Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes (original) (raw)
Abstract
Aims/hypothesis
Seroconversion to islet autoantibodies precedes type 1 diabetes. This study aimed to identify periods of high seroconversion incidence, which could be targeted for mechanistic and therapeutic studies.
Methods
Incidence of islet autoantibodies was calculated in 1,650 genetically at-risk children followed with measurements of islet autoantibodies and thyroid autoantibodies at age 9 months and 2, 5, 8, 11, 14 and 17 years. Peak incidence periods were confirmed in a second cohort of 150 children followed until age 6 years with three-monthly samples up to age 3 years.
Results
Islet autoantibody incidence (per 1,000 person-years) was 18.5 until age 9 months, 21 from 9 months to 2 years and <10 for intervals after age 2 years. The second cohort confirmed peak incidence around age 9 months and demonstrated an absence of seroconversion before this age. Seroconversion to insulin autoantibodies occurred earlier than other autoantibodies (p < 0.01 against glutamic acid decarboxylase [GAD]-, insulinoma-associated protein 2 [IA-2]- and zinc transporter 8 [ZnT8]-autoantibodies). Early peak seroconversion incidence was most evident in children with high-risk HLA DR3/4-DQ8 or DR4/4-DQ8 genotypes.
Conclusion
The age period 9 months to 2 years is associated with a high incidence of activation of type 1 diabetes-associated autoimmunity in genetically at-risk children and should be targeted for effective primary prevention strategies.
Similar content being viewed by others
Introduction
Type 1 diabetes is an autoimmune disorder in which disease onset is preceded by a pre-clinical period of islet autoimmunity [1]. The initiation of islet autoimmunity is heralded by seroconversion for islet autoantibodies directed against insulin, glutamic acid decarboxylase (GAD), insulinoma-associated protein 2 (IA-2) and zinc transporter 8 (ZnT8) [2–5]. Progression to clinical disease after seroconversion is variable and associated with the diversity and specificity of islet autoantibodies [6, 7] as well as genetic determinants of type 1 diabetes [8, 9]. While much is known with respect to the incidence of type 1 diabetes with the notable rise over recent decades and a peak age of incidence around puberty [10], little is known about the incidence of islet autoimmunity and its relationship to age. Understanding periods of high and low incidence of seroconversion and their relationships with demographics and genes will provide insights into potential pathogenetic mechanisms and environmental triggers, and identify ages at which prevention strategies are most likely to be needed. We have prospectively followed from birth children who are genetically at risk for type 1 diabetes for more than 20 years, providing the opportunity to examine the incidence of seroconversion during childhood and adolescence. Here we show that seroconversion is greatest in the age period 9 months to 2 years, is rare at ages 6 months or younger, occurs earlier in boys and occurs earliest for insulin autoantibodies. These data emphasise the importance of developing immune therapies that can be safely used in neonates and infants.
Methods
Study cohort, participants and samples
The study was performed in children from the BABYDIAB Study, a longitudinal study examining the natural history of islet autoimmunity and type 1 diabetes in 1,650 children born to a mother or father with type 1 diabetes [11]. Recruitment began in 1989 and ended in 2000. All children (840 boys, 810 girls) were recruited from Germany. The cohort is not population based and 97% of families are German and of European descent. Venous blood samples were obtained from children at study visits scheduled at 9 months and at 2, 5, 8, 11, 14 and 17 years of age. Islet autoantibodies (insulin autoantibodies [IAA], GAD autoantibodies [GADA], IA-2 autoantibodies [IA-2A] and ZnT8 autoantibodies [ZnT8-A]) were measured in samples taken at all scheduled visits, and every 6 months in children positive for islet autoantibodies. The median follow-up time from birth to last sample was 11.1 years (interquartile range, 5.7–14.0 years) and from birth to last contact was 13.9 years (interquartile range, 11.9–15.6 years).
As a confirmatory cohort, islet autoantibody incidence was examined in 150 children who participated in the BABYDIET Study [12]. These children were selected for having both a first-degree relative with type 1 diabetes and type 1 diabetes risk HLA genotypes. Children were recruited between 2000 and 2004 and followed from the age of 3 months with three-monthly blood samples until age 3 years and subsequently with yearly blood samples. The median follow-up time from birth to last sample was 5.2 years (interquartile range, 3.2–6.6 years). The BABYDIET Study was a dietary intervention study in which children were randomised to be first exposed to gluten-containing foods at age 6 or 12 months (ClinicalTrials.gov NCT01115621). No difference in islet autoantibody development was observed with respect to randomisation group [13].
