Common carotid intima-media thickness does not add to Framingham risk score in individuals with diabetes mellitus: the USE-IMT initiative (original) (raw)
Abstract
Aims/hypothesis
The aim of this work was to investigate whether measurement of the mean common carotid intima-media thickness (CIMT) improves cardiovascular risk prediction in individuals with diabetes.
Methods
We performed a subanalysis among 4,220 individuals with diabetes in a large ongoing individual participant data meta-analysis involving 56,194 subjects from 17 population-based cohorts worldwide. We first refitted the risk factors of the Framingham heart risk score on the individuals without previous cardiovascular disease (baseline model) and then expanded this model with the mean common CIMT (CIMT model). The absolute 10 year risk for developing a myocardial infarction or stroke was estimated from both models. In individuals with diabetes we compared discrimination and calibration of the two models. Reclassification of individuals with diabetes was based on allocation to another cardiovascular risk category when mean common CIMT was added.
Results
During a median follow-up of 8.7 years, 684 first-time cardiovascular events occurred among the population with diabetes. The C statistic was 0.67 for the Framingham model and 0.68 for the CIMT model. The absolute 10 year risk for developing a myocardial infarction or stroke was 16% in both models. There was no net reclassification improvement with the addition of mean common CIMT (1.7%; 95% CI −1.8, 3.8). There were no differences in the results between men and women.
Conclusions/interpretation
There is no improvement in risk prediction in individuals with diabetes when measurement of the mean common CIMT is added to the Framingham risk score. Therefore, this measurement is not recommended for improving individual cardiovascular risk stratification in individuals with diabetes.
Similar content being viewed by others
Introduction
Diabetes mellitus has long been recognised as a cardiovascular risk factor. Individuals with diabetes are at increased risk of cardiovascular morbidity and mortality as their risk is twofold higher than that of individuals with normal glucose metabolism [1, 2]. Guidelines for the management of diabetes advocate assessing the absolute risk of developing cardiovascular disease (CVD) to guide the initiation of appropriate treatment in those at high risk [3–5]. Over past decades many prediction models (or risk scores) have been developed to predict the risk for CVD, of which only a small number have been specifically developed for individuals with diabetes [6]. Some recommend using the Framingham risk score, which includes diabetes as a risk factor; others recommend the Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe (DECODE) risk, which includes a plasma glucose measure, or the United Kingdom Prospective Diabetes Study (UKPDS), which incorporates the duration of diabetes. A recent report reviewed the majority of cardiovascular risk prediction models used in individuals with diabetes with respect to performance, validation and impact [7]. Only a minority of these risk prediction models have been validated and tested for their predictive accuracy. The Framingham risk prediction model is the only risk score to have been studied for its effect on patients’ management and on CVD [7, 8].
Since individuals with diabetes have an accelerated development of atherosclerosis, improvement in risk prediction may be established by including a measure of preclinical atherosclerosis. Measurement of carotid intima-media thickness (CIMT) has been proposed to be added to cardiovascular risk factors to improve individual risk assessment [9, 10]. Thus far, the value of CIMT measurements in cardiovascular risk prediction has primarily been studied in the general population and not in specific high-risk groups, such as individuals with diabetes. We therefore assessed whether measurement of mean common CIMT, on top of the Framingham risk score, improves cardiovascular risk prediction in individuals with diabetes by using the USE-IMT. The USE-IMT collaboration is an ongoing global individual participant data meta-analysis project, based on prospective cohort studies, set up to determine the added value of the CIMT to current risk prediction models in individuals without pre-existing CVD [11]. In this analysis we determined the added value of the mean common CIMT as this measurement is referred to in guidelines and may be the most feasible in clinical practice.
Methods
Study population and design
USE-IMT is an ongoing individual participant data meta-analysis of which the selection of cohorts has been described in detail elsewhere [11]. In short, eligible cohorts were identified through literature searches of databases and through expert suggestion. Sixteen cohorts were included in the current analyses; these cohorts were required to have available baseline data on age, sex, cigarette smoking status, antihypertensive medication use, blood pressure, cholesterol fractions, CIMT measurements, history of CVD, diabetes status and follow-up information on occurrence of CVD. Individual data from cohorts were collected and harmonised for the statistical analyses using SPSS version 17 (SPSS Inc, Chicago, IL, USA).
We then selected 56,194 individuals (out of 74,937) with information on mean common CIMT to whom the risk scores apply (i.e. individuals aged 45–75 years with systolic blood pressure of <180 mmHg, a total cholesterol level of <8 mmol/l and who were free from previous symptomatic cardiovascular disease) [12, 13]. Incomplete data on mean common CIMT, cardiovascular risk factors and (time to) events (approximately 12% of total values), were imputed for each cohort separately (single imputation in the multivariate imputation by chained equations [MICE] package of R statistical software, version 2.10.0, Vienna, Austria). Predictors in our imputation model included all variables in our database, including the outcome of interest [14].
Diabetes, mean common CIMT and events
Diabetes status was known in all cohorts included in this analysis. The total number of individuals with diabetes mellitus in USE-IMT was 4,220. The definition of diabetes mellitus varied by cohort and is shown in Table 1.
Table 1 Cohorts included in the present analysis and criteria used for diagnosis of diabetes mellitus in each cohort
Average mean common CIMT was calculated for each individual using the maximum set of carotid angles, near and/or far wall measurements and left and/or right side measurements, that were assessed within each cohort. Information on the CIMT methodology used in each study is displayed in electronic supplementary material (ESM) Table 1. Plaques and CIMT measurements from other locations than the common carotid segment were not considered in this analysis. However, when a plaque was present in the common carotid segment at the place where the CIMT measurements were taken it was included in the CIMT measurement and in the analysis. Time to first fatal or non-fatal myocardial infarction or stroke was used as a primary endpoint in this analysis.
Statistical analysis
The original variables of the Framingham risk score (age, sex, cigarette smoking status, blood pressure, antihypertensive medication use, total cholesterol, high-density-lipoprotein cholesterol and presence of diabetes) [8] were refit on the complete USE-IMT cohort using a multivariable Cox proportional-hazards model. Subsequently, this baseline model was then extended with the mean common CIMT variable as described previously [11].
The discriminative value of both models was expressed with Harrell’s c-index [15]. The 10 year absolute risk to develop a first-time myocardial infarction or stroke was calculated from the model with and without mean common CIMT.
Individuals with diabetes were selected and their predicted risks (with either the baseline model or the CIMT model) were used to classify them into a risk category. The following cut-offs were used: <5% (low risk), ≥5 to <10% (low-to-intermediate risk), ≥10 to <20% (intermediate-to-high risk) or ≥20% (high risk).
For individuals with diabetes, the net reclassification improvement (NRI), which takes survival time into account [16], was calculated using the percentage of correct movement across categories for those with and without events. Correct movement is upward classification by a new marker in those with events and downward classification for those without events. In addition, we assessed improvement without cut-off by risk categories using the integrated discrimination improvement, which can be seen as equal to differences in discrimination slopes [17] and expressed the relative integrated discrimination improvement (rIDI). This rIDI represents the increase in discrimination slopes divided by the slope of the old model [18].
Since the Atherosclerosis Risk in Communities (ARIC) study concerned a large proportion of individuals with diabetes, we performed a sensitivity analysis by excluding the individuals with diabetes from the ARIC study. All analyses were performed in the statistical environment R (version 2.10.0). All statistical testing was two-sided and p < 0.05 was considered statistically significant.
Results
General characteristics
Table 1 shows the studies that were used for this analysis, and the definitions used in each study to identify individuals with diabetes. A total of 4,220 individuals with diabetes were included in USE-IMT; of these, 71% were derived from North American cohorts. Characteristics of the USE-IMT population and population with diabetes are shown in Table 2. The mean common CIMT (SD) in the individuals with diabetes was 0.79 mm (0.19) compared with 0.74 mm in the total USE-IMT population. The median follow-up of the individuals with diabetes mellitus was 8.7 years, during which 684 first-time myocardial infarctions or first-time strokes occurred (Table 2).
Table 2 Characteristics of the participants of the cohorts in USE-IMT and those with diabetes
Risk factors and CIMT in diabetes for cardiovascular events
The relations between the classical cardiovascular risk factors used in the Framingham risk score, mean common CIMT and the outcome for the general USE-IMT population and the population with diabetes are shown in Table 3, adjusted for the Framingham risk variables. In both populations, risk factors were strongly related with the occurrence of first-time stroke or myocardial infarction. Yet, among those with diabetes, the magnitude of the relation with events for smoking, blood pressure, age and sex were less strong than in the overall general population. The association between mean common CIMT and outcome was similar (magnitude and direction) for the general USE-IMT population and for the population with diabetes.
Table 3 Relation of risk factors and CIMT with incident cardiovascular events, adjusted for Framingham risk variables
Calibration
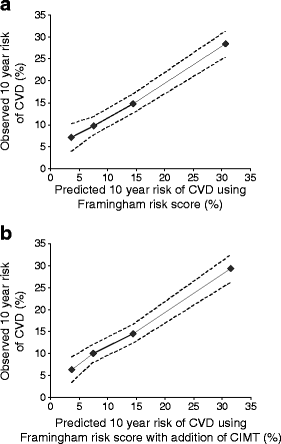
The addition of mean common CIMT improved the baseline model in the whole USE-IMT population (Wald test and the likelihood ratio test, both p < 0.001) [11]. For individuals with diabetes, the 10 year predicted risk was in close agreement with the 10 year cardiovascular disease risk as estimated with Kaplan–Meier (Fig. 1).
