Incidence and remission of type 2 diabetes in relation to degree of obesity at baseline and 2 year weight change: the Swedish Obese Subjects (SOS) study (original) (raw)
Abstract
Aims/hypothesis
The aim of this work was to analyse the rates of incidence and remission of type 2 diabetes in relation to baseline BMI and weight change in the prospective, controlled Swedish Obese Subjects (SOS) study.
Methods
Three-thousand four-hundred and eighty-five obese individuals receiving bariatric surgery or conventional treatment were grouped into four baseline BMI categories (<35, 35–40, 40–45 or ≥45 kg/m2) and five weight-change categories according to their BMI at 2 years (increase [≥1 BMI unit increase], no change [less than 1 BMI unit change], minor reduction [−1 to −9 BMI units], medium reduction [−10 to −14 BMI units] and major reduction [< −15 BMI units]). The incidence and remission of diabetes at 2 years was assessed.
Results
Among individuals with no weight change, diabetes incidence rates were 5.5%, 7.4%, 8.3% and 5.2%, in the four baseline BMI categories, respectively. In those with an initial BMI of 35–40, 40–45 and ≥45 kg/m2 who attained a minor reduction in weight, the corresponding rates were 1.3%, 1.2% and 3.4%, respectively. In both the medium- and major-weight-reduction groups, diabetes incidence was ≤0.5%. Among individuals with diabetes at baseline, the remission rates were 15.3–26.9% in the no-weight-change groups, and 48.1–70% for individuals who attained a minor weight reduction. In the medium- and major-weight-reduction groups, the remission rate was 77–97%. There were no differences in 2 year incidence and remission rates between different baseline BMI groups that achieved the same degree of weight reduction.
Conclusions/interpretation
In obese individuals, the favourable effect of weight reduction on type 2 diabetes incidence and remission is independent of initial BMI.
Trial registration ClinicalTrials.gov number NCT01479452
Similar content being viewed by others
Introduction
Obesity is associated with an increased risk for type 2 diabetes [1]. In severely obese individuals, surgically induced weight loss significantly lowers the incidence of type 2 diabetes [2–4] and primary prevention trials have shown that moderate weight reduction can reduce the risk for type 2 diabetes in obese individuals with impaired glucose tolerance [5–8]. Furthermore, in many patients with type 2 diabetes, bariatric surgery results in diabetes remission over 2 years [9, 10], although relapses may later occur [9].
Although obesity and weight loss are important determinants of type 2 diabetes, little is known about the influence of baseline BMI and degree of weight loss on diabetes prevention and remission. Current guidelines for bