Adiponectin: mechanistic insights and clinical implications (original) (raw)
Abstract
Adiponectin is an adipocyte-derived secretory protein that has been very widely studied over the past 15 years. A multitude of different functions have been attributed to this adipokine. It has been characterised in vitro at the level of tissue culture systems and in vivo through genetic manipulation of rodent models. It is also widely accepted as a biomarker in clinical studies. Originating in adipose tissue, generally positive metabolic effects have been attributed to adiponectin. In this review, we briefly discuss the key characteristics of this interesting but very complex molecule, highlight recent results in the context of its mechanism of action and summarise some of the key epidemiological data that helped establish adiponectin as a robust biomarker for insulin sensitivity, cardiovascular disease and many additional disease phenomena.
Similar content being viewed by others
Introduction
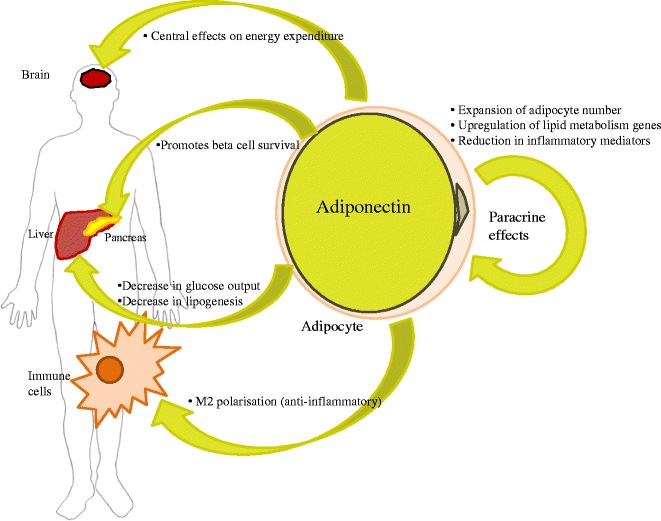
Since the first report of its discovery in the mid-1990s [1], adiponectin has been the focus of an increasing amount of research given its pleiotropic, beneficial effects on insulin sensitivity and systemic inflammation. More than 9,000 publications identified in PubMed focus on adiponectin action, or minimally use it as a key biomarker. The protein was originally known as Acrp30 [1], but several additional laboratories soon thereafter identified this protein in a different context and referred to it as adipoQ [2], apM1 [3] and GBP28 [4], until the consensus name ‘adiponectin’ found widespread acceptance [5]. Since these early days, a number of different functions have been attributed to this molecule. Even though the overabundance of papers on the topic can be a source of confusion regarding the role of this molecule in cellular and systemic physiology, there is consensus in the literature that adiponectin generally exerts insulin sensitising, anti-inflammatory and anti-apoptotic actions on a number of different cell types (Fig. 1). That these beneficial metabolic effects are associated with an adipocyte-derived secretory molecule is surprising and rather unique in the context of adipokines. However, consistent with these unusual properties, adiponectin release from the adipocyte is generally downregulated under metabolically unfavourable conditions, resulting in reduced circulating levels in the plasma. This type of regulation in which an unfavourable adipose tissue status triggers a more limited release of adiponectin allows it to be used as a powerful biomarker that integrates many environmental cues on the adipocyte to a circulating, easily measured plasma variable. This property is at the core of adiponectin’s widespread popularity in clinical and preclinical settings.
Fig. 1
Major mechanisms of adiponectin’s actions on peripheral tissues to impact insulin sensitivity and metabolism
Structure
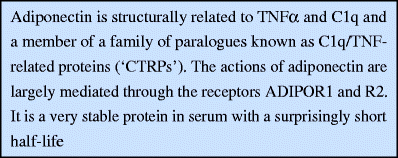
Adiponectin is a 30 kDa protein with a globular C-terminal domain and a collagenous N-terminal domain [1]. The collagen domain allows adiponectin to form trimers, hexamers, and higher order complexes prior to being secreted. A cysteine residue (Cys39 in the mouse) in the collagenous stalk is a critical mediator of higher order complexes [6], which may represent the most biologically active forms of the protein [7, 8]. Adiponectin is structurally related to both C1q and TNFα, which are prototypical members of a growing family of paralogues known as C1q/TNF-related proteins (‘CTRPs’) [9].
Adiponectin receptors
Adiponectin’s actions are mediated through a number of receptors, such as adiponectin receptors 1 and 2 (ADIPOR1 and ADIPOR2) [10]. These receptors are predicted to have seven transmembrane portions but are functionally distinct from G-protein-coupled receptors, particularly as they have the opposite polarity (i.e. N-terminus is facing the intracellular compartment). Single- and double-knockout mice for the adiponectin receptors suggest a significant amount of functional redundancy between them [11]. Although relative ratios of ADIPOR1 and ADIPOR2 may vary from tissue to tissue, in general, both are fairly ubiquitously expressed. An additional cell surface molecule with significant affinity for adiponectin, referred to as T-cadherin, has been identified [12]. Although it binds adiponectin, it is not a signalling receptor per se, as it lacks an intracellular signalling domain. Nevertheless, T-cadherin is required for adiponectin to exert its full cardioprotective potential [13].
Other pathways may exist. There is early evidence for additional, as-yet-unidentified adiponectin receptors that may mediate improvements in insulin sensitivity [14]. Also, in some cell types, the adaptor protein APPL1 has been implicated as a downstream mediator of adiponectin signalling through its interaction with the adiponectin receptors [15].
Secretion and clearance
Adiponectin is an adipose-specific secretory protein and its transcript is abundant in the adipocyte. It is constitutively produced and constitutes approximately 0.01–0.05% of plasma protein (usual range, 2–20 μg/ml), so it is a relatively abundant plasma factor. Adiponectin is a very stable protein in the circulation, with minimal degradation observed while circulating [16], but it has a surprisingly short plasma half-life of ~45–75 min, depending on the complex and the conditions [17]. The liver is the primary site of adiponectin clearance from the circulation [17], but other cell types also bind adiponectin quite avidly, in particular, pancreatic beta cells as well as some cell types in the heart and kidney.
Although adiponectin is secreted by adipose tissue, circulating levels are, paradoxically, decreased with increasing central adiposity [5, 18]. However, higher degrees of lower extremity and truncal adiposity are associated with higher concentrations of adiponectin. The location of adipose depots, therefore, differentially influences circulating adiponectin concentrations. While there are differences in adiponectin levels across different ethnic groups, these trends of an association between increased truncal and lower extremity adiposity and higher adiponectin levels (and higher insulin sensitivity) are preserved. While the inverse correlations to overall BMI certainly hold up across many populations, these gross measures of body mass alone do not adequately account for adiponectin levels. Furthermore, cohort studies in many different groups have consistently shown an inverse relationship between adiponectin levels and the presence of (or propensity to develop) glucose intolerance and type 2 diabetes [19]. Below we will briefly summarise recent mechanistic insights that may underlie the relationship between adiponectin and insulin sensitivity.
Adiponectin promotes beta cell function and survival
The adiponectin receptors ADIPOR1 and ADIPOR2 are expressed in pancreatic islets, specifically, on the beta cells [20–22]. Although both ADIPOR1 and ADIPOR2 are present, ADIPOR1 is the predominant form, at least in the mouse. Not only is the receptor expressed here, but exogenously administered recombinant adiponectin given to adiponectin-null mice avidly binds to beta cells, suggesting that circulating adiponectin directly targets this tissue.
Adiponectin may act to increase glucose-mediated insulin secretion and transcription of insulin and related gene products [23]. While the data are not clear on the effect of adiponectin on insulin release under conditions of normal insulin sensitivity [22, 24], it does, however, appear to increase glucose-mediated insulin secretion in high-fat diet-fed mice (i.e. under insulin-resistant conditions) [18].
Several studies have demonstrated that adiponectin has anti-apoptotic effects on beta cells, both in cell culture and islet preparations [23, 25–27]. This effect in the pancreas does not appear to be mediated through activation of AMP-activated protein kinase (AMPK), but may be, at least in part, due to activation of other pro-survival kinases, such as extracellular signal-regulated kinases 1 and 2 (ERK1/2) and Akt [23, 26].
Recent data have highlighted the role of sphingolipid metabolism in the pleiotropic effects of adiponectin, including its effect on the pancreas [25]. Activation of the ADIPOR1 and ADIPOR2 receptors increases ceramidase activity, leading to decreased intracellular ceramide concentrations and increased levels of the anti-apoptotic metabolite sphingosine-1 phosphate. In beta cell culture, adiponectin is able to mitigate the apoptotic effects of either palmitate- or ceramide-induced cell death—an effect that may critically depend on the formation of the downstream conversion product of ceramide, sphingosine-1 phosphate. It is still unknown whether this ceramidase activity resides with the receptor itself or is a result of activation of downstream effectors.

