetes was considered present if the age at onset of diabetes was ≤35 years and the time to definitive insulin therapy ≤1 year. Established diabetic nephropathy (cases) was defined by persistent albuminuria (>300 mg/24 h or >200 μg/min or >200 mg/l) in two out of three consecutive measurements made on sterile urine samples, after >5 years’ diabetes duration. In patients using ongoing regimens of angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker (ARB) therapy, the last measurements of urinary albumin excretion before treatment initiation were used for classification. Patients with clinical suspicion of non-diabetic renal or urinary tract disease were excluded. Absence of diabetic nephropathy (controls) was defined as persistent normoalbuminuria (urinary albumin excretion rate [uAER] <30 mg/24 h or <20 μg/min or <20 mg/l) after at least 15 years of diabetes in patients not treated with angiotensin-converting enzyme inhibitors or ARBs.
The study was performed in accordance with the Declaration of Helsinki. The local ethics committee approved the study and all patients gave their informed consent.
Baseline clinical laboratory investigations
All patients had blood samples and phenotypic characteristics collected as part of the European Rational Approach for the Genetics of Diabetic Complications (EURAGEDIC) project [18]. Blood pressure was measured twice in the resting state. HbA1c was determined by standard HPLC techniques with normal values in the range 4.1% to 6.4%. Plasma creatinine concentration was determined by modified Jaffe’s method. Timed urine collections were used to obtain uAER. Diabetic retinopathy was assessed by fundus photography or direct ophthalmoscopy carried out by an experienced ophthalmologist. Based on standardised questionnaires, former or current smokers of one or more cigarettes/cigars/pipes per day were classified as smokers and all others as non-smokers. Non-fatal cardiovascular disease was considered present in patients with a history of admission for stroke, myocardial infarction or vascular amputations. DNA material was genotyped as part of the EURAGEDIC project as follows. Genomic DNA was isolated from human leucocytes using standard methods. D18S880 marker genotyping was performed at the French National Genotyping Centre using an automated high-throughput method. All liquid handling was performed robotically in 384 well plates with a BasePlate Robot (The Automation Partnership, Royston, UK). PCR amplification was carried out in a 5 μl volume with 5 ng of genomic DNA and primers AGGCAGCTGTGTGAGGTAAC (forward) labelled with the fluorescent dye Fam and GGGTGAGGAGAACATGCC (reverse) using a standard protocol. The annealing temperature was 55°C. Fluorescent PCR products were analysed on a MegaBace TM 1000 Sequencer (Amersham Biosciences, Little Chalfont, UK) using appropriate software. Automatic genotyping was performed using Genetic Profiler software (version 3.1; Amersham Biosciences). Before statistical analysis, rigorous genotype quality assurance was performed to ensure accurate binning of alleles. Three alleles were observed with fragment sizes of 167, 170 and 173 base pairs corresponding to five, six and seven leucine repeats respectively. The 5L–5L homozygous genotype was compared with all other genotypes (i.e. genotypes with six or more leucine repeats).
Follow-up
In this prospective observational study, patients were followed: until an endpoint was reached; to the last visit at the outpatient department; or until 1 September 2006 for the Danish population, 1 December 2009 for the Finnish population or 1 February 2007 for the French population. The endpoints were all-cause mortality and ESRD, defined as need to start chronic dialysis or kidney transplantation. All patients were traced through the national register. If a patient died before the last update, the date of death was recorded. Information about date of ESRD was obtained from patient records or discharge letters from other hospitals.
Statistical analysis
Variables with normal distribution are presented as mean±SD and variables with skewed distribution were log-transformed before analysis and presented as median and interquartile range (IQR). For variables with normal distribution, comparisons between groups were performed using unpaired Student’s t tests, whereas for variables with skewed distribution Mann–Whitney U tests were used. χ 2 tests were used to compare non-continuous variables. Time-to-event analyses were performed using Kaplan–Meier plots and logrank testing. Tests for a non-zero slope of scaled Schoenfeld residuals on functions of time were performed to explore the proportional hazards (PH) assumption in Cox regression models [19]. If the PH assumption was met (test based on Schoenfeld residuals p > 0.05), Cox regression models were used to estimate unadjusted and adjusted hazard ratios with 95% confidence intervals. Otherwise, Cox models with time-dependent covariates were used to calculate HRs over time [20]. After an initial crude analysis, two subsequent models were constructed in which the associations were adjusted for potential confounders and covariates defined a priori. In the first multivariate model, adjustment was performed for age, sex and centre of inclusion. In the other model, further adjustment was performed for duration of diabetes, HbA1c, blood pressure, plasma creatinine and uAER. In this study, we were able to detect a genotype relative risk for mortality of 1.2 with a power of 95% at the level of significance p = 0.05. In those with DN, we were able to detect a genotype RR for ESRD of 1.3 with a power of 95% at p = 0.05. The PS program of Dupont and Plummer [21] was used to calculate power. Analysis of the Danish, Finnish and French populations separately gave comparable results, and thus pooled data are presented.