Fig. 1
The goodness of fit of Framingham risk score in individuals with diabetes. The predicted and the observed risks are plotted for the population with diabetes of the baseline model (a) and the model in which the mean common CIMT has been added (b). The dotted lines indicate the upper and the lower limits of the observed risks as estimated with Kaplan–Meier
Discrimination
For individuals with diabetes, the C statistic for the baseline model was 0.671 (95% CI 0.649, 0.693) and 0.678 (95% CI 0.656, 0.700) for the CIMT model.
Distribution of Framingham risk score in individuals with diabetes
The distribution of the predicted risk among the individuals with diabetes, according to the Framingham risk score, was as follows: 9.7% were classified as being at low risk (<5%), 26.9% were classified as being at low-to-intermediate risk, (≥5 to <10%), 35.5% were classified as being at intermediate-to-high risk (≥10 to <20%) and 27.8% were classified as being at high risk (≥20%).
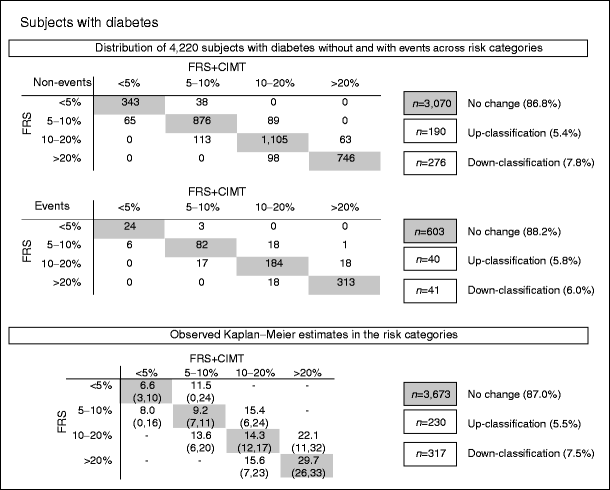
Net reclassification of individuals with diabetes
The upper section of Fig. 2 shows the distribution of the number of individuals with diabetes without and with events across risk categories based on the Framingham risk score (rows), and the distribution of individuals with diabetes after the addition of the mean common CIMT (columns). In those with and without events, the percentage of individuals with diabetes that remained in the same risk category was high (>85%). The observed mean risk of the individuals with diabetes who remained in the same risk categories (shaded values, lower section of Fig. 2) corresponded well to the risk categories. In those who did not experience an event, more individuals were correctly classified to a lower risk category than to a higher risk category. Yet, for those individuals with events, a similar number was correctly up-classified as was incorrectly down-classified. In the lower section of Fig. 2, the observed risks in those who shifted risk category show much uncertainty, as indicated by the wide range of the upper and lower limits. The NRI value indicated that the added value of mean common CIMT was small and non-significant (1.7%; 95% CI −1.8, 3.8). For sexes separately, the result was 1.6% (95% CI −7.1, 4.5) for men and 1.9% (95% CI −3.0, 4.6) for women. The integrated discrimination improvement was 0.005195% (95% CI 0.0011, 0.0091) for the individuals with diabetes, with no differences between men and women. To put the integrated discrimination improvement into perspective, we calculated the rIDI, which reflects the increase in discrimination slope divided by the slope of the baseline model. The rIDI with CIMT for the individuals with diabetes was 6.88%. This value may be seen as the predictive contribution of CIMT to the model (compared with the average contribution of 12.5% of the existing eight predictors).
Fig. 2
Reclassification of individuals with diabetes after addition of CIMT to Framingham risk score. The upper section of the figure shows the numbers of individuals with diabetes with and without events classified according to their 10 year absolute risk of developing a myocardial infarction or stroke predicted using the Framingham risk score (FRS, rows) or classified according to their 10 year absolute risk of developing a first-time myocardial infarction or stroke predicted using the Framingham risk score plus mean common CIMT measurement (FRS + CIMT, columns). The lower section of the figure shows the observed Kaplan–Meier estimates with the lower and upper bounds for absolute risk in all individuals (with and without events)
For stroke and myocardial infarction as separate endpoints, the NRI was 4.0% and 2.2%, respectively.
Sensitivity analysis
In our sensitivity analysis, the ARIC study participants were excluded from the diabetic population, resulting in 2,690 individuals with a mean (SD) common CIMT of 0.83 (0.19). These individuals had a median follow-up of 6.6 years during which 379 first-time strokes and myocardial infarctions occurred. Harrell’s c-index was 0.727 (95% CI 0.699, 0.755) without and 0.730 (95% CI 0.702, 0.759) with addition of mean common CIMT. The NRI was −6.7% (95% CI −20.9, 3.7) and not significantly different from our main analysis.
Discussion
This meta-analysis based on individual data of 4,220 subjects with diabetes obtained from 16 cohort studies worldwide, showed that measurement of mean common CIMT, on top of Framingham risk factors does not provide additional information in CVD risk in subjects with diabetes. When Framingham risk score is used to predict the absolute 10 year risk of developing CVD in an individual with diabetes, adding a mean common CIMT measure does not contribute to the risk prediction.
Several guidelines for the management of diabetes support assessment of cardiovascular risk to guide preventive treatment in individuals with diabetes. This is of particular importance since not all individuals with diabetes are at high risk [5, 6]. We used the Framingham risk score to assess the added value of mean common CIMT in individuals with diabetes. Framingham is one of the most widely used risk scores in primary care. Also, a recent systematic review recommend the use of the Framingham risk score to predict CVD risk in individuals with diabetes because this was the only risk score that had been externally validated and had adequate discriminative capacity [7].
Yet, improvement is possible in predicting the 10 year risk of developing CVD in individuals with diabetes. Measurement of subclinical atherosclerosis by mean common CIMT may identify those at high risk. The literature on the added value of CIMT measurements within the diabetic population is very limited. This is mostly due to the fact that individuals with diabetes are often included in general-population-based cohorts and are analysed as a subgroup. Consequently, these analyses are based on a limited sample size and a limited number of events, which hampers the precision in determining the NRI gained with CIMT measurement. Even within our collaboration, the number of individuals that shifted risk categories was small, and this limited precise estimates of mean observed risks, as can be seen from the lower section of Fig. 2.
Recently, results from two cohorts, one not being part of USE-IMT, addressed this issue [19, 20]. Yoshida and co-workers followed a cohort of 783 Japanese patients (aged 30–75 years, with diabetes, free from previous CVD) for 5.4 years and reported that an increased common CIMT (including plaques) was related to the risk of CVD (n = 85) [19]. The C statistic of the Framingham risk score model was 0.645 and rose to 0.656 with addition of common CIMT. No information on net reclassification was presented. The second report followed 881 patients with diabetes, aged 45–84 years, participating in the Multi-Ethnic Study of Atherosclerosis (MESA), for 6.4 years [20]. CIMT was expressed as the combined maximal intima-media thickness of the internal and common carotid sites using a z score [20]. In those with diabetes, the CIMT was statistically significantly related to increased risk of coronary heart disease events, but not to CVD, when risk factors were taken into account. The C statistic increased from 0.72 to 0.74 with the addition of CIMT measurements of coronary events, but did not change for CVD. No information on reclassification was presented. These findings are in agreement with ours. Common CIMT relates to risk of coronary events or CVD, but does not improve individual risk stratification in clinical practice.
It may well be that other measures of subclinical atherosclerosis, such as carotid plaque, CIMT measured at the carotid bifurcation or internal carotid segments, ankle brachial index or coronary calcium, may be of greater value in the identification of individuals with diabetes who are at high risk of future CVD [21, 22]. Nevertheless, studies presenting clinically useful indices of the added value of markers on top of classical risk factors have not yet been published and are needed to improve individual CVD risk stratification in subjects with diabetes.
One may argue whether there is a need for risk stratification in patients with diabetes mellitus. The current guidelines on cardiovascular risk factor management vary in their recommendations. The NCEP ATP III, Canadian dyslipidemia and WHO guidelines put individuals with type 1 or type 2 diabetes in the highest risk category, implying that pharmacological therapy is needed for all, irrespective of the risk factor level [23, 24]. The American Heart Association guideline for women advocates the classification of women with diabetes mellitus at high risk [25]. The UK National Institute for Health and Clinical Excellence guidelines recommend drug treatment in individuals with diabetes when blood pressure or lipid levels are elevated. Recent European guidelines indicate that individuals with diabetes mellitus with one or more risk factors are seen as very high risk and those with no other risk factors are considered as high risk [26, 27]. Given these guidelines, the rationale for further risk stratification in individuals with diabetes mellitus seems unnecessary, let alone measuring common CIMT for that purpose.
The strength of our study lies in the large sample size from multiple cohorts that collaborate in the ongoing USE-IMT initiative. Furthermore, our main model was based on individuals in USE-IMT who were eligible for cardiovascular risk stratification. As this population may be very different from that of Framingham, we refit all the Framingham variables with and without addition of mean common CIMT. Next, we separately analysed the value of CIMT in those with diabetes. Moreover, to test whether our results were driven by the ARIC study, which had a major contribution to the population with diabetes in USE-IMT, we performed a sensitivity analysis by excluding the subjects with diabetes from the ARIC study. This showed that even though the discriminative value of the baseline and CIMT model without the ARIC study was reasonable to good, there was no NRI achieved by adding the mean common CIMT measures. This strengthens our conclusions that mean common CIMT does not add information on cardiovascular risk in individuals with diabetes, when added to the variables of the Framingham model.
However, a meta-analysis like USE-IMT has limitations. Joining data from multiple cohorts has inevitably led to variations that we cannot account for, e.g. in the definition of diabetes across the cohorts. In addition, there was no information available on the severity or duration of the disease or the use of medication. This certainly limits our ability to further characterise the population with diabetes included in our study. At the same time, the cohorts in USE-IMT were all based on the general population, which suggests that they are probably similar across the studies. In addition, we are also unable to account for differences in measurement (e.g. the number of sonographers/readers, whether or not the analysis was performed using semi-automated software) in the studies included in USE-IMT. One way in which we have compensated for this is by using a frailty model of studies in our Cox proportional-hazards model. By adding this term to our model, we have ‘corrected’ for the differences in CIMT and outcome across studies.
Plaque burden and intima-media thickness in other carotid segments were not taken into account. Individuals with diabetes may have more atherosclerotic plaques and more severe coronary atherosclerosis than individuals without diabetes, either of which may have remained undetected when analysing the mean common CIMT only. Finally, the protocols to assess and measure mean common CIMT may have varied by study. This may have led to an increase in the variability of the CIMT measurement and thus an underestimation of the relations under study.
In conclusion, mean common CIMT measurement provides no added value to the Framingham risk score in individuals with diabetes. Therefore, this measurement is not recommended for improving individual cardiovascular risk stratification in individuals with diabetes.
Abbreviations
ARIC:
Atherosclerosis Risk in Communities
CIMT:
Carotid intima-media thickness
CVD:
Cardiovascular disease
NRI:
Net reclassification improvement
rIDI:
Relative integrated discrimination improvement
USE-IMT:
USE Intima Media Thickness collaboration
References
- Asia Pacific Cohort Studies Collaboration (2003) The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia-Pacific region. Diabetes Care 26:360–366
Article Google Scholar - The Emerging Risk Factors Collaboration (2010) Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375:2215–2222
Article Google Scholar - British Cardiac Society British Hypertension Society, Diabetes UK HEART UK Primary Care Cardiovascular Society Stroke Association (2005) JBS 2: Joint British Societies’ guidelines on prevention of cardiovascular disease in clinical practice. Heart 91(Suppl 5):v1–v52
Google Scholar - National Collaborating Center for Chronic Conditions. Type 2 Diabetes National Clinical Guideline for Management in Primary and Secondary Care (update). www.nice.org.uk/nicemedia/live/11983/40803/40803.pdf (accessed 14 December 2012).
- Rydén L, Standl E, Bartnik M et al (2007) Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. Eur Heart J 28:88–136
Article PubMed Google Scholar - Chamnan P, Simmons RK, Sharp SJ, Griffin SJ, Wareham NJ (2009) Cardiovascular risk assessment scores for people with diabetes: a systematic review. Diabetologia 52:2001–2014
Article PubMed CAS Google Scholar - van Dieren S, Beulens JWJ, Kengne AP et al (2011) Prediction models for the risk of cardiovascular disease in patients with type 2 diabetes: a systematic review. Heart 98:360–369
Article PubMed Google Scholar - D’Agostino RB Sr, Vasan RS, Pencina MJ et al (2008) General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 117:743–753
Article PubMed Google Scholar - Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M (2007) Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 115:459–467
Article PubMed Google Scholar - The Society of Atherosclerosis Imaging and Prevention (2011) Appropriate use criteria for carotid intima media thickness testing. Atherosclerosis 214:43–46
Article Google Scholar - Den Ruijter HM, Peters SA, Anderson TJ et al (2012) Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA 308:796–803
Article Google Scholar - De Backer G, Ambrosioni E, Borch-Johnsen K et al (2003) European guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 24:1601–1610
Article PubMed Google Scholar - Naghavi M, Falk E, Hecht HS et al (2006) From vulnerable plaque to vulnerable patient—part III: executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) task force report. Am J Cardiol 98:2–15
Article Google Scholar - Moons KGM, Donders RART, Stijnen T, Harrell J (2006) Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol 59:1092–1101
Article PubMed Google Scholar - Harrell FE Jr, Lee KL, Califf RM, Pryor DB, Rosati RA (1984) Regression modelling strategies for improved prognostic prediction. Stat Med 2:143–152
Article Google Scholar - Pencina MJ, D’Agostino RB, Steyerberg EW (2011) Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 30:11–21
Article PubMed Google Scholar - Pencina MJ, D’Agostino RB, D’Agostino RB Jr, Vasan RS (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27:157–172
Article PubMed Google Scholar - Pencina MJ, D’Agostino RB, Vasan RS (2010) Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med 12:1703–1711
Google Scholar - Yoshida M, Mita T, Yamamoto R et al (2012) Combination of the Framingham risk score and carotid intima-media thickness improves the prediction of cardiovascular events in patients with type 2 diabetes. Diabetes Care 35:178–180
Article PubMed Google Scholar - Malik S, Budoff MJ, Katz R et al (2011) Impact of subclinical atherosclerosis on cardiovascular disease events in individuals with metabolic syndrome and diabetes. Diabetes Care 34:2285–2290
Article PubMed Google Scholar - Bagheri R, Schutta M, Cumaranatunge RG et al (2007) Value of electrocardiographic and ankle brachial index abnormalities for prediction of coronary atherosclerosis in asymptomatic subjects with type 2 diabetes mellitus. Am J Cardiol 99:951–955
Article PubMed Google Scholar - Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD (2011) Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. JACC 57:1690–1696
Article PubMed Google Scholar - Mendis S, Lindholm LH, Mancia G, Whitworth J, Alderman M, Lim S, Heagerty T (2007) World Health Organization (WHO) and International Society of Hypertension (ISH) risk prediction charts: assessment of cardiovascular risk for prevention and control of cardiovascular disease in low and middle-income countries. J Hypertens 8:1578–1585
Article Google Scholar - Grundy SM, Becker D, Clarke LT (2001) Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. JAMA 285:2486–2497
Article Google Scholar - Mosca L, Benjamin EJ, Berra K et al (2011) Effectiveness-based guidelines for the prevention of cardiovascular disease in women 2011 update: a guideline from the American Heart Association. J Am Coll Cardiol 57:1404–1423
Article PubMed Google Scholar - Perk J, de Backer G, Gohlke H et al (2012) European Guidelines on cardiovascular disease prevention in clinical practice. Prev Cardiol 19:585–668
Article Google Scholar - Centre for Clinical Practice at NICE (UK). (2009) Type 2 diabetes: newer agents for blood glucose control in type 2 diabetes. National Institute for Health and Clinical Excellence (UK), London.
- Nambi V, Chambless L, Folsom AR et al (2010) Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol 55:1600–1607
Article PubMed CAS Google Scholar - Lorenz MW, Schaefer C, Steinmetz H, Sitzer M (2010) Is carotid intima media thickness useful for individual prediction of cardiovascular risk? Ten-year results from the Carotid Atherosclerosis Progression Study (CAPS). Eur Heart J 31:2041–2048
Article PubMed Google Scholar - Cao JJ, Arnold AM, Manolio TA et al (2007) Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: The Cardiovascular Health Study. Circulation 116:32–38
Article PubMed Google Scholar - Hedblad B, Nilsson P, Janzon L, Berglund G (2000) Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects. Results from a cross-sectional study in Malmö, Sweden. Diabet Med 17:299–307
Article PubMed CAS Google Scholar - Stensland-Bugge E, Bonaa KH, Joakimsen O, Njolstad I (2000) Sex differences in the relationship of risk factors to subclinical carotid atherosclerosis measured 15 years later: The Tromsø study. Stroke 31:574–581
Article PubMed CAS Google Scholar - Mora S, Szklo M, Otvos JD et al (2007) LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 192:211–217
Article PubMed CAS Google Scholar - Salonen R, Salonen JT (1991) Determinants of carotid intima-media thickness: a population-based ultrasonography study in eastern Finnish men. J Intern Med 229:225–231
Article PubMed CAS Google Scholar - Price JF, Tzoulaki I, Lee AJ, Fowkes FG (2007) Ankle brachial index and intima media thickness predict cardiovascular events similarly and increased prediction when combined. J Clin Epidemiol 60:1067–1075
Article PubMed Google Scholar - Anderson TJ, Charbonneau F, Title LM et al (2011) Microvascular function predicts cardiovascular events in primary prevention: long-term results from the firefighters and their endothelium (FATE) study. Circulation 123:163–169
Article PubMed Google Scholar - Ali YS, Rembold KE, Weaver B et al (2006) Prediction of major adverse cardiovascular events by age-normalized carotid intimal medial thickness. Atherosclerosis 187:186–190
Article PubMed CAS Google Scholar - Prabhakaran S, Singh R, Zhou X, Ramas R, Sacco RL, Rundek T (2007) Presence of calcified carotid plaque predicts vascular events: the Northern Manhattan Study. Atherosclerosis 195:e197–e201
Article PubMed CAS Google Scholar - Henry RMA, Kostense PJ, Spijkerman AMW et al (2003) Arterial stiffness increases with deteriorating glucose tolerance status: The Hoorn Study. Circulation 107:2089–2095
Article PubMed Google Scholar - Hofman A, van Duijn CM, Franco OH et al (2011) The Rotterdam Study: 2012 objectives and design update. Eur J Epidemiol 8:657–686
Article Google Scholar - Kitagawa K, Hougaku H, Yamagami H et al (2007) Carotid intima-media thickness and risk of cardiovascular events in high-risk patients. Cerebrovasc Dis 24:35–42
Article PubMed Google Scholar - Halcox JPJ, Donald AE, Ellins E et al (2009) Endothelial function predicts progression of carotid intima-media thickness. Circulation 119:1005–1012
Article PubMed Google Scholar - Holewijn S, den Heijer M, Swinkels DW, Stalenhoef AFH, de Graaf J (2009) The metabolic syndrome and its traits as risk factors for subclinical atherosclerosis. J Clin Endocrinol Metab 94:2893–2899
Article PubMed CAS Google Scholar
Funding
This project is supported by a grant from the Netherlands Organisation for Health Research and Development (ZonMw 200320003).
Duality of interest
J. de Graaf received a Dutch Heart Foundation grant to perform the NBS2 study (Nijmegen Biomedical Study). G. Engström was employed by AstraZeneca as a senior epidemiologist. K. Kitagawa was employed by Osaka University Hospital, received a grant from the Ministry of Education, Culture, Sports, and Technology of Japan and received lecture fees from sanofi-aventis. E. M. Lonn was a consultant for Merck and Hoffman-La Roche, provided expert testimony for Merck, received grants from AstraZeneca, sanofi-aventis, Novartis and GlaxoSmithKline and received lecture fees from Merck and Novartis. E. B. Mathiesen received a grant from the North Norwegian Health Authorities. D. H. O’Leary owns stock in Medpace. J. F. Polak received a grant from the National Heart, Lung, and Blood Institute. J. F. Price received a grant from the British Heart Foundation. T. Rundek received grants from the National Institutes of Health. J. T. Salonen received a grant from the University of Eastern Finland for the funding of the Kuopio Ischaemic Heart Disease Risk Factor study. D. E. Grobbee and M. L. Bots reported having been a consultant, for and having received grants and lecture fees from, AstraZeneca. All other authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
All authors made substantial contributions to conception and design, acquisition of data and interpretation of data. HMdR and SP analysed the data. MLB drafted the manuscript and all other authors were involved in revising the content of the manuscript for important intellectual content. HMdR and MLB take full responsibility for the work as a whole, including the study design, access to the data and the decision to submit and publish the manuscript. All authors approved the final, submitted version of the manuscript.
Author information
Authors and Affiliations
- Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Heidelberglaan 100, 3584 CX, Utrecht, the Netherlands
H. M. den Ruijter, S. A. E. Peters, K. A. Groenewegen, M. J. Eijkemans, D. E. Grobbee, H. Koffijberg, K. G. Moons & M. L. Bots - Department of Experimental Cardiology, University Medical Center Utrecht, Utrecht, the Netherlands
H. M. den Ruijter - Department of Cardiac Sciences and Libin Cardiovascular Institute of Alberta, University of Calgary, Alberta, Canada
T. J. Anderson - Department of Epidemiology and Public Health University College London, London, UK
A. R. Britton - Institute for Health and Care Research, VU University Medical Center, Amsterdam, the Netherlands
J. M. Dekker & G. Nijpels - Dept of Clinical Sciences in Malmö, Lund University, Skåne University Hospital, Malmö, Sweden
G. Engström, B. Hedblad & M. Rosvall - Department of Biostatistical Sciences and Neurology, Wake Forest School of Medicine, Winston-Salem, NC, USA
G. W. Evans - Department of General Internal Medicine, Division of Vascular Medicine, Radboud University Nijmegen Medical Centre, Nijmegen, the Netherlands
J. de Graaf & S. Holewijn - University of Malaya Medical Center, Kuala Lumpur, Malaysia
D. E. Grobbee - Department of Epidemiology, Erasmus University Medical Center, Rotterdam, the Netherlands
A. Hofman, M. Kavousi, M. A. Ikram & J. C. Witteman - Osaka Medical Center for Health Science and Promotion, Osaka, Japan
A. Ikeda & A. Kitamura - Stroke Center, Department of Neurology, Osaka University Graduate School of Medicine, Osaka, Japan
K. Kitagawa & S. Okazaki - Department of Radiology, Erasmus University Medical Center, Rotterdam, the Netherlands
M. A. Ikram - Department of Neurology, Erasmus University Medical Center, Rotterdam, the Netherlands
M. A. Ikram - Department of Medicine, Division of Cardiology and Population Health Research Institute, McMaster University, Hamilton, Ontario, Canada
E. M. Lonn - Department of Neurology, University Hospital, Goethe-University, Frankfurt am Main, Germany
M. W. Lorenz - Brain and Circulation Research Group, Institute of Clinical Medicine, University of Tromsø, Tromsø, Norway
E. B. Mathiesen - Department of Radiology, Tufts Medical Center, Boston, MA, USA