The BABYDIAB and BABYDIET studies were approved by the ethical committee of Bavaria, Germany (Bayerische Landesärztekammer No. 95357 and Ludwig-Maximilians University No. 329/00, respectively). All families gave written informed consent to participate in the studies. Investigations were carried out in accordance with the principles of the Declaration of Helsinki, as revised in 2000.
Autoantibody measurements
IAA, GADA, IA-2A and ZnT8-A were determined centrally by the Institute of Diabetes Research Munich using radiobinding assays as previously described [11, 14]. Briefly, IAA were measured by protein A/G radiobinding assays using [125I]-recombinant human insulin labelled at A-14 tyrosine, and GADA, IA-2A and ZnT8-A were measured separately by protein A radiobinding assays using [35S]methionine in vitro transcribed/translated recombinant human GAD65, IA-2ic and the carboxy terminal portion of ZnT8 for the two major variants at amino acid 325 (arginine and tryptophan), respectively. The upper limit of normal for each assay was determined using Q–Q plots and corresponded to the 99th percentile of 836 control children. Islet autoantibody assays were evaluated by the Diabetes Autoantibody Standardization Program (laboratory 121) [15, 16]. Thyroid peroxidase (TPO) autoantibodies were measured using a direct radiobinding assay according to the manufacturer’s instructions (CentAK anti-TPO, Medipan, Dahlewitz/Berlin, Germany) as previously described [17].
Genotyping
HLA-DRB1, HLA-DQA1 and HLA-DQB1 alleles were typed using PCR-amplified DNA and non-radioactive sequence-specific oligonucleotide probes as described previously [18]. Children with the high-risk HLA genotypes _DR4-DQA1*030X-DQB1*0302/DR3_-DQA1*0501-DQB1*0201 or DR4-DQA1*030X-DQB1*0302/DR4-DQA1*030X-DQB1*0302 were compared with children with other HLA genotypes.
Statistical analysis
Children were classified as islet autoantibody positive if they were positive for one or more islet autoantibodies in at least two consecutive samples. The age of the first autoantibody-positive sample was considered the seroconversion age in children. If a child was positive in the first sample measured, autoantibodies in the child’s mother were compared and, if positive, the child sample was classified as negative on the basis that the positive result was likely to be due to placental transfer of maternal autoantibodies. For some analyses, children were also classified as multiple islet autoantibody positive if they were positive for more than one of the antibodies measured on at least one occasion, or as single islet autoantibody positive if they were not. For these subanalyses, bias due to different follow-up times in children who seroconverted at different ages was reduced by excluding single islet autoantibody-positive children who had not been followed for at least 3 years after seroconversion (n = 19), and children who converted to multiple islet autoantibodies after 3 years of initial seroconversion (n = 8).
Islet autoantibody incidence and TPO autoantibody incidence in the BABYDIAB cohort was calculated from the number of children who newly seroconverted to islet autoantibody positivity in samples obtained at 9 months (mean age of sample, 0.85 years), 2 years (mean age of sample, 2.0 years), 5 years (mean age of sample, 5.0 years), 8 years (mean age of sample, 8.0 years), 11 years (mean age of sample, 11.0 years) and 14 years (mean age of sample, 14.0 years) and the number of children followed at each time point, and by subsequently calculating incidence as cases per 1,000 per year (per 1,000 person-years) for each time interval. As an example, if 1,500 children remained negative at their 9 month visit (median age at 9 month visit was 0.85 years) and reached their 2 year follow-up (median age at 2 year visit was 2.0 years), and 25 of these children became positive at age 2 years, the incidence for the period 9 months to 2 years would be 25/1,500/(2–0.85) × 1,000 = 14.5 per 1,000 person-years. Intervals used for the BABYDIET cohort were 0 to 6, 6.1 to 12, 12.1 to 18, 18.1 to 24, 24.1 to 36, and 36.1 to 60 months. The 95% CIs are provided around the antibody incidences.
Comparisons of islet autoantibody incidence between groups were performed using Fisher’s exact test using the actual number of cases. Time to event analyses (Kaplan–Meier) were used to examine progression from first islet autoantibody to diabetes. Kaplan–Meier analysis was used to calculate risk and to compare probabilities of type 1 diabetes in children stratified for age of seroconversion. The time from the age of seroconversion to the age at diagnosis of diabetes or the age at last follow-up was used as the event time. The logrank test was used to compare categories in the Kaplan–Meier analysis. All statistical analyses were performed using the Statistical Package for Social Science (SPSS 17.0; Chicago, IL, USA).
Results
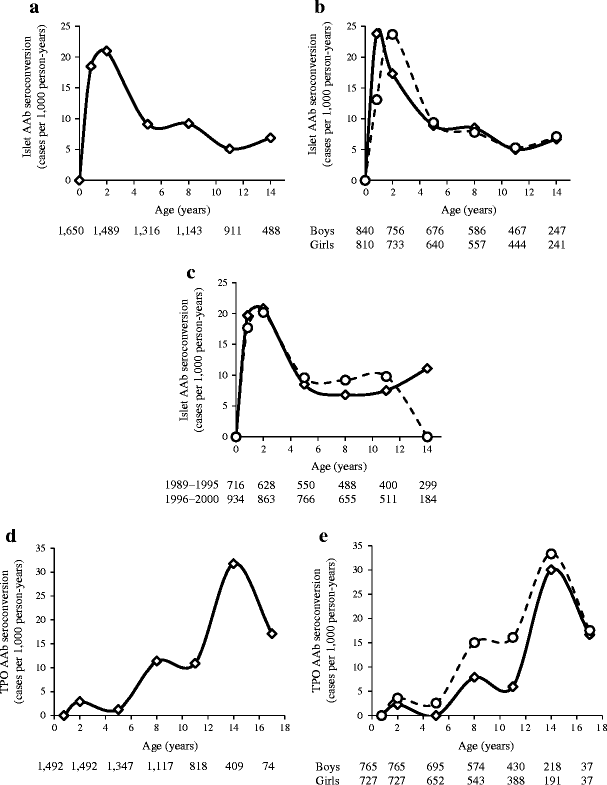
A total of 152 children in the BABYDIAB cohort developed islet autoantibodies during follow-up; 26 seroconverted at age 9 months, 34 at age 2 years, 38 at 5 years, 28 at 8 years, 14 at 11 years, 10 at 14 years and two after 17 years. The islet autoantibody incidence (95% CI) was high at age 9 months (18.5 [12.7, 27] per 1,000 person-years) and 2 years (21 [15.9, 29] per 1,000 person-years) and decreased thereafter (9.1 [6.3, 12.5] per 1,000 person-years at 5 years; 9.2 [5.7, 11.7] per 1,000 person-years at 8 years; 5.1 [3.1, 8.5] per 1,000 person-years at 11 years; 6.9 [3.8, 12.4] per 1,000 person-years at 14 years; Fig. 1a). Although boys and girls had similar overall risks for islet autoantibodies, the peak incidence in boys was observed at the 9 month sample and in girls at the 2 year sample (Fig. 1b). The year of birth did not affect islet autoantibody incidence (Fig. 1c). As a comparison, the incidence of seroconversion to thyroid autoimmunity (TPO autoantibodies) did not rise until puberty and reached peak levels at adolescence (Fig. 1d). Unlike islet autoantibodies, the incidence of TPO autoantibodies at early age was higher in girls than in boys (p = 0.002; Fig. 1e).
Fig. 1
Autoantibody incidence (cases per 1,000 person-years) in offspring of parents with type 1 diabetes (BABYDIAB Study). Incidence is shown at the ages of islet autoantibody testing (9 months and 2, 5, 8, 11 and 14 years) and refers to the age intervals between these time points. a Incidence of islet autoantibodies (AAb; any of IAA, GADA, IA-2A and ZnT8-A). b Incidence of islet autoantibodies in boys (solid line) and girls (dashed line). c Incidence of islet autoantibodies in offspring born between 1989 and 1995 (solid line) and 1996 and 2000 (dashed line). d Incidence of TPO autoantibodies (AAb). e Incidence of TPO autoantibodies in boys (solid line) and girls (dashed line). Numbers under the _x_-axes are the numbers of children still followed at each time point
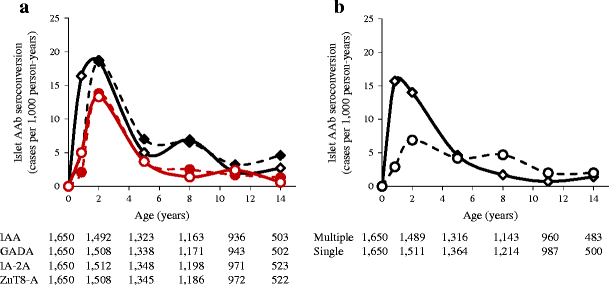
Similar changes in incidence by age (95% CI) were seen for all four islet autoantibodies with the highest respective incidence at age 2 years (IAA, 18.7 [13.3, 26.2] per 1,000 person-years; GADA, 18.5 [13.1, 25.9] per 1,000 person-years; IA2A, 13.8 [9.3, 20.4] per 1,000 person-years; ZnT8-A, 13.3 [8.9, 19.8] per 1,000 person-years). However, IAA developed earlier with a higher incidence at age 9 months than other islet autoantibodies (16.4 [10.9, 24.5] per 1,000 person-years vs 5 [2.5, 10.2] for GADA, 2.1 [0.8, 6.2] for IA-2A and 5 [2.5, 10.2] for ZnT8-A; p = 0.005, p < 0.0001 and p = 0.005, respectively; Fig. 2a). The incidence of multiple islet autoantibodies was high and pronounced at 9 months and 2 years compared with age 5, 8, 11 and 14 years (Fig. 2b). In contrast, the incidence of single islet autoantibodies was relatively stable throughout follow-up, and at age 9 months and 2 years was lower that the incidence of multiple islet autoantibodies (Fig. 2b).
Fig. 2
Incidence (cases per 1,000 person-years) of specific islet autoantibodies (AAb). a IAA (solid black line), GADA (dashed black line), IA-2A (solid coloured line) and ZnT8-A (dashed coloured line) in offspring of parents with type 1 diabetes (BABYDIAB Study). b Incidence of multiple (solid line) and single (dashed line) islet autoantibodies. Incidence is shown at the ages of islet autoantibody testing (9 months and 2, 5, 8, 11 and 14 years). Numbers under the _x_-axes are the numbers of children still followed at each time point
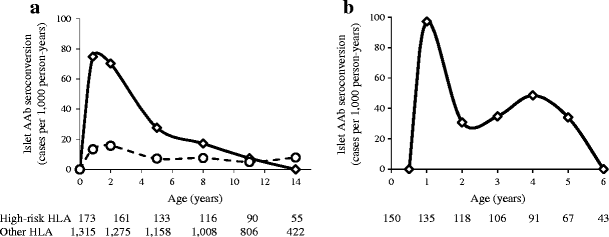
Children with the HLA DR3/4-DQ8 or DR4/4-DQ8 genotypes had a much higher incidence of islet autoantibodies from age 9 months to 5 years compared with children without these genotypes (p < 0.0001 for 9 months, p < 0.0001 for 2 years, p = 0.0006 for 5 years; Fig. 3a). A clear peak incidence was observed at 9 months to 2 years of age in children with the high-risk genotypes.
Fig. 3
a Incidence of islet autoantibodies (AAb; cases per 1,000 person-years) in offspring of parents with type 1 diabetes relative to HLA genotype (BABYDIAB Study). Children with the DR3/4-DQ8 or DR4/4-DQ8 genotypes (solid line) had higher incidence levels than children with other HLA genotypes (dashed line) during early childhood. Numbers under the _x_-axis are the numbers of children still followed at each time point. b Incidence of islet autoantibodies (any of IAA, GADA, IA-2A or ZnT8-A) in 150 children with first-degree relatives with type 1 diabetes and high-risk HLA genotypes participating in the BABYDIET Study. Incidence is shown at ages 6 and 12 months and 2, 3, 4, 5 and 6 years and refer to the age intervals between these points. Numbers under the _x_-axis are the numbers of children still followed at each time point
In order to confirm the findings from the BABYDIAB cohort and to refine incidence from birth to age 3 years, islet autoantibody incidence was determined in the BABYDIET cohort (Fig. 3b). These children had a higher overall increased risk of developing islet autoantibodies than BABYDIAB children and had samples collected at 3 month intervals until age 3 years. No child seroconverted to islet autoantibody positivity at age 3 or 6 months. Peak incidence was observed in the 6.1 to 12 months age period (97 [95% CI 46, 186] per 1,000 person-years; p = 0.006 vs 0 to 6 months). Incidences for each 12 month period after age 12 months were less than 50 per 100 person-years (p = 0.02; 6.1 to 12 months vs 12.1 to 72 months). Similarly to observations in the BABYDIAB cohort, the incidence in the 6 to 12 month age period (95% CI) tended to be higher in boys than in girls (159 [66, 326] vs 48 [15, 166] per 1,000 person-years; p = 0.2).
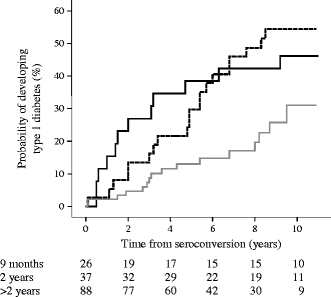
To determine whether seroconversion during the early peak incidence period was associated with a high risk for type 1 diabetes, we analysed the rate of progression to type 1 diabetes in BABYDIAB children who developed islet autoantibodies at the age of 9 months, at the age of 2 years and after the age of 2 years (Fig. 4). Progression was high and faster in children who seroconverted during the peak incidence period compared with children who developed islet autoantibodies during a low incidence period (progression within 10 years [95% CI]: 46% [27%, 65%] for seroconverters at 9 months; 54% [38%, 70%] for seroconverters at age 2 years; and 31% [16%, 46%] for seroconverters after age 2 years; p = 0.03 and p = 0.006 for comparisons against seroconverters after age 2 years, respectively). Moreover, 30 (83%) of 36 children who developed diabetes by age 10 years had seroconverted to islet autoantibody positivity at 9 months or 2 years.
Fig. 4
Risk for type 1 diabetes relative to the age of islet autoantibody seroconversion. Kaplan–Meier analysis for the progression to type 1 diabetes from the age of first appearance of islet antibodies in children who seroconverted at age 9 months (solid line), 2 years (dashed line) and after age 2 years (grey line). Numbers under the _x_-axis are the numbers of children still followed at each time point
Discussion
The incidence of type 1 diabetes has its peak around puberty. Here, we show that, in genetically at-risk individuals, the incidence of islet autoantibody seroconversion has a peak at 9 months to 2 years of age. This peak incidence period is preceded by a period during which islet autoantibody seroconversion is rare, and is followed by a relatively low incidence period in which seroconversion is often characterised by single islet autoantibodies. The findings imply a heightened propensity to activate type 1 diabetes relevant autoimmunity between the ages of 9 months and 2 years, and suggest an increased susceptibility of the immune system to autoimmunity and/or increased exposure to factors that trigger autoimmunity in this period.
These are the first data providing the incidence of seroconversion to autoimmunity during childhood and adolescence. While the incidence estimates are approximate, as evidenced by relatively wide confidence intervals, there are some important and consistent messages in the data. The early peak incidence of islet autoantibody seroconversion seen at 9 months to 2 years was consistently observed in two time periods and was confirmed in a second cohort. Seroconversion at this early age was associated with a high rate of progression to diabetes and accounted for the large majority of children who developed type 1 diabetes by age 10 years, indicating that the increased seroconversion was disease relevant. The findings apply to genetically at-risk children and were pronounced in those children with both a first-degree family history of type 1 diabetes and high type 1 diabetes risk HLA class II genotypes, and thus may not be representative of all children. Nevertheless, these genetically at-risk children are those in whom first prevention therapies are tested and therefore the findings are highly relevant to prevention.