Cardioprotective and renal effects of adiponectin
The potent correlations and strong predictive value of adiponectin measurements for the future development of cardiovascular disease have been appreciated for many years. In a 2004 landmark study, Pischon and colleagues established in a very large cohort that high plasma adiponectin concentrations are associated with a lower risk of myocardial infarction in men [28]. Potent positive correlations with HDL-cholesterol can be seen, and in preclinical studies the Walsh group elegantly established potent effects of recombinant adiponectin on cardiomyocyte survival in the context of ischaemia/reperfusion studies [29]. Based on our in vitro and in vivo data, these effects may be, at least in part, explained on the basis of the ceramide-lowering effects of adiponectin [25]. It remains an unsolved issue why end-stage cardiovascular disease, in contrast to early stages, displays a strong positive correlation between all-cause and cardiovascular mortality and high levels of adiponectin [30]. A similar situation prevails in the kidney. Low adiponectin correlates with albuminuria in both mice and humans [31, 32]. Lack of adiponectin has been linked to increased podocyte injury and albuminuria in adiponectin gene knockout (Adipoq −/−) mouse models, with adiponectin therapies potentially reversing some renal dysfunction [33, 34]. Similar to the observations in cardiovascular disease, these positive adiponectin effects on renal physiology are in contrast to the elevated levels observed in both children and adults with chronic kidney disease, whose adiponectin levels are positively correlated with proteinuria [32, 35]. Similar to end-stage cardiovascular disease, it is not known which mechanisms lead to this upregulation, particularly since adiponectin is not cleared through the kidney except in cases of severe proteinuria [36]. In both cases, however, we assume that this reflects a compensatory upregulation of adiponectin production, although it is possible that in the end stages of these conditions, the low fat mass associated with cachexia begins to significantly confound the relationship between adiponectin and survival. At the current time, the reasons for these unexpected findings are unclear and will need to be elucidated by further studies.
Adiponectin improves peripheral insulin sensitivity
Adiponectin and skeletal muscle
Being a major source of systemic glucose, skeletal muscle plays a key role in insulin sensitivity. Not surprisingly, therefore, there has been considerable attention paid to the possible metabolic effects adiponectin has on this tissue.
ADIPOR1 is the predominant adiponectin receptor found in skeletal muscle [10, 37, 38]. Most studies have utilised binding with globular adiponectin, which appears to have higher binding and more biological activity in skeletal muscle than the full-length form [39–41]. The in vivo physiological significance of the globular form remains quite unclear, however, as the vast majority, if not all, circulating adiponectin exists in full-length, multimeric complexes.
Bearing this important limitation in mind, globular adiponectin appears to act, at least in part, through AMPK (and, subsequently, by inhibiting acetyl-CoA carboxylase) [39, 40, 42] and PPARα [11, 43] to exert its metabolic effects in muscle. AMPK phosphorylation in C2C12 myotubes can be prevented by ceramidase inhibition, again supporting a role for sphingolipid metabolism in adiponectin signalling in this tissue [25]. Adiponectin binding results in an increase in glucose uptake (via GLUT4 translocation) and non-oxidative glycolysis, while at the same time reducing intramyocellular triacylglycerol content and promoting fatty acid oxidation [40–44]. Furthermore, adiponectin has effects on mitochondrial number and oxidative fibre types [42, 45].
In the diseased state, the effects of adiponectin on skeletal muscle are dampened. The binding of both globular and full-length adiponectin is decreased in the obese, insulin-resistant ob/ob mouse, which may be due to a lower adiponectin receptor density [46]. While human studies do not demonstrate altered ADIPOR1/ADIPOR2 mRNA levels associated with insulin-resistant states [37, 38], cultured myotubes from obese patients with and without diabetes demonstrate impaired adiponectin-stimulated AMPK phosphorylation and fatty acid oxidation [37]. This suggests impaired adiponectin action downstream of the adiponectin receptor. Since these effects can be seen in in vitro patient samples, they are independent of other potentially confounding circulating hormonal factors (e.g. insulin).
