Common variants in CNDP1 and CNDP2, and risk of nephropathy in type 2 diabetes (original) (raw)
Abstract
Aims/hypothesis
Several genome-wide linkage studies have shown an association between diabetic nephropathy and a locus on chromosome 18q harbouring two carnosinase genes, CNDP1 and CNDP2. Carnosinase degrades carnosine (β-alanyl-l-histidine), which has been ascribed a renal protective effect as a scavenger of reactive oxygen species. We investigated the putative associations of genetic variants in CNDP1 and CNDP2 with diabetic nephropathy (defined either as micro- or macroalbuminuria) and estimated GFR in type 2 diabetic patients from Sweden.
Methods
We genotyped nine single nucleotide polymorphisms (SNPs) and one trinucleotide repeat polymorphism (D18S880, five to seven leucine repeats) in CNDP1 and CNDP2 in a case–control set-up including 4,888 unrelated type 2 diabetic patients (with and without nephropathy) from Sweden (Scania Diabetes Registry).
Results
Two SNPs, rs2346061 in CNDP1 and rs7577 in CNDP2, were associated with an increased risk of diabetic nephropathy (rs2346061 p = 5.07 × 10−4; rs7577 p = 0.021). The latter was also associated with estimated GFR (β = −0.037, p = 0.014), particularly in women. A haplotype including these SNPs (C-C-G) was associated with a threefold increased risk of diabetic nephropathy (OR 2.98, 95% CI 2.43–3.67, p < 0.0001).
Conclusions/interpretation
These data suggest that common variants in CNDP1 and CNDP2 play a role in susceptibility to kidney disease in patients with type 2 diabetes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.
Introduction
Diabetic nephropathy is one of the most severe complications of type 1 and type 2 diabetes mellitus, and the leading cause of end-stage renal disease (ESRD) and renal replacement therapy [1, 2]. Diabetic nephropathy results from a complex interaction between genetic susceptibility and the diabetic environment characterised by poor glycaemic control and hypertension [3–6].
About 35% of type 2 diabetic patients develop diabetic nephropathy [6]. Familial clustering has been observed, supporting the presence of a genetic component [4, 7]. Increased urinary albumin excretion is a hallmark of diabetic nephropathy and heritability for albuminuria has been estimated to be about 0.4 [7, 8]. Recent genome-wide linkage scans in different ethnic groups have shown an association between diabetic nephropathy and a locus on chromosome 18q [9–11], which also harbours two carnosine dipeptidase (CNDP) genes, CNDP1 and CNDP2.
CNDP1 and CNDP2 lie adjacent to each other on chromosome 18q. The former encodes a dipeptidase that hydrolyses the substrate l-carnosine (_β_-alanyl-l-histidine) specifically, while CNDP2 encodes a non-specific dipeptidase [12]. Carnosine has been ascribed a protective role in diabetic nephropathy, since it serves as a scavenger of oxygen radicals and thus can inhibit formation of AGEs [13, 14].
Janssen et al. provided the first evidence of an association between a tri-nucleotide repeat variant (D18S880: five to seven leucine repeats) in the signal peptide of exon 2 of CNDP1 and diabetic nephropathy in type 2 diabetic patients of European and Arab ancestry [15]. This finding was subsequently replicated in type 2 diabetic patients with diabetic nephropathy and/or ESRD of European origin in the USA [16]. In a follow-up study in African-Americans the same team identified two single nucleotide polymorphism (SNPs) in CNDP1 and CNDP2 that were associated with type 2 diabetes and ESRD [17]. However, several studies have not been able to replicate these findings [18, 19], nor has any association been shown between the CNDP locus and diabetic nephropathy in type 1 diabetes [20–22]. The 5L-5L genotype of the D18S880 marker has been reported to be associated with low serum CNDP concentrations in diabetic patients [15], making the CNDP genes interesting candidates for diabetic nephropathy susceptibility. The possibility that CNDP1 and CNDP2 play a role in diabetic nephropathy is supported by a finding that these genes are differentially expressed in kidney of animal models of diabetes [23].
Against this background of discrepant results, this study was designed to explore whether there is an association between SNPs (including the repeat variant) in the CNDP locus and diabetic nephropathy in a large well characterised population of patients with type 2 diabetes from southern Sweden (Scania Diabetes Registry [SDR]).
Methods
Study population: The Scania Diabetes Registry
All patients were from the SDR in southern Sweden (Table 1). At the time of investigation, the registry included 1264 type 1 diabetes and 5123 type 2 diabetic patients with mean disease duration of 14 years (Table 1). The registry contains information on age at onset of diabetes, mode of treatment and time insulin therapy was started, as well as follow-up data on change in BMI, HbA1c, lipids, blood pressure and development of diabetic complications.
Table 1 Clinical characteristics of type 2 diabetic patients with and without nephropathy in the SDR
Inclusion criteria in the present study were: Scandinavian origin, age at onset of diabetes >35 years, diabetes duration of ≥10 years, C-peptide ≥0.3 nmol/l and GAD antibody negativity. Diabetes diagnosis was based upon WHO criteria with fasting plasma glucose ≥7.0 mmol/l. In total, 4,888 type 2 diabetic patients were included in the study. Diabetic patients with other kidney diseases (n = 35) were excluded.
Albumin excretion rate was measured from timed overnight urine collections or as albumin : creatinine ratio (ACR) in morning spot urine tests on at least two of three occasions at 6 month intervals during follow-up. Albumin concentration in urine was determined by immunonephelometry (Beckman Instruments, Brea, CA, USA) until 1998 and thereafter by an immunoturbimetric method (Beckman Coulter, Beckman Instruments, Brea, CA, USA).
Diabetic nephropathy was subdivided into incipient (microalbuminuria) and manifest (macroalbuminuria) diabetic nephropathy. Microalbuminuria was defined as: (1) AER 20–200 μg/min in at least two timed overnight urine samples; or (2) ACR 2.0–25 g/mol in men and 3.5–35 g/mol in women. Values above the upper limit of the definition for microalbuminuria were indicative of macroalbuminuria. Based upon this definition, 880 type 2 diabetic patients were considered to have diabetic nephropathy in the SDR (Table 1).
Renal function was estimated from serum creatinine concentrations and expressed as estimated GFR. The formula for GFR (Cockroft and Gault) was: (ml min−1 1.73 m−2) = ([140 − age in years] × weight in kg × 1.73)/(plasma creatinine in μmol/l × F × BSA), where F is 0.8 in men and 0.85 in women [24].
All patients gave their written informed consent and the local ethics committees approved the study.
Selection of SNPs and haplotyping
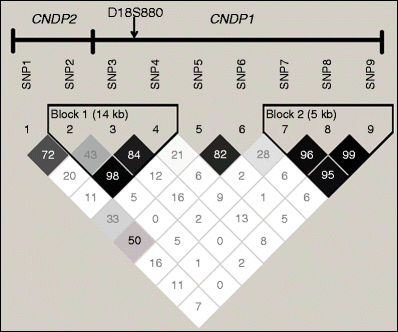
We selected nine SNPs and one microsatellite marker (D18S880) in CNDP1 and CNDP2 (chromosome 18 position: 70321335 to 70395500 bps, accessed 23 January 2010) (Fig. 1) with a minor allele frequency (MAF) >0.05 in Europeans. Selection was from the dbSNP (www.ncbi.nlm.nih.gov/snp/) and HapMap (www.hapmap.org/) databases. These SNPs capture 50% of variants (36 neighbouring SNPs) with MAF > 5% at r 2 ≥ 0.8 from the CNDP1 gene region (HapMap 1000 genomes version) [25] (see also electronic supplementary material [ESM] Table 1).