The BABYDIET cohort was informative as to when seroconversion starts to occur. No seroconversion occurred at 3 and 6 months in these children. Because there were relatively few children in this cohort, we cannot conclude that seroconversion does not occur in this period. Nevertheless, the observed lack of seroconversion is intriguing with respect to pathogenesis. Several scenarios could explain the finding. It is possible that triggers relevant for activating islet autoreactive cells are infrequent in this period. Alternatively, they may be present but there is protection against activation of autoreactive cells. Such protection could be in the form of other environmental factors or by an immune system that is downregulated or incomplete in its antigen presentation capacity, as has been suggested to be the case at birth [19–21].
In contrast to the period up to age 6 months, the subsequent period up to age 2 years presented the highest risk of developing islet autoimmunity. This was particularly evident for autoimmunity that was associated with multiple islet autoantibodies, and in children with HLA genotypes conferring high type 1 diabetes risk. The high incidence at this age may reflect exposure to environmental factors relevant to type 1 diabetes and/or susceptibility of the immune system to activation against self. It is, however, interesting to note that another type of autoimmunity has high incidence periods at a later age; seroconversion to TPO autoantibodies was frequent in this cohort, but did not reach high levels until puberty and appeared to peak in adolescence. The thyroid organ undergoes remodelling around this age as a result of increased activity [22–24], potentially explaining the increased rate of TPO autoimmunity. Interestingly, neonatal remodelling of the pancreas has been suggested to be involved in the pathogenesis of diabetes in the NOD mouse [25]. While in humans beta cell apoptosis appears to be restricted to the first months of life [26], it is conceivable that beta cell growth or insulin demands after weaning could contribute to the high incidence of islet autoimmunity after age 6 months.
Of further interest with respect to pathogenesis, we found that boys tended to have an earlier peak incidence of islet autoantibody seroconversion than girls. The reason for this sex-based effect is unclear. There are several differences in hormones between boys and girls in the first years of life, including markedly increased oestradiol concentrations in girls [27]. These differences could play a role in immune response per se [28] or affect beta cell remodelling or function.
Finally, the incidence data have implications for prevention in genetically at-risk children. A substantial proportion of children who develop type 1 diabetes will have seroconverted between 9 months and 2 years of age. Thus, if we are to prevent type 1 diabetes, primary prevention will be most needed in the first months of life. Of potential benefit, this period may be associated with protection against autoimmunity and may be ideal for therapies aiming to induce autoantigen-specific immune tolerance. The earlier seroconversion of IAA suggests that (pro)insulin is an important antigen to target in this early period. An alternative to inducing early immune tolerance could be to protect high-risk children during the high incidence period with treatments that inhibit T cell activation or protect beta cells from apoptosis. If successful, such short-term treatments could reduce the incidence of childhood autoimmune diabetes.
Abbreviations
GAD:
Glutamic acid decarboxylase
GADA:
Glutamic acid decarboxylase autoantibodies
IA-2:
Insulinoma-associated protein 2
IA-2A:
Insulinoma-associated protein 2 autoantibodies
IAA:
Insulin autoantibodies
TPO:
Thyroid peroxidase
ZnT8:
Zinc transporter 8
ZnT8-A:
Zinc transporter 8 autoantibodies
References
- Atkinson MA, Eisenbarth GS (2001) Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet 358:221–229
Article PubMed CAS Google Scholar - Palmer JP, Asplin CM, Clemons P et al (1983) Insulin antibodies in insulin-dependent diabetics before insulin treatment. Science 222:1337–1339
Article PubMed CAS Google Scholar - Baekkeskov S, Aanstoot HJ, Christgau S et al (1990) Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 347:151–156
Article PubMed CAS Google Scholar - Rabin DU, Pleasic SM, Shapiro JA et al (1994) Islet cell antigen 512 is a diabetes-specific islet autoantigen related to protein tyrosine phosphatases. J Immunol 152:3183–3188
PubMed CAS Google Scholar - Wenzlau JM, Juhl K, Yu L et al (2007) The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 104:17040–17045
Article PubMed CAS Google Scholar - Achenbach P, Warncke K, Reiter J et al (2004) Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes 53:384–392
Article PubMed CAS Google Scholar - Achenbach P, Warncke K, Reiter J et al (2006) Type 1 diabetes risk assessment: improvement by follow-up measurements in young islet autoantibody-positive relatives. Diabetologia 49:2969–2976