Although there are a considerable number of papers dedicated to adiponectin action in muscle, the authors remain somewhat sceptical of the relevance of the published data on the physiology of this protein in vivo. The vast majority of papers either focus on in vitro or in vivo studies predominantly performed with globular adiponectin. Can the relatively large adiponectin complexes in vivo actually effectively cross the endothelium? Muscle does not seem to be a major target of labelled adiponectin injected into mice [17]. Neither transgenic mice overexpressing Adipoq nor _Adipoq_-null mice injected with recombinant adiponectin appear to have a phenotype in muscle when carefully clamped. Rather, the major differences are consistently found at the level of the liver in all these models [47–49].
Adiponectin and the liver
Adiponectin has several effects on the liver. One of the most prominent of these is suppression of hepatic glucose output, lowering systemic glucose levels. Adiponectin acts to sensitise hepatocytes to the effects of insulin, such that glucose production is significantly suppressed for any given dose of insulin in the presence of physiological doses of adiponectin [50]. Adiponectin acts to suppress the expression [40, 50, 51] and activity [50] of key regulators in gluconeogenesis, such as phosphoenolpyruvate carboxykinase and glucose-6-phosphatase. As mentioned above, murine euglycaemic clamp experiments have shown no difference in rates of glucose disappearance, glycolysis or glycogen synthesis in the presence or absence of intravenous adiponectin infusion [49], again highlighting adiponectin’s main effect, which is to lower blood sugar by suppressing hepatic glucose output rather than disposal.
Adiponectin also acts on fatty acid metabolism in the liver, which has secondary effects on circulating levels of triacylglycerol and NEFA. Even moderate overproduction of adiponectin in transgenic mice leads to substantial protection from hepatic steatosis in the setting of obesity [52] or high-fat feeding [53], while the Adipor1/Adipor2 double-knockout mouse tends to accumulate increased liver fat [11]. Similarly, studies in humans have consistently shown an association between low adiponectin levels and the presence and degree of liver fat [54]. Transgenic mice overexpressing Adipoq show reduced lipogenic activity at the level of the liver [55], but may be predominantly protected as a result of a healthy, non-inflamed expansion of adipose tissue that can readily accommodate any excess lipids [52, 53].
Increases in the phosphorylation state of the insulin receptor, as well as downstream mediators in the insulin pathway (e.g. Akt, glycogen synthase kinase 3β), are seen with modest overproduction of adiponectin and in part mediate its effects on hepatic insulin sensitivity [52]. There are other mechanisms at play beyond sensitisation to insulin. AMPK activation, somewhere downstream from the adiponectin receptor, has been recognised as a mechanism of adiponectin action in the liver. It has become increasingly clear in recent years, however, that many of this hormone’s effects are independent of AMPK. Liver-specific deletion of liver kinase B1 (LKB1), the major upstream activator of AMPK, blunts, but does not abolish, adiponectin’s ability to decrease serum glucose concentrations by reducing hepatic glucose output or gluconeogenic gene expression [51]. This suggests both an LBK1/AMPK-dependent and -independent pathway of adiponectin action on the liver. Importantly, adiponectin has also been shown to lower hepatic ceramide content independently of LBK1/AMPK, supporting the key role for sphingolipid metabolism and highlighting the relevance of the sphingolipid pathway in the adiponectin-mediated effects on the liver [25]. Overexpression of AdipoR1 and AdipoR2 in the liver has been shown to significantly increase hepatic ceramidase activity, lower high-fat diet-induced hepatic ceramide accumulation and improve insulin resistance.

Adiponectin has local effects on adipose tissue
Adipocytes are known to express the adiponectin receptors (ADIPOR1>R2) [56]. This finding suggests that adiponectin is able to act locally in an autocrine or paracrine fashion to influence adipose tissue function.
One of the most striking effects of adiponectin’s effect on adipose tissue is seen in the _Adipoq_-overexpressing ob/ob mouse. This mouse displays greater adiposity than the ob/ob mouse, with the excess weight accounted for by a greater subcutaneous fat mass (while the intra-abdominal and hepatic fat matched that in wild-type mice) [52]. Histological examination has shown that the expanded adipose tissue consists of a larger number of adipocytes with a significantly lower average cell size compared with the lipid-engorged adipocytes seen on the ob/ob background. Furthermore, mRNA levels of several key genes involved in lipid metabolism, including Pparg2 and Pgc1a, are upregulated in the white adipose tissue of these mice, suggesting a generalised improvement in lipid metabolism [52, 53].