Fig. 1
LD analysis of nine SNPs in CNDP2 and CNDP1, measured by _D_′. Dark squares, high LD values. SNP1, rs4891558; SNP 2, rs7577; SNP 3, rs2346061; SNP 4, rs7244370; SNP 5, rs7239132; SNP 6, rs12604675; SNP 7, rs12964454; SNP 8, rs12456388; SNP 9, rs9953129
Genotyping
We genotyped nine SNPs (seven in CNDP1, two in CNDP2) in 4,888 patients from the SDR using an allelic discrimination method on the ABI 7900 platform (TaqMan assay; Applied Biosystems, Foster City, CA, USA). We obtained an average genotyping success rate of 98% and a 99.9% concordance rate, based on 780 duplicate comparisons using Taq Man assays. Genotyping for the D18S880 microsatellite marker was performed in all study participants by sequence analysis using a capillary sequencer (ABI 3130xl; Applied Biosystems, Darmstadt, Germany). The primers used were: AGGCAGCTGTGTGAGGTAAC (forward) and GGGTGAGGAGAACATGCC (reverse), where the forward primer was FAM-labelled at the 5′ end (Eurofins MWG Operon, Edersberg, Germany) and a PCR product length of 167 bp confirmed the presence of five CTG repeat units (five leucine codons). Random samples were sequenced later to confirm a genotyping concordance rate of 99.9%.
Statistical analyses
Data are presented as means ± SD. Non-normally distributed variables (ACR, and estimated GFR) were logarithmically (natural) transformed for analyses. Variables showing skewed distribution were compared using the Mann–Whitney _U_-test. The risk of developing diabetic nephropathy expressed as OR and 95% CI was calculated by logistic regression analyses adjusted for age and sex. Genotype–phenotype correlations were assessed using linear regression analyses adjusted for age, sex, diabetes duration, HbA1c, smoking status, systolic blood pressure and BMI (where appropriate). ANOVA with Bonferroni’s test as post hoc test were used to evaluate differences between means.
Deviations from Hardy–Weinberg equilibrium were evaluated with a Pearson’s χ 2 goodness-of-fit test. Correction for multiple testing was performed using QVALUE software package (http://genomics.princeton.edu/storeylab/qvalue/accessed 10 August2010). Haplotypes were reconstructed using PHASE (version 2.1; http://stephenslab.uchicago.edu/software.html, accessed 10 August 2010) [26].
All statistical genetic analyses were performed using an additive model (dominant and recessive models were also used) with the Statistical Package for the Social Sciences version 17.0 (SPSS, Chicago, IL, USA) and PLINK (http://pngu.mgh.harvard.edu/~purcell/plink/) [27].
Power analysis
The study was able to detect a genotype RR of 1.15 with a power of at least 70% at p < 0.05. To calculate power for SNPs in our study, we used the PS program of Dupont and Plummer (available at http://biostat.mc.vanderbilt.edu/twiki/bin/view/Main/PowerSampleSize, accessed 24 January 2010) [28].
Results
Characteristics of patients
Table 1 compares the clinical characteristics of the 4,008 type 2 diabetic patients with normoalbuminuria with those of the 880 type 2 diabetic patients with diabetic nephropathy. While there was no difference in age, the diabetic nephropathy patients had longer duration of diabetes than normoalbuminuric counterparts. The diabetic nephropathy patients had lower estimated GFR, HDL and cholesterol concentrations, but higher blood pressure, ACR, HbA1c and triacylglycerol concentrations than normoalbuminuric type 2 diabetic patients. Smoking (51% vs 36%; p < 0.01) and retinopathy (51% vs 32%; p < 0.01) were more common in diabetic nephropathy than normoalbuminuric patients.