Article PubMed CAS Google Scholar - Winkler C, Lauber C, Adler K et al (2011) An interferon-induced helicase (IFIH1) gene polymorphism associates with different rates of progression from autoimmunity to type 1 diabetes. Diabetes 60:685–690
Article PubMed CAS Google Scholar - Lipponen K, Gombos Z, Kiviniemi M et al (2010) Effect of HLA class I and class II alleles on progression from autoantibody positivity to overt type 1 diabetes in children with risk-associated class II genotypes. Diabetes 59:3253–3256
Article PubMed CAS Google Scholar - Dabelea D, Bell RA, D’Agostino RB Jr et al (2007) Incidence of diabetes in youth in the United States. JAMA 297:2716–2724
Article PubMed Google Scholar - Ziegler AG, Hummel M, Schenker M, Bonifacio E (1999) Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes 48:460–468
Article PubMed CAS Google Scholar - Schmid S, Buuck D, Knopff A, Bonifacio E, Ziegler AG (2004) BABYDIET, a feasibility study to prevent the appearance of islet autoantibodies in relatives of patients with type 1 diabetes by delaying exposure to gluten. Diabetologia 47:1130–1131
Article PubMed CAS Google Scholar - Hummel S, Pflueger M, Hummel M, Bonifacio E, Ziegler AG (2011) Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET Study. Diabetes Care 34:1301–1305
Article PubMed Google Scholar - Achenbach P, Lampasona V, Landherr U et al (2009) Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 52:1881–1888
Article PubMed CAS Google Scholar - Torn C, Mueller PW, Schlosser M, Bonifacio E, Bingley PJ (2008) Diabetes Antibody Standardization Program: evaluation of assays for autoantibodies to glutamic acid decarboxylase and islet antigen-2. Diabetologia 51:846–852
Article PubMed CAS Google Scholar - Schlosser M, Mueller PW, Torn C, Bonifacio E, Bingley PJ (2010) Diabetes Antibody Standardization Program: evaluation of assays for insulin autoantibodies. Diabetologia 53:2611–2620
Article PubMed CAS Google Scholar - Bonifacio E, Mayr A, Knopff A, Ziegler AG (2009) Endocrine autoimmunity in families with type 1 diabetes: frequent appearance of thyroid autoimmunity during late childhood and adolescence. Diabetologia 52:185–192
Article PubMed CAS Google Scholar - Schenker M, Hummel M, Ferber K et al (1999) Early expression and high prevalence of islet autoantibodies for DR3/4 heterozygous and DR4/4 homozygous offspring of parents with type I diabetes: the German BABYDIAB Study. Diabetologia 42:671–677
Article PubMed CAS Google Scholar - Mold JE, Michaëlsson J, Burt TD et al (2008) Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 322:1562–1565
Article PubMed CAS Google Scholar - Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D (2002) Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol 128:118–123
Article PubMed CAS Google Scholar - Velilla PA, Rugeles MT, Chougnet CA (2006) Defective antigen-presenting cell function in human neonates. Clin Immunol 121:251–259
Article PubMed CAS Google Scholar - Fleury Y, van Melle G, Woringer V, Gaillard RC, Portmann L (2001) Sex-dependent variations and timing of thyroid growth during puberty. J Clin Endocrinol Metab 86:750–754
Article PubMed CAS Google Scholar - Kaloumenou I, Mastorakos G, Alevizaki M et al (2008) Thyroid autoimmunity in schoolchildren in an area with long-standing iodine sufficiency: correlation with gender, pubertal stage, and maternal thyroid autoimmunity. Thyroid 18:747–754
Article PubMed CAS Google Scholar - Loviselli A, Velluzzi F, Mossa P et al (2001) The Sardinian Autoimmunity Study. 3. Studies on circulating antithyroid antibodies in Sardinian schoolchildren: relationship to goiter prevalence and thyroid function. Thyroid 11:849–857
Article PubMed CAS Google Scholar - Turley S, Poirot L, Hattori M, Benoist C, Mathis D (2003) Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med 198:1527–1537
Article PubMed CAS Google Scholar - Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B (2000) Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes 49:1325–1333
Article PubMed CAS Google Scholar - Andersson AM, Toppari J, Haavisto AM et al (1998) Longitudinal reproductive hormone profiles in infants: peak of inhibin B levels in infant boys exceeds levels in adult men. J Clin Endocrinol Metab 83:675–681
Article PubMed CAS Google Scholar - Giannoni E, Guignard L, Knaup Reymond M et al (2011) Estradiol and progesterone strongly inhibit the innate immune response of mononuclear cells in newborns. Infect Immun 79:2690–2698
Article PubMed CAS Google Scholar
Acknowledgements
We thank A. Knopff and J. Stock (Forschergruppe Diabetes e.V. at Helmholtz Center Munich, Neuherberg, Germany) and M. Zwilling (Forschergruppe Diabetes, Klinikum Rechts der Isar, University of Technology, Munich) for data collection and expert technical assistance, and K. Adler (Forschergruppe Diabetes, Klinikum Rechts der Isar, University of Technology, Munich) for laboratory management. We also thank all paediatricians and family doctors in Germany for participating in the BABYDIAB Study.