Concurrently, overexpression of Adipoq leads to decreased infiltration of macrophages into adipose tissue [52] and adiponectin acts locally on adipose tissue to suppress the release of a number of pro-inflammatory cytokines (e.g. TNFα, IL-6) from adipocytes and surrounding stromal vascular cells [52, 57–60]. This demonstrates the complex interplay between local (e.g. adipose tissue) and systemic inflammation.
How adiponectin achieves its potent local effects on adipose tissue remains an unsolved mystery. It will be interesting to see to what extent adiponectin-mediated changes on the various sphingolipid pathways in adipocytes drive the phenotype. Alternatively, since adipocytes accumulate large amounts of adiponectin intracellularly, the role of this intracellular pool of adiponectin remains to be analysed in detail.

Adiponectin has systemic anti-inflammatory effects
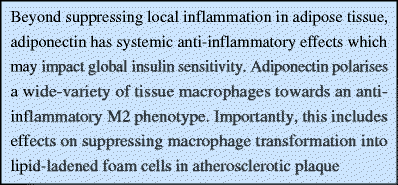
Unquestionably, adiponectin exerts important effects on local inflammation in adipose tissue. However, there are significant anti-inflammatory properties of adiponectin that extend beyond this microenvironment. Insofar as systemic inflammation may promote insulin-resistance, these effects are relevant.
Adiponectin suppresses the growth and proliferation of bone marrow-derived granulocyte and macrophage progenitors while not affecting other haematopoietic cell lines [61]. In addition, inflammatory functions in macrophages are affected: phagocytic activity is inhibited in human macrophages incubated with adiponectin [61], as is the release of pro-inflammatory cytokines [61, 62]. Lipopolysaccharide-stimulated TNFα and IL-6 release from macrophages is inhibited through decreased nuclear factor-κB translocation into the nucleus [59, 62]. Also, production of the anti-inflammatory cytokine, IL-10, is increased [60, 63, 64]. Conversely, the macrophages from adiponectin-null mice display an activated phenotype, with higher levels of TNFα, MCP-1 and IL-6 production than macrophages from wild-type mice. The increases in these cytokines can be reversed by exogenous administration of recombinant adiponectin [60]. Collectively, these data suggest that adiponectin promotes the polarisation of macrophages towards an M2 (i.e. anti-inflammatory) phenotype.
Adiponectin influences macrophage function in a diverse array of tissues, not only in adipose tissue. Peritoneal [60], alveolar [60] and hepatic macrophages [65] all display M2 polarisation under the influence of adiponectin. In the context of atherogenesis, adiponectin inhibits macrophage transformation into lipid-laden foam cells [66, 67]. Ectopic production of adiponectin by macrophages in transgenic mice leads to improved systemic insulin sensitivity after high-fat diet feeding and protection from atherosclerosis when crossed with LDL-deficient mice [68]. These data highlight the place of adiponectin at the intersection of systemic immune responses and metabolic disease. It is tempting to speculate that the sphingolipid pathways described for beta cells, cardiac myocytes and the liver may also be the driving force for the phenotype in macrophages, but this remains to be studied in detail.
Conclusions
Adiponectin is a key mediator of systemic insulin sensitivity and glucose homeostasis. These effects are achieved by a diverse set of effects on several important targets, including the liver, pancreas, cardiac myocytes and the immune system, and even the adipose tissue itself. The main metabolic effects of adiponectin are to suppress hepatic glucose output while suppressing inflammatory responses in many other cell types, including macrophages. Moreover, through its action on the sphingolipid pathways, adiponectin exerts potent anti-apoptotic activities that have been documented particularly for beta cells and cardiac myocytes [25].
Despite the tremendous interest in adiponectin and a meteoric rise in the number of publications on this protein, there remain several key areas. How relevant are the actions in the brain? Adiponectin can easily be detected in cerebrospinal fluid [69], and has potent central effects pharmacologically to decrease body weight and increase energy expenditure [70, 71]. Much still remains to be investigated in this area. Additional questions arise regarding signal transduction distal to the adiponectin receptor. Although traditionally the mechanism has been attributed to AMPK phosphorylation, how this activation occurs in relation to the activation of cell surface receptors is unclear. Furthermore, recent evidence has suggested that many adiponectin-mediated effects are independent of the AMPK pathway. Attention has turned to the role of ceramide catabolism as the intracellular mediator of the effects of adiponectin. There is still room for additional mechanisms of action—and hopefully the next couple of years will yield a more complete picture of how this protein functions and why the adipocyte critically depends on releasing this hormone at relatively high levels with a rather short resident time in the plasma.
Abbreviations
AMPK:
AMP-activated protein kinase
References
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270:26746–26749
Article PubMed CAS Google Scholar - Hu E, Liang P, Spiegelman BM (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271:10697–10703
Article PubMed CAS Google Scholar - Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 221:286–289
Article PubMed CAS Google Scholar - Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M (1996) Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem 120:803–812
Article PubMed CAS Google Scholar - Arita Y, Kihara S, Ouchi N et al (1999) Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257:79–83
Article PubMed CAS Google Scholar - Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE (2008) Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology 149:2270–2282
Article PubMed CAS Google Scholar - Hara K, Horikoshi M, Yamauchi T et al (2006) Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care 29:1357–1362
Article PubMed CAS Google Scholar - Zhu N, Pankow JS, Ballantyne CM et al (2010) High-molecular-weight adiponectin and the risk of type 2 diabetes in the ARIC Study. J Clin Endocrinol Metab 92:5097–5104
Article Google Scholar - Davis KE, Scherer PE (2008) Adiponectin: no longer the lone soul in the fight against insulin resistance? Biochem J 416:e7–e9
Article PubMed CAS Google Scholar - Yamauchi T, Kamon J, Ito Y et al (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769
Article PubMed CAS Google Scholar - Yamauchi T, Nio Y, Maki T et al (2007) Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13:332–339
Article PubMed CAS Google Scholar - Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF (2004) T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA 101:10308–10313
Article PubMed CAS Google Scholar - Denzel MS, Scimia MC, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B (2010) T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest 120:4342–4352
Article PubMed CAS Google Scholar - Awazawa M, Ueki K, Inabe K et al (2011) Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab 13:401–412
Article PubMed CAS Google Scholar - Mao X, Kikani CK, Riojas RA et al (2006) APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8:516–523
Article PubMed CAS Google Scholar - Pischon T, Hotamisligil GS, Rimm EB (2003) Adiponectin: stability in plasma over 36 hours and within-person variation over 1 year. Clin Chem 49:650–652
Article PubMed CAS Google Scholar - Halberg N, Schraw TD, Wang ZV et al (2009) Systemic fate of the adipocyte-derived factor adiponectin. Diabetes 58:1961–1970
Article PubMed Google Scholar - Turer AT, Khera A, Ayers CR et al (2011) Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia 54:2515–2524
Article PubMed CAS Google Scholar - Li S, Shin HJ, Ding EL, van Dam RM (2009) Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 302:179–188
Article PubMed CAS Google Scholar - Huypens P, Moens K, Heimberg H, Ling Z, Pipeleers D, van de Casteele M (2005) Adiponectin-mediated stimulation of AMP-activated protein kinase (AMPK) in pancreatic beta cells. Life Sci 77:1273–1282
Article PubMed CAS Google Scholar - Kharroubi I, Rasschaert J, Eizirik DL, Cnop M (2003) Expression of adiponectin receptors in pancreatic beta cells. Biochem Biophys Res Commun 312:1118–1122
Article PubMed CAS Google Scholar - Staiger K, Stefan N, Staiger H et al (2005) Adiponectin is functionally active in human islets but does not affect insulin secretory function or beta-cell lipoapoptosis. J Clin Endocrinol Metab 90:6707–6713
Article PubMed CAS Google Scholar - Wijesekara N, Krishnamurthy M, Bhattacharjee A, Suhail A, Sweeney G, Wheeler MB (2010) Adiponectin-induced ERK and Akt phosphorylation protects against pancreatic beta cell apoptosis and increases insulin gene expression and secretion. J Biol Chem 285:33623–33631
Article PubMed CAS Google Scholar - Winzell MS, Nogueiras R, Dieguez C, Ahrén B (2004) Dual action of adiponectin on insulin secretion in insulin-resistant mice. Biochem Biophys Res Commun 321:154–160
Article PubMed Google Scholar - Holland WL, Miller RA, Wang ZV et al (2011) Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 17:55–63
Article PubMed CAS Google Scholar - Brown JE, Conner AC, Digby JE et al (2010) Regulation of beta-cell viability and gene expression by distinct agonist fragments of adiponectin. Peptides 31:944–949
Article PubMed CAS Google Scholar - Rakatzi I, Mueller H, Ritzeler O, Tennagels N, Eckel J (2004) Adiponectin counteracts cytokine- and fatty acid-induced apoptosis in the pancreatic beta-cell line INS-1. Diabetologia 47:249–258