Association between CNDP SNPs and risk of diabetic nephropathy and estimated GFR
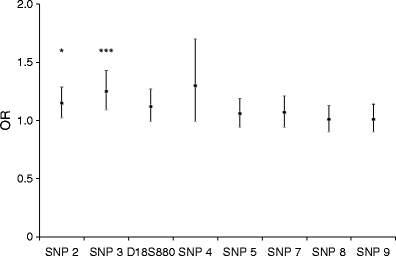
Of the genotyped SNPs and microsatellite marker from the CNDP genes, only rs4891558 (CNDP2) was monomorphic (MAF < 0.05) in this cohort; moreover, all SNPs except rs12604675 from _CNDP1_) were in Hardy–Weinberg equilibrium (_p_ > 0.10). The rs2346061 SNP in CNDP1 showed a significant association with diabetic nephropathy in additive (OR 1.25, 95% CI 1.1–1.4, p = 5.07 × 10−4) (Table 2 and Fig. 2) and alternate models (Table 2), but not with estimated GFR (Table 3).
Table 2 Risk of diabetic nephropathy predicted by various genotypic models for rs2346061 and rs7577 in the study participants
Fig. 2
ORs (95% CIs, error bars) for diabetic nephropathy vs type 2 diabetes (normoalbuminuria) patients associated with CNDP1 and CNDP2 SNPs. *p < 0.05, ***p < 0.001 for the logistic regression analysis. Age, sex, BMI, systolic blood pressure, smoking, duration of diabetes and HbA1c were included in this model for nephropathy. SNP 2, rs7577; SNP 3, rs2346061; D18S880 polymorphism; SNP 4, rs7244370; SNP 5, rs7239132; SNP 7, rs12964454; SNP 8, rs12456388; SNP 9, rs9953129
Table 3 Effects of CNDP SNPs on kidney function (estimated GFR) and HbA1c in the study population
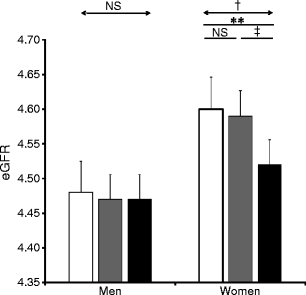
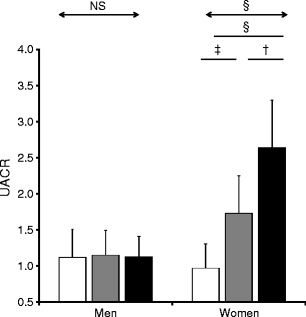
The rs7577 SNP in CNDP2 was nominally associated with diabetic nephropathy in additive (OR 1.15, 95% CI 1.02–1.3, p = 0.021) and recessive models (p = 0.016) (Table 2 and Fig. 2). The same SNP was also associated with estimated GFR in all type 2 diabetes participants with lower estimated GFR, and CC and TC compared with TT genotypes (rs7577) (p = 0.014) (Table 3). This was particularly the case in women. The difference in estimated GFR between CC and TT/TC genotype carriers (log eGFRwomen CC 4.52 ± 0.35, TC 4.58 ± 0.34, TT 4.59 ± 0.34; β = 0.036, p = 0.031) (Fig. 3) was associated with a higher urinary ACR (UACR) (log UACRwomen CC 2.01 ± 0.55, TC 1.72 ± 0.54, TT 1.23 ± 0.63; β = 0.11, p = 0.0001) (Fig. 4).
Fig. 3
Genotype-based association between CNDP2 and estimated GFR (eGFR). Estimated GFR (ml min−1 1.73 m−2) was log-transformed and expressed as mean±SD. **p < 0.01, † p < 0.031 and ‡ p < 0.04 for comparison in rs7577 genotype by ANOVA with Bonferroni’s test as post hoc test. White bars, T/T; grey bars, T/C; black bars, C/C
Fig. 4
The association between rs7577 genotype and urinary albumin excretion measured as urinary ACR (UACR). Urinary ACR values were log-transformed. † p = 0.04, ‡ p = 0.004 and § p = 0.0001 for comparison in rs7577 genotypes by ANOVA with Bonferroni’s test as post hoc test. White bars, T/T; grey bars, T/C; black bars, C/C
Table 4 shows genotype frequencies for the D18S880 microsatellite marker. We identified the 5L, 6L or 7L leucine repeats, whereas the 4L and 8L repeats were very rare (<0.005%) and thus not considered for analysis. There were no significant differences in D18S880 genotype frequencies (p = 0.17; df = 2) between diabetic nephropathy and type 2 diabetic patients, and this marker was not associated with diabetic nephropathy in the SDR cohort (ESM Table 2, ESM Table 3).