Funding
The work was supported by grants from the Helmholtz Zentrum München, the Competence Network for Diabetes Mellitus funded by the Federal Ministry of Education and Research (FKZ 01GI0805-07), the European Union (EP7-HEALTH-2007, DIAPREPP N202013), the Deutsche Forschungsgemeinschaft (DFG ZI-310/14-1 to 14-4) and the Juvenile Diabetes Research Foundation (JDRF; no 1-2006-665). E. Bonifacio is supported by the DFG Research Center and Cluster of Excellence–Center for Regenerative Therapies, Dresden (FZ 111).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
A-GZ is the principal investigator of the BABYDIAB and BABYDIET studies, designed the studies and concept, undertook statistical analyses, interpreted the results, wrote the manuscript and critically reviewed it for intellectual content. EB performed the statistical analysis, interpreted the results, wrote the manuscript, and critically reviewed it for intellectual content. The BABYDIAB-BABYDIET Study group members contributed to cohort contact and follow-up (MP, CW, EG, MH, SH), autoantibody measurements (PA, SK), data preparation, assembly and quality check (MP, CW, SH) and manuscript writing (MP, CW, EG). All the authors have read and edited the manuscript and approved the version to be published.
Author information
Authors and Affiliations
- Institute of Diabetes Research, Helmholtz Zentrum München, German Research Center for Environmental Health, Ingolstaedter Landstrasse 1, 85764, Neuherberg, Germany
A.-G. Ziegler - Forschergruppe Diabetes e.V. at Helmholtz Zentrum München, Neuherberg, Germany
A.-G. Ziegler & E. Bonifacio - Forschergruppe Diabetes, Klinikum rechts der Isar, University of Technology, Munich, Germany
A.-G. Ziegler - Center for Regenerative Therapies, Dresden University of Technology, Dresden, Germany
E. Bonifacio
Authors
- A.-G. Ziegler
You can also search for this author inPubMed Google Scholar - E. Bonifacio
You can also search for this author inPubMed Google Scholar
Consortia
the BABYDIAB-BABYDIET Study Group
Corresponding author
Correspondence toA.-G. Ziegler.
Additional information
A list of the BABYDIAB-BABYDIET Study Group authors is provided in the Appendix.
Appendix
Appendix
BABYDIAB-BABYDIET Study Group authors: A.-G. Ziegler, P. Achenbach, M. Pflüger, C. Winkler (Institute of Diabetes Research, Helmholtz Zentrum München, Neuherberg, Germany); E. Giannopoulou (Forschergruppe Diabetes, Klinikum Rechts der Isar, University of Technology, Munich, Germany); M. Hummel (Forschergruppe Diabetes e.V. at Helmholtz Zentrum München, Neuherberg, Germany); S. Hummel (Institute of Diabetes Research, Helmholtz Zentrum München, Neuherberg, Germany); S. Krause (Forschergruppe Diabetes, Klinikum Rechts der Isar, University of Technology, Munich, Germany).
Rights and permissions
About this article
Cite this article
Ziegler, AG., Bonifacio, E. & the BABYDIAB-BABYDIET Study Group. Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes.Diabetologia 55, 1937–1943 (2012). https://doi.org/10.1007/s00125-012-2472-x
- Received: 31 October 2011
- Accepted: 04 January 2012
- Published: 31 January 2012
- Issue Date: July 2012
- DOI: https://doi.org/10.1007/s00125-012-2472-x