Article PubMed CAS Google Scholar - Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB (2004) Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 291:1730–1737
Article PubMed CAS Google Scholar - Shibata R, Sato K, Pimentel DR et al (2005) Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med 11:1096–1103
Article PubMed CAS Google Scholar - Cavusoglu E, Ruwende C, Chopra V et al (2006) Adiponectin is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction in patients presenting with chest pain. Eur Heart J 27:2300–2309
Article PubMed CAS Google Scholar - Sharma K (2009) The link between obesity and albuminuria: adiponectin and podocyte dysfunction. Kidney Int 76:145–148
Article PubMed CAS Google Scholar - Zoccali C, Mallamaci F (2011) Adiponectin and leptin in chronic kidney disease: causal factors or mere risk markers? J Ren Nutr 21:87–91
Article PubMed CAS Google Scholar - Ohashi K, Iwatani H, Kihara S et al (2007) Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler Thromb Vasc Biol 27:1910–1917
Article PubMed CAS Google Scholar - Sharma K, Ramachandrarao S, Qiu G et al (2008) Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118:1645–1656
PubMed CAS Google Scholar - Lo MM, Salisbury S, Scherer PE, Furth SL, Warady BA, Mitsnefes MM (2011) Serum adiponectin complexes and cardiovascular risk in children with chronic kidney disease. Pediatr Nephrol 26:2009–2017
Article PubMed Google Scholar - von Eynatten M, Liu D, Hock C et al (2009) Urinary adiponectin excretion: a novel marker for vascular damage in type 2 diabetes. Diabetes 58:2093–2099
Article Google Scholar - Chen MB, McAinch AJ, Macaulay SL et al (2005) Impaired activation of AMP-kinase and fatty acid oxidation by globular adiponectin in cultured human skeletal muscle of obese type 2 diabetics. J Clin Endocrinol Metab 90:3665–3672
Article PubMed CAS Google Scholar - Debard C, Laville M, Berbe V et al (2004) Expression of key genes of fatty acid oxidation, including adiponectin receptors, in skeletal muscle of type 2 diabetic patients. Diabetologia 47:917–925
Article PubMed CAS Google Scholar - Tomas E, Tsao TS, Saha AK et al (2002) Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci USA 99:16309–16313
Article PubMed CAS Google Scholar - Yamauchi T, Kamon J, Minokoshi Y et al (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8:1288–1295
Article PubMed CAS Google Scholar - Fruebis J, Tsao TS, Javorschi S et al (2001) Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98:2005–2010
PubMed CAS Google Scholar - Civitarese AE, Ukropcova B, Carling S et al (2006) Role of adiponectin in human skeletal muscle bioenergetics. Cell Metab 4:75–87
Article PubMed CAS Google Scholar - Yamauchi T, Kamon J, Waki H et al (2003) Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem 278:2461–2468
Article PubMed CAS Google Scholar - Ceddia RB, Somwar R, Maida A, Fang X, Bikopoulos G, Sweeney G (2005) Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia 48:132–139
Article PubMed CAS Google Scholar - Iwabu M, Yamauchi T, Okada-Iwabu M et al (2010) Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature 464:1313–1319
Article PubMed CAS Google Scholar - Tsuchida A, Yamauchi T, Ito Y et al (2004) Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem 279:30817–30822
Article PubMed CAS Google Scholar - Nawrocki AR, Rajala MW, Tomas E et al (2006) Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. J Biol Chem 281:2654–2660
Article PubMed CAS Google Scholar - Combs TP, Pajvani UB, Berg AH et al (2004) A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145:367–383
Article PubMed CAS Google Scholar - Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L (2001) Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest 108:1875–1881
PubMed CAS Google Scholar - Berg AH, Combs TP, Du X, Brownlee M, Scherer PE (2001) The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7:947–953
Article PubMed CAS Google Scholar - Miller RA, Chu Q, Le Lay J et al (2011) Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. J Clin Invest 121:2518–2528
Article PubMed CAS Google Scholar - Kim JY, van de Wall E, Laplante M et al (2007) Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117:2621–2637
Article PubMed CAS Google Scholar - Asterholm IW, Scherer PE (2010) Enhanced metabolic flexibility associated with elevated adiponectin levels. Am J Pathol 176:1364–1376
Article PubMed CAS Google Scholar - Polyzos SA, Toulis KA, Goulis DG, Zavos C, Kountouras J (2011) Serum total adiponectin in nonalcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism 60:313–326