Table 4 The genotype frequencies for D18S880 repeat variant in SDR
The other genotyped SNPs (rs7244370, rs7239132, rs12964454, rs12456388 and rs9953129) did not show any significant association with risk of diabetic nephropathy or estimated GFR (ESM Table 2, ESM Table 3).
rs7239132 SNP in CNDP1 was associated with HbA1c levels. The AA genotype carriers had significantly lower HbA1c levels than CA/CC carriers (Table 3).
Haplotype analysis
The three SNPs with linkage disequilibrium (LD) values _D_′ = 0.98 (r 2 = 0.75) between rs7577 and rs7244370, _D_′ = 0.84 (r 2 = 0.24) between rs2346061 and rs7244370, and _D_′ = 0.43 (r 2 = 0.14) between rs7577and rs2346061, were used for haplotype reconstruction to study the risk associated with different allelic combinations of the common variants. Their frequencies and risk associated with diabetic nephropathy are presented in Table 5.
Table 5 Haplotype frequencies in type 2 diabetic patients (with and without nephropathy) and risk associated with diabetic nephropathy
Haplotype C-C-G (including alleles from rs7577, rs2346061 and rs7244370) was associated with a threefold increased risk of diabetic nephropathy (Table 5) as well as reduced estimated GFR (β = −0.039; p = 0.011, adjusted).
Discussion
The key finding of the present study was that the SNP in the 3′ untranslated region of CNDP2 (rs7577) was associated with increased risk of diabetic nephropathy as shown by increased ACR and decreased estimated GFR, particularly in women. Another SNP in the CNDP1 (rs2346061) promoter was associated with diabetic nephropathy. However, it did not influence estimated GFR. A haplotype consisting of these alleles was associated with increased risk of diabetic nephropathy and reduced estimated GFR.
The SNPs rs2346061 and rs7577are located in the regulatory region of CNDP1 and CNDP2, and could thereby modulate carnosinase activity in the same way as reported for another SNP in this region [15]. In previous studies of African-American type 2 diabetic patients [17], as well as of European type 1 diabetic patients [22], no association was seen between this SNP and diabetic nephropathy. It was recently claimed that the association between diabetic nephropathy and CNDP1, was sex-specific [29] and restricted to women. In support of this finding, women with the CC genotype (rs7577) had reduced estimated GFR as compared with women with the TC/TT genotypes. This finding was further supported by 1.5-fold higher ACRs in women with the CC than in those with TC/TT genotypes. It is not surprising to find sex-specific differences for associations with the CNDP genes, as women have lower carnosine levels in muscle than men due to their higher serum carnosinase levels [30]. Also, in female mice, carnosine levels increased >250% in muscle after testosterone administration [31].
The D18S880 microsatellite marker has been reported to be associated with diabetic nephropathy in some [15, 16] but not all [17, 22] studies. Janssen et al. reported for the first time that in individuals homozygous for the allele with the lowest number of leucine repeats (5L), this was associated with lower serum carnosinase concentrations, conferring protection from nephropathy [15]. This was further supported by another study showing higher carnosinase concentrations with increasing number of leucine repeats in COS cells [32]. Since carnosine has been ascribed anti-oxidant effects, and is a potential inhibitor of ACE activity and AGEs [32], the activity of the enzyme carnosinase may be important in the development of nephropathy.
Our data did not support an association between the leucine repeat and diabetic nephropathy in this cohort of Scandinavian patients with type 2 diabetes.