A two-tailed p value of 0.05 or less was considered statistically significant. Statistical analyses were performed using a commercially available program (SPSS for Windows, version 16.0, Chicago, IL, USA).
Results
Baseline characteristics
In total, 2,487 patients (900 from Denmark, 687 from France and 900 from Finland) were included. The CNDP1 genotype could not be determined in 401 patients: DNA samples from 74 patients from France were missing, and poor-quality DNA samples were obtained from 65 patients from Denmark, 88 patients from France and 174 from Finland. The baseline characteristics of these patients were not significantly different from those who were genotyped. Table 1 shows the baseline characteristics of the study population (n = 2,086). Patients with DN were younger, more commonly men, and had higher HbA1c, blood pressure and serum creatinine levels than controls. The frequency of the 5L–5L genotype was not significantly different between patients with DN and patients without DN [22] and also not between men and women. The OR for the presence of DN according to 5L–5L genotype was 0.99 (95% CI 0.82–1.18) for the whole population, with an OR of 1.05 (95% CI 0.83–1.34) for men and 0.90 (95% CI 0.69–1.18) for women (p = 0.40 for interaction).
Table 1 Baseline characteristics of the study population
Prospective analyses for mortality
Median (IQR) follow-up was 8.8 (6.1–10.5) years. Of patients with DN, 81 of 334 patients (24.3%) with 5L–5L died during the follow-up vs 142 of 582 patients (24.4%) with other genotypes (logrank test p = 0.73). In controls without DN, these numbers were 26 of 430 (6.0%) and 40 of 740 (5.4%), respectively (logrank test p = 0.66). The PH assumption for Cox regression was met (test based on Schoenfeld residuals, p = 0.17). After adjustment for potential confounders, including age, sex, blood pressure and history of cardiovascular events in Cox regression analyses, HR for mortality in 5L–5L vs other genotypes was 0.95 (95% CI 0.73–1.22, p = 0.67).
There was no significant interaction between 5L–5L and presence of DN for prediction of mortality (p = 0.57). However, there was a trend towards interaction between 5L–5L and sex for prediction of mortality (p = 0.08). After stratification for sex, adjusted HR for mortality in 5L–5L vs other genotypes was 0.87 (95% CI 0.62–1.21, p = 0.41) in men and 1.31 (95% CI 0.87–1.98, p = 0.31) in women.
Prospective analyses for end-stage renal disease
No events occurred in controls without nephropathy. We therefore limited further analyses to patients with DN (n = 916). At baseline, 52 patients were already diagnosed with ESRD and were not included in the prospective analyses.
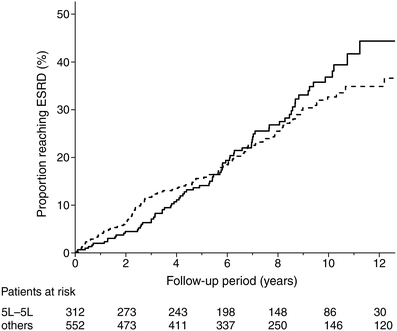
During follow-up, 77 out of 312 patients (24.7%) with 5L–5L developed ESRD vs 121 of 552 (21.9%) patients with other genotypes. Figure 1 shows a Kaplan–Meier plot of development of ESRD according to CNDP1 genotype (logrank test p = 0.57). The PH assumption for Cox regression was not met (p = 0.02). Further Cox-regression analysis with a time-dependent covariate showed that the hazard ratio for ESRD was not constant over time (p = 0.03). As shown in Table 2, within the first 6 years, the hazard ratios were not significantly different between patients with the 5L–5L genotype and those with other genotypes. For example, the HR for ESRD in patients with the 5L–5L genotype compared with other genotypes was 0.82 (95% CI 0.55–1.22) at 2 years of follow-up. However, after 8 years of follow-up, patients with the 5L–5L genotype appeared to have an increased risk of ESRD compared with those with other genotypes, with an HR of 1.53 (95% CI 1.01–2.34) at 8 years of follow-up and an HR of 1.89 (95% CI 1.07–3.36) at 10 years of follow-up. Adjustment for possible confounders, including duration of diabetes, baseline HbA1c, blood pressure, plasma creatinine and urinary AER did not materially change the results of the analyses for 5L–5L: HR at 8 years, 1.71 (95% CI 1.11–2.65); and at 10 years, 2.19 (95% CI 1.21–4.01).
Fig. 1
Kaplan–Meier curves of ESRD in 864 patients with type 1 diabetes and diabetes nephropathy according to CNDP1 genotype: five leucine repeat homozygous (5L–5L) vs all other genotypes (logrank test p = 0.57). Solid line, 5L–5L genotype; dotted line, all other genotypes
Table 2 Hazard ratios for ESRD from Cox models with time-dependent variables
Discussion
In this study of a large cohort of white European patients with type 1 diabetes, the prevalence of homozygosity for 5L–5L repeats in the CNDP1 gene was not significantly different between patients with DN and patients without DN at study entry. In prospective analyses, we found neither an association of 5L–5L with mortality nor an interaction of 5L–5L with DN for prediction of mortality. Patients with 5L–5L have shown a trend towards a sex-dependent association with risk for mortality, with higher risk associated with 5L–5L in women than in men, but no significant associations with mortality were present in the sexes separately. Patients with DN and 5L–5L were at increased risk of progression to ESRD compared with those with other allelic variations, but this increase in risk became apparent only after 8 years of follow-up. The increase in risk was independent of potential confounders.
In an earlier study in a large cohort of patients with type 1 diabetes and DN (n = 445), Wanic et al. [23] found no significant association between 5L–5L and susceptibility for progression to ESRD after a mean follow-up of 5.6 years. In that study, it was not investigated whether the proportional hazards assumption of Cox-regression analyses was violated. If we had performed similar analyses as performed in the study of Wanic et al., we would have found similar results. However, taking into account the change in hazards over time unmasked an increased risk associated with 5L–5L after prolonged follow-up.
The CNDP1 gene, which is located on chromosome 18, encodes a secreted serum carnosinase that degrades carnosine specifically, whereas CNDP2 encodes tissue carnosinase [24]. Carnosine is a naturally occurring dipeptide that has been shown to have beneficial actions as, for example, a scavenger of free oxygen radicals and an inhibitor of formation of advanced glycation end products [25–27]. Individuals with genotypes containing a higher number of leucine repeats (six or seven repeats) in their CNDP1 gene were found to have higher serum carnosinase activity compared with those with five leucine repeats [28]. High serum carnosine levels resulting from low carnosinase activity were considered to underlie findings from cross-sectional studies that suggested a protective role of the 5L–5L genotype against DN, mainly in type 2 diabetes [10, 11].
Findings from cross-sectional studies should be interpreted carefully. For instance, interaction between 5L–5L and DN resulting in a survival disadvantage in these patients could falsely suggest a protective effect of 5L–5L for DN in a cross-sectional study [14]. Our study is the first to investigate such a potential interaction prospectively. We found no evidence for an interaction between 5L–5L and DN for mortality. Another interaction that we considered was an interaction by sex, given a recent finding from cross-sectional data that in patients with type 2 diabetes the association between CNDP1 and DN is sex specific, with a decreased risk in women with the 5L–5L genotype [13]. In the current study, we found a trend towards a different risk of mortality between men and women, with a trend towards increased risk in women with the 5L–5L genotype compared with men. In contrast to these findings, however, our study did not indicate an interaction by sex for the prospective relationship of the 5L–5L genotype with ESRD. Furthermore, a protective role of 5L–5L for progression to ESRD in patients with DN could not be confirmed in our large study nor in a previous study in type 1 diabetes [23]. Differences in factors or genes predisposing for DN or mortality between type 1 and type 2 diabetes may underlie discrepant findings of cross-sectional analyses of associations of 5L–5L with DN in patients with type 1 and type 2 diabetes [29].
Although CNDP1 polymorphism was not associated with DN in our population, it should be noted that once DN is established, the 5L–5L genotype appeared to be significantly associated with increased rather than decreased risk of progression to ESRD beyond 6 years of follow-up. A possible explanation for this discrepancy from previous findings that suggested a protective role of 5L–5L could be that other unidentified risk alleles may be involved in deterioration of renal function in diabetic patients. For example, McDonough et al. [30] have shown that in African-Americans, a population in which there is no association of CNDP1 and CNDP2 with ESRD, other variants in this region of chromosome 18 contribute to the risk of DN. These other variants may mask or modify the effect of 5L–5L. Another potential explanation for an inverse association between CNDP1 and ESRD that only appears after prolonged follow-up could lie in environmental factors that alter over time, such as glycaemic control. Indeed, Riedl et al. [31] have shown that hyperglycaemia enhances secretion of carnosinase and its activity. Additionally, tissue carnosinase could have an important role in susceptibility to DN and ESRD. Whether the 5L–5L genotype also determines the consequent availability of the intracellular precursor of carnosinase and whether this may have consequences for intracellular carnosine concentrations remains to be investigated.
The strength of our study is its prospective design. It was also performed in three independent, but relatively homogeneous, populations of white patients with type 1 diabetes mellitus, and it included more cases and controls than all previous studies on the CNDP1 polymorphism.
In conclusion, this study provides evidence that CNDP1 polymorphism is neither related to risk for mortality nor interacts with DN regarding survival in white Europeans with type 1 diabetes. Homozygosity for the 5L–5L genotype was associated with increased risk of progression from nephropathy to ESRD after prolonged follow-up. Based on these results, we suggest that possible interactions between CNDP1 polymorphism and other candidate genes or environmental factors in different populations should be further investigated, preferably in prospective studies, in order to elucidate the role of carnosine and its gene in DN.
Abbreviations
5L–5L:
Homozygosity for five leucine repeat
ARB:
Angiotensin-receptor blocker
DN:
Diabetic nephropathy
ESRD:
End-stage renal disease
EURAGEDIC:
European Rational Approach for the Genetics of Diabetic Complications
IQR:
Interquartile range
PH:
Proportional hazards
uAER:
Urinary albumin excretion rate
References
- Harvey JN (2003) Trends in the prevalence of diabetic nephropathy in type 1 and type 2 diabetes. Curr Opin Nephrol Hypertens 12:317–322
Article PubMed Google Scholar - Stengel B, Billon S, van Dijk PCW et al (2003) Trends in the incidence of renal replacement therapy in end-stage renal disease in Europe, 1990–1999. Nephrol Dial Transplant 18:1824–1833
Article PubMed Google Scholar - Collins AJ, Kasiske B, Herzog C, Chen S et al (2001) US Renal Data System Excerpts from the USRDS 2001 Annual Data Report. Am J Kid Dis 38(Suppl 3):S1–S248
Google Scholar - Parving HH, Osterby R, Ritz E (2000) Diabetic nephropathy. In: Brenner BM (ed) The kidney. Saunders, Philadelphia, pp 1713–1773
Google Scholar - Seaquist ER, Goetz FC, Rich S, Barbosa J (1989) Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med 320:1161–1165
Article CAS PubMed Google Scholar - Borch-Johnsen K, Norgaard K, Hommel E et al (1992) Is diabetic nephropathy an inherited complication? Kidney Int 41:719–722
Article CAS PubMed Google Scholar - Fava S, Hattersley AT (2002) The role of genetic susceptibility in diabetic nephropathy: evidence from family studies. Nephrol Dial Transplant 17:1543–1546
Article CAS PubMed Google Scholar - Vardarli I, Baier LJ, Hanson RL et al (2002) Gene for susceptibility to diabetic nephropathy in type 2 diabetes maps to 18q22.3-23. Kidney Int 62:2176–2183
Article CAS PubMed Google Scholar - Bowden DW, Colicigno CJ, Langefeld CD et al (2004) A genome scan for diabetic nephropathy in African Americans. Kidney Int 66:1517–1526
Article CAS PubMed Google Scholar - Janssen B, Hohenadel D, Brinkkoetter P et al (2005) Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 54:2320–2327
Article CAS PubMed Google Scholar - Freedman BI, Hicks PJ, Sale MM et al (2007) A leucine repeat in the carnosinase gene CNDP1 is associated with diabetic end-stage renal disease in European Americans. Nephrol Dial Transplant 22:1131–1135
Article CAS PubMed Google Scholar - Mooyaart AL, van Valkengoed IG, Shaw PK et al (2009) Lower frequency of the 5/5 homozygous CNDP1 genotype in South Asian Surinamese. Diabetes Res Clin Pract 85:272–278