Article PubMed CAS Google Scholar - Shetty S, Ramos-Roman MA, Cho YR et al (2012) Enhanced fatty acid flux triggered by adiponectin overexpression. Endocrinology 153:113–122
Article PubMed CAS Google Scholar - Rasmussen MS, Lihn AS, Pedersen SB, Bruun JM, Rasmussen M, Richelsen B (2006) Adiponectin receptors in human adipose tissue: effects of obesity, weight loss, and fat depots. Obesity (Silver Spring) 14:28–35
Article CAS Google Scholar - Dietze-Schroeder D, Sell H, Uhlig M, Koenen M, Eckel J (2005) Autocrine action of adiponectin on human fat cells prevents the release of insulin resistance-inducing factors. Diabetes 54:2003–2011
Article PubMed CAS Google Scholar - Ge Q, Ryken L, Noel L, Maury E, Brichard SM (2011) Adipokines identified as new downstream targets for adiponectin: lessons from adiponectin-overexpressing or -deficient mice. Am J Physiol Endocrinol Metab 301:E326–E335
Article PubMed CAS Google Scholar - Ajuwon KM, Spurlock ME (2005) Adiponectin inhibits LPS-induced NF-κB activation and IL-6 production and increases PPARγ2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol 288:R1220–R1225
Article PubMed CAS Google Scholar - Ohashi K, Parker JL, Ouchi N et al (2010) Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 285:6153–6160
Article PubMed CAS Google Scholar - Yokota T, Oritani K, Takahashi I et al (2000) Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 96:1723–1732
PubMed CAS Google Scholar - Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME (2004) Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun 316:924–929
Article PubMed CAS Google Scholar - Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H (2004) Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun 323:630–635
Article PubMed CAS Google Scholar - Kumada M, Kihara S, Ouchi N et al (2004) Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation 109:2046–2049
Article PubMed CAS Google Scholar - Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE (2011) Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. J Biol Chem 286:13460–13469
Article PubMed CAS Google Scholar - Tian L, Luo N, Klein RL, Chung BH, Garvey WT, Fu Y (2009) Adiponectin reduces lipid accumulation in macrophage foam cells. Atherosclerosis 202:152–161
Article PubMed CAS Google Scholar - Ouchi N, Kihara S, Arita Y et al (2001) Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation 103:1057–1063
Article PubMed CAS Google Scholar - Luo N, Liu J, Chung BH et al (2010) Macrophage adiponectin expression improves insulin sensitivity and protects against inflammation and atherosclerosis. Diabetes 59:791–799
Article PubMed CAS Google Scholar - Kusminski CM, McTernan PG, Schraw T et al (2007) Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia 50:634–642
Article PubMed CAS Google Scholar - Qi Y, Takahashi N, Hileman SM et al (2004) Adiponectin acts in the brain to decrease body weight. Nat Med 10:524–529
Article PubMed CAS Google Scholar - Park S, Kim DS, Kwon DY, Yang HJ (2011) Long-term central infusion of adiponectin improves energy and glucose homeostasis by decreasing fat storage and suppressing hepatic gluconeogenesis without changing food intake. J Neuroendocrinol 23:687–698
Article PubMed CAS Google Scholar
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
ATT and PES contributed to the design and drafting of this article. Both authors approved the final version of this manuscript.
Author information
Authors and Affiliations
- Department of Medicine, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX, 75390-8521, USA
A. T. Turer & P. E. Scherer - Division of Cardiology, University of Texas Southwestern Medical Center, Dallas, TX, USA
A. T. Turer - Touchstone Diabetes Center, University of Texas Southwestern Medical Center, Dallas, TX, USA
P. E. Scherer - Department of Cell Biology, University of Texas Southwestern Medical Center, Dallas, TX, USA
P. E. Scherer
Authors
- A. T. Turer
You can also search for this author inPubMed Google Scholar - P. E. Scherer
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toA. T. Turer.
Rights and permissions
About this article
Cite this article
Turer, A.T., Scherer, P.E. Adiponectin: mechanistic insights and clinical implications.Diabetologia 55, 2319–2326 (2012). https://doi.org/10.1007/s00125-012-2598-x
- Received: 11 January 2012
- Accepted: 09 March 2012
- Published: 12 June 2012
- Issue Date: September 2012
- DOI: https://doi.org/10.1007/s00125-012-2598-x