The next question is whether the promoter SNP (rs2346061) in CNDP1 really is associated with diabetic nephropathy, since we observed no effect of this variant on kidney function (estimated GFR). This SNP was not associated with diabetic nephropathy (proteinuria or ESRD) in white type 1 diabetic patients [11]. However, in that study [11] there was a modest association between two other SNPs in the CNDP1 promoter region (rs12954438, rs890332) and proteinuria but not ESRD, supporting the view that promoter SNPs in CNDP1 might influence albuminuria but that this may not translate into a progressive deterioration of kidney function. Notably, we excluded patients with ESRD from our study. Also, none of these SNPs are in LD (r 2 = 0.03; HapMap version 2 release 24) with rs2346061.
Further support for the view that different SNPs in CNDP1 influence albuminuria and progression to ESRD comes from a study in African-American type 2 diabetic patients, which did not find any association between a proxy SNP rs2346061 and ESRD [17].
However, we have no evidence that these are the causal SNPs; functional studies are needed to define the role of potential functional effects of these SNPs. Lacking such information, we also tested whether haplotypes including these two SNPs conferred a stronger effect on diabetic nephropathy than the individual SNPs.
We found that the haplotype C-C-G in block 2 (including alleles from rs7577, rs2346061 and rs7244370) was associated with a threefold increased risk of diabetic nephropathy as well as reduced estimated GFR. Another study has also reported stronger association between haplotypes in the CNDP2 region and ESRD than between individual alleles and ESRD [17].
Our study has some pros and cons. Thus while the well characterised patient groups were large enough to have a power of 75–80% to detect an association between the two key SNPs rs7577 and rs2346061 and diabetic nephropathy, our study was still underpowered for low frequency SNPs, as well as for haplotypes.
In conclusion, we provide evidence of an association between a common SNP rs2346061 in CNDP1 and diabetic nephropathy. As this SNP was not associated with kidney function, it is possible that it merely increases risk of albuminuria, rather than of progression of kidney disease, as other studies have also failed to demonstrate an association between this SNP and ESRD. The SNP rs7577 in CNDP2 confers increased risk of nephropathy by altering kidney function, particularly in women. A three-allelic haplotype in the regulatory region of the CNDP genes was associated with a threefold increased risk of diabetic nephropathy and reduced estimated GFR, suggesting that other modifying SNPs are needed to increase risk of progression towards kidney dysfunction.
Abbreviations
ACR:
Albumin : creatinine ratio
CNDP:
Carnosine dipeptidase
ESRD:
End-stage renal disease
LD:
Linkage disequilibrium
MAF:
Minor allele frequency
SDR:
Scania Diabetes Registry
SNP:
Single nucleotide polymorphism
UACR:
Urinary albumin creatinine ratio
References
- No authors listed (2010) US Renal Data System 2010 Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda
- No authors listed (2010) ERA-EDTA Registry Annual Report 2008. Academic Medical Center, Amsterdam
- Schmidt S, Ritz E (1997) Genetic determinants of diabetic renal disease and their impact on therapeutic interventions. Kidney Int Suppl 63:S27–S31
PubMed CAS Google Scholar - Conway BR, Maxwell AP (2009) Genetics of diabetic nephropathy: are there clues to the understanding of common kidney diseases? Nephron Clin Pract 112:c213–c221
Article PubMed CAS Google Scholar - Sallinen R, Kaunisto MA, Forsblom C et al (2010) Association of the SLC22A1, SLC22A2, and SLC22A3 genes encoding organic cation transporters with diabetic nephropathy and hypertension. Ann Med 42:296–304