Article CAS PubMed Google Scholar - Mooyaart AL, Zutinic A, Bakker SJ et al (2010) Association between CNDP1 genotype and diabetic nephropathy is sex-specific. Diabetes 59:1555–1559
Article CAS PubMed Google Scholar - Bakker SJ, Alkhalaf A, Tarnow L, Navis G (2008) Re: Exclusion of polymorphisms in carnosinase genes (CNDP1 and CNDP2) as a cause of diabetic nephropathy in type 1 diabetes: results of large case–control and follow-up studies. Diabetes 57:e16
Article CAS PubMed Google Scholar - Lajer M, Tarnow L, Fleckner J et al (2004) Association of aldose reductase gene Z+2 polymorphism with reduced susceptibility to diabetic nephropathy in Caucasian type 1 diabetic patients. Diabet Med 21:867–873
Article CAS PubMed Google Scholar - Hadjadj S, Pean F, Gallois Y et al (2004) Different patterns of insulin resistance in relatives of type 1 diabetic patients with retinopathy or nephropathy: the Genesis France–Belgium Study. Diab Care 27:2661–2668
Article Google Scholar - Marre M, Jeunemaitre X, Gallois Y et al (1997) Contribution of genetic polymorphism in the renin-angiotensin system to the development of renal complications in insulin-dependent diabetes: Genetique de la Nephropathie Diabetique (GENEDIAB) study group. J Clin Invest 99:1585–1595
Article CAS PubMed Google Scholar - Tarnow L, Groop PH, Hadjadj S et al (2008) European rational approach for the genetics of diabetic complications—EURAGEDIC: patient populations and strategy. Nephrol Dial Transplant 23:161–168
Article CAS PubMed Google Scholar - Therneau T, Grambsch P (2000) Anonymous modeling survival data: extending the Cox Model. Springer, Rochester, pp 127–152
Google Scholar - Kleinbaum D, Klein M (2005) Anonymous survival analysis: a self-learning text, 2nd edn. Springer, Rochester, pp 216–256
Google Scholar - Dupont WD, Plummer WD Jr (1990) Power and sample size calculations. A review and computer program. Control Clin Trials 11:116–128
Article CAS PubMed Google Scholar - Tregouet DA, Groop PH, McGinn S et al (2008) G/T substitution in intron 1 of the UNC13B gene is associated with increased risk of nephropathy in patients with type 1 diabetes. Diabetes 57:2843–2850
Article CAS PubMed Google Scholar - Wanic K, Placha G, Dunn J, Smiles A, Warram JH, Krolewski AS (2008) Exclusion of polymorphisms in carnosinase genes (CNDP1 & CNDP2) as cause of diabetic nephropathy in type 1 diabetes mellitus. Results of large case–control and follow-up studies. Diabetes 57:2547–2551
Article CAS PubMed Google Scholar - Teufel M, Saudek V, Ledig JP et al (2003) Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J Biol Chem 278:6521–6531
Article CAS PubMed Google Scholar - Hipkiss AR, Worthington VC, Himsworth DT, Herwig W (1998) Protective effects of carnosine against protein modification mediated by malondialdehyde and hypochlorite. Biochim Biophys Acta 1380:46–54
CAS PubMed Google Scholar - Boldyrev A, Bulygina E, Leinsoo T, Petrushanko I, Tsubone S, Abe H (2004) Protection of neuronal cells against reactive oxygen species by carnosine and related compounds. Comp Biochem Physiol B Biochem Mol Biol 137:81–88
Article PubMed Google Scholar - Sauerhofer S, Yuan G, Braun GS et al (2007) l-Carnosine, a substrate of carnosinase-1, influences glucose metabolism. Diabetes 56:2425–2432
Article PubMed Google Scholar - Riedl E, Koeppel H, Brinkkoetter P et al (2007) A CTG polymorphism in the CNDP1 gene determines the secretion of serum carnosinase in Cos-7 transfected cells. Diabetes 56:2410–2413
Article CAS PubMed Google Scholar - Freedman BI, Bostrom M, Daeihagh P, Bowden DW (2007) Genetic factors in diabetic nephropathy. Clin J Am Soc Nephrol 2:1306–1316
Article CAS PubMed Google Scholar - McDonough CW, Hicks PJ, Lu L, Langefeld CD, Freedman BI, Bowden DW (2009) The influence of carnosinase gene polymorphisms on diabetic nephropathy risk in African-Americans. Hum Genet 126:265–275
Article CAS PubMed Google Scholar - Riedl E, Koeppel H, Pfister F et al (2010) _N_-Glycosylation of carnosinase influences protein secretion and enzyme activity: implications for hyperglycemia. Diabetes 59:1984–1990
Article CAS PubMed Google Scholar
Acknowledgements
The EURAGEDIC study was supported by the European Commission (contract QLG2-CT-2001-01669). This study was partly funded by a grant from the European Union to the Prevention of Diabetes Complications (PREDICTIONS) network. FinnDiane was funded by the Folkhälsan Research Foundation, Wilhelm and Else Stockmann Foundation, Medicinska understödsföreningen Liv och Hälsa, Signe and Ane Gyllenberg Foundation and EVO governmental grants. We acknowledge all the physicians and nurses at each participating FinnDiane centre for their invaluable roles in patient recruitment, collection of samples and data (see Electronic supplementary material [ESM]).
Duality of interest
All authors declare that they have no duality of interest associated with this manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
- Department of Internal Medicine, University Medical Center Groningen, Hanzeplein 1, P.O. Box 30.001, 9700, RB, Groningen, The Netherlands
A. Alkhalaf, S. J. L. Bakker, H. J. G. Bilo, R. O. B. Gans & G. J. Navis - Diabetes Center, Isala Clinics, Zwolle, the Netherlands
A. Alkhalaf & H. J. G. Bilo - Department of Epidemiology, University Medical Center Groningen, Groningen, the Netherlands
D. Postmus - Folkhälsan Institute of Genetics, Folkhälsan Research Center, Biomedicum Helsinki, Helsinki, Finland
C. Forsblom & P. H. Groop - Division of Nephrology, Department of Medicine, Helsinki University Central Hospital, Helsinki, Finland
C. Forsblom & P. H. Groop - Institut National de la Santé et de la Recherche Médicale (INSERM), Unité Mixte de Recherche en Santé (UMRS) 937, Pierre and Marie Curie University, Paris, France
N. Vionnet - Department of Endocrinology and Diabetology, Centre Hospitalier Universitaire Poitiers, Poitiers, France
S. Hadjadj - INSERM, Unit 927 and CIC802, Poitiers, France
S. Hadjadj - Department of Endocrinology, Diabetology and Nutrition, Bichat-Claude Bernard University Hospital, Paris, France
M. Marre - INSERM Unit 695, University of Paris, Paris, France
M. Marre - Department of Medical Endocrinology, University of Copenhagen, Copenhagen, Denmark
H. H. Parving - Faculty of Health Science, Aarhus University, Aarhus, Denmark
H. H. Parving - Steno Diabetes Center, Gentofte, Denmark
P. Rossing & L. Tarnow
Authors
- A. Alkhalaf
You can also search for this author inPubMed Google Scholar - S. J. L. Bakker
You can also search for this author inPubMed Google Scholar - H. J. G. Bilo
You can also search for this author inPubMed Google Scholar - R. O. B. Gans
You can also search for this author inPubMed Google Scholar - G. J. Navis
You can also search for this author inPubMed Google Scholar - D. Postmus
You can also search for this author inPubMed Google Scholar - C. Forsblom
You can also search for this author inPubMed Google Scholar - P. H. Groop
You can also search for this author inPubMed Google Scholar - N. Vionnet
You can also search for this author inPubMed Google Scholar - S. Hadjadj
You can also search for this author inPubMed Google Scholar - M. Marre
You can also search for this author inPubMed Google Scholar - H. H. Parving
You can also search for this author inPubMed Google Scholar - P. Rossing
You can also search for this author inPubMed Google Scholar - L. Tarnow
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toA. Alkhalaf.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Alkhalaf, A., Bakker, S.J.L., Bilo, H.J.G. et al. A polymorphism in the gene encoding carnosinase (CNDP1) as a predictor of mortality and progression from nephropathy to end-stage renal disease in type 1 diabetes mellitus.Diabetologia 53, 2562–2568 (2010). https://doi.org/10.1007/s00125-010-1863-0
- Received: 12 June 2010
- Accepted: 05 July 2010
- Published: 14 August 2010
- Issue Date: December 2010
- DOI: https://doi.org/10.1007/s00125-010-1863-0