D. H. O’Leary & J. F. Polak - Centre for Population Health Sciences, University of Edinburgh, Edinburgh, UK
J. F. Price & C. Robertson - Cardiology Division, Department of Internal Medicine, University of Virginia, Charlottesville, VA, USA
C. M. Rembold - Department of Neurology, Miller School of Medicine, University of Miami, Miami, FL, USA
T. Rundek - MAS-Metabolic Analytical Services Oy, Helsinki, Finland
J. T. Salonen - Department of Neurology, University Hospital, Goethe-University, Frankfurt am Main, Germany
M. Sitzer - Department of Neurology Klinikum, Herford, Germany
M. Sitzer - Department of Internal Medicine and Cardiovascular Research Institute Maastricht, Maastricht University Medical Center, Maastricht, the Netherlands
C. D. A. Stehouwer
Authors
- H. M. den Ruijter
You can also search for this author inPubMed Google Scholar - S. A. E. Peters
You can also search for this author inPubMed Google Scholar - K. A. Groenewegen
You can also search for this author inPubMed Google Scholar - T. J. Anderson
You can also search for this author inPubMed Google Scholar - A. R. Britton
You can also search for this author inPubMed Google Scholar - J. M. Dekker
You can also search for this author inPubMed Google Scholar - G. Engström
You can also search for this author inPubMed Google Scholar - M. J. Eijkemans
You can also search for this author inPubMed Google Scholar - G. W. Evans
You can also search for this author inPubMed Google Scholar - J. de Graaf
You can also search for this author inPubMed Google Scholar - D. E. Grobbee
You can also search for this author inPubMed Google Scholar - B. Hedblad
You can also search for this author inPubMed Google Scholar - A. Hofman
You can also search for this author inPubMed Google Scholar - S. Holewijn
You can also search for this author inPubMed Google Scholar - A. Ikeda
You can also search for this author inPubMed Google Scholar - M. Kavousi
You can also search for this author inPubMed Google Scholar - K. Kitagawa
You can also search for this author inPubMed Google Scholar - A. Kitamura
You can also search for this author inPubMed Google Scholar - H. Koffijberg
You can also search for this author inPubMed Google Scholar - M. A. Ikram
You can also search for this author inPubMed Google Scholar - E. M. Lonn
You can also search for this author inPubMed Google Scholar - M. W. Lorenz
You can also search for this author inPubMed Google Scholar - E. B. Mathiesen
You can also search for this author inPubMed Google Scholar - G. Nijpels
You can also search for this author inPubMed Google Scholar - S. Okazaki
You can also search for this author inPubMed Google Scholar - D. H. O’Leary
You can also search for this author inPubMed Google Scholar - J. F. Polak
You can also search for this author inPubMed Google Scholar - J. F. Price
You can also search for this author inPubMed Google Scholar - C. Robertson
You can also search for this author inPubMed Google Scholar - C. M. Rembold
You can also search for this author inPubMed Google Scholar - M. Rosvall
You can also search for this author inPubMed Google Scholar - T. Rundek
You can also search for this author inPubMed Google Scholar - J. T. Salonen
You can also search for this author inPubMed Google Scholar - M. Sitzer
You can also search for this author inPubMed Google Scholar - C. D. A. Stehouwer
You can also search for this author inPubMed Google Scholar - J. C. Witteman
You can also search for this author inPubMed Google Scholar - K. G. Moons
You can also search for this author inPubMed Google Scholar - M. L. Bots
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toM. L. Bots.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
den Ruijter, H.M., Peters, S.A.E., Groenewegen, K.A. et al. Common carotid intima-media thickness does not add to Framingham risk score in individuals with diabetes mellitus: the USE-IMT initiative.Diabetologia 56, 1494–1502 (2013). https://doi.org/10.1007/s00125-013-2898-9
- Received: 18 December 2012
- Accepted: 08 March 2013
- Published: 09 April 2013
- Issue Date: July 2013
- DOI: https://doi.org/10.1007/s00125-013-2898-9