Article PubMed CAS Google Scholar - Forbes JM, Fukami K, Cooper ME (2007) Diabetic nephropathy: where hemodynamics meets metabolism. Exp Clin Endocrinol Diab 115:69–84
Article CAS Google Scholar - Freedman BI, Bostrom M, Daeihagh P, Bowden DW (2007) Genetic factors in diabetic nephropathy. Clin J Am Soc Nephrol 2:1306–1316
Article PubMed CAS Google Scholar - Forsblom CM, Kanninen T, Lehtovirta M, Saloranta C, Groop LC (1999) Heritability of albumin excretion rate in families of patients with type II diabetes. Diabetologia 42:1359–1366
Article PubMed CAS Google Scholar - Iyengar SK, Abboud HE, Goddard KA et al (2007) Genome-wide scans for diabetic nephropathy and albuminuria in multiethnic populations: the Family Investigation of Nephropathy and Diabetes (FIND). Diabetes 56:1577–1585
Article PubMed CAS Google Scholar - Vardarli I, Baier LJ, Hanson RL et al (2002) Gene for susceptibility to diabetic nephropathy in type 2 diabetes maps to 18q22.3-23. Kidney Int 62:2176–2183
Article PubMed CAS Google Scholar - Bowden DW, Colicigno CJ, Langefeld CD et al (2004) A genome scan for diabetic nephropathy in African Americans. Kidney Int 66:1517–1526
Article PubMed CAS Google Scholar - Teufel M, Saudek V, Ledig JP et al (2003) Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J Biol Chem 278:6521–6531
Article PubMed CAS Google Scholar - Hipkiss AR, Chana H (1998) Carnosine protects proteins against methylglyoxal-mediated modifications. Biochem Biophys Res Commun 248:28–32
Article PubMed CAS Google Scholar - Hipkiss AR, Preston JE, Himsworth DT et al (1998) Pluripotent protective effects of carnosine, a naturally occurring dipeptide. Ann NY Acad Sci 854:37–53
Article PubMed CAS Google Scholar - Janssen B, Hohenadel D, Brinkkoetter P et al (2005) Carnosine as a protective factor in diabetic nephropathy: association with a leucine repeat of the carnosinase gene CNDP1. Diabetes 54:2320–2327
Article PubMed CAS Google Scholar - Freedman BI, Hicks PJ, Sale MM et al (2007) A leucine repeat in the carnosinase gene CNDP1 is associated with diabetic end-stage renal disease in European Americans. Nephrol Dial Transplant 22:1131–1135
Article PubMed CAS Google Scholar - McDonough CW, Hicks PJ, Lu L, Langefeld CD, Freedman BI, Bowden DW (2009) The influence of carnosinase gene polymorphisms on diabetic nephropathy risk in African-Americans. Hum Genet 126:265–275
Article PubMed CAS Google Scholar - Kilis-Pstrusinska K, Zwolinska D, Grzeszczak W (2010) Is carnosinase 1 gene (CNDP1) polymorphism associated with chronic kidney disease progression in children and young adults? Results of a family-based study. Arch Med Res 41:356–362
Article PubMed CAS Google Scholar - Kim S, Abboud HE, Pahl MV et al (2010) Examination of association with candidate genes for diabetic nephropathy in a Mexican American population. Clin J Am Soc Nephrol 5:1072–1078
Article PubMed CAS Google Scholar - Alkhalaf A, Bakker SJ, Bilo HJ et al (2010) A polymorphism in the gene encoding carnosinase (CNDP1) as a predictor of mortality and progression from nephropathy to end-stage renal disease in type 1 diabetes mellitus. Diabetologia 53:2562–2568
Article PubMed CAS Google Scholar - Craig DW, Millis MP, DiStefano JK (2009) Genome-wide SNP genotyping study using pooled DNA to identify candidate markers mediating susceptibility to end-stage renal disease attributed to type 1 diabetes. Diabet Med 26:1090–1098
Article PubMed CAS Google Scholar - Wanic K, Placha G, Dunn J, Smiles A, Warram JH, Krolewski AS (2008) Exclusion of polymorphisms in carnosinase genes (CNDP1 and CNDP2) as a cause of diabetic nephropathy in type 1 diabetes: results of large case–control and follow-up studies. Diabetes 57:2547–2551
Article PubMed CAS Google Scholar - Hu Y, Kaisaki PJ, Argoud K et al (2009) Functional annotations of diabetes nephropathy susceptibility loci through analysis of genome-wide renal gene expression in rat models of diabetes mellitus. BMC Med Genomics 2:41
Article PubMed Google Scholar - Farbom P, Wahlstrand B, Almgren P et al (2008) Interaction between renal function and microalbuminuria for cardiovascular risk in hypertension: the Nordic Diltiazem Study. Hypertension 52:115–122
Article PubMed Google Scholar - Johnson AD, Handsaker RE, Pulit SL, Nizzari MM, O'Donnell CJ, de Bakker PI (2008) SNAP: a web-based tool for identification and annotation of proxy SNPs using HapMap. Bioinformatics 24:2938–2939
Article PubMed CAS Google Scholar - Stephens M, Donnelly P (2003) A comparison of Bayesian methods for haplotype reconstruction from population genotype data. Am J Hum Genet 73:1162–1169
Article PubMed CAS Google Scholar - Purcell S, Neale B, Todd-Brown K et al (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575
Article PubMed CAS Google Scholar - Dupont WD, Plummer WD Jr (1998) Power and sample size calculations for studies involving linear regression. Control Clin Trials 19:589–601
Article PubMed CAS Google Scholar - Mooyaart AL, Zutinic A, Bakker SJ et al (2010) Association between CNDP1 genotype and diabetic nephropathy is sex specific. Diabetes 59:1555–1559
Article PubMed CAS Google Scholar - Bando K, Shimotsuji T, Toyoshima H, Hayashi C, Miyai K (1984) Fluorometric assay of human serum carnosinase activity in normal children, adults and patients with myopathy. Ann Clin Biochem 21:510–514
PubMed CAS Google Scholar - Penafiel R, Ruzafa C, Monserrat F, Cremades A (2004) Gender-related differences in carnosine, anserine and lysine content of murine skeletal muscle. Amino Acids 26:53–58
Article PubMed CAS Google Scholar - Riedl E, Koeppel H, Brinkkoetter P et al (2007) A CTG polymorphism in the CNDP1 gene determines the secretion of serum carnosinase in Cos-7 transfected cells. Diabetes 56:2410–2413
Article PubMed CAS Google Scholar
Acknowledgements
T. S. Ahluwalia is supported by a grant from the Bo and Kerstin Hjelt Diabetes Foundation (www.hjeltfoundations.org/). The study was further supported by grants from the Swedish Research Council, including a Linné grant (2008-6589) and a Strategic Research Grant (Exodiab; 2009-1039), the Heart-Lung Foundation, the Swedish Diabetes Research Foundation, the Knut and Alice Wallenberg Foundation, the Novo Nordisk Foundation, and European Community 7th Framework Programme grants (ENGAGE Health-2007-201413 and an Innovative Medicines Initiative grant: SUMMIT 2009-115006)). Special thanks go to M. Svensson for her help in genotyping the D18S880 polymorphism.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
- Department of Clinical Sciences—Diabetes and Endocrinology, Lund University Diabetes Centre, Clinical Research Centre (CRC), University Hospital Skane (UMAS), 20502, Malmo, Sweden
T. S. Ahluwalia, E. Lindholm & L. C. Groop
Authors
- T. S. Ahluwalia
- E. Lindholm
- L. C. Groop
Corresponding author
Correspondence toT. S. Ahluwalia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
Proxy SNPs captured by the SNPs studied and their MAF in the CEU population (PDF 75 kb)
ESM Table 2
Genotype frequencies for rs7577, D18S880, rs2346061, rs7244370, rs7239132, rs12964454, rs12456388 and rs9953129 in SDR study of type 2 diabetic patients with and without nephropathy (PDF 31 kb)
ESM Table 3
Risk of diabetic nephropathy predicted by the conventional (additive) genotypic model for the CNDP variants in the SDR (PDF 27 kb)
Rights and permissions
About this article
Cite this article
Ahluwalia, T.S., Lindholm, E. & Groop, L.C. Common variants in CNDP1 and CNDP2, and risk of nephropathy in type 2 diabetes.Diabetologia 54, 2295–2302 (2011). https://doi.org/10.1007/s00125-011-2178-5
- Received: 20 October 2010
- Accepted: 13 April 2011
- Published: 15 May 2011
- Issue Date: September 2011
- DOI: https://doi.org/10.1007/s00125-011-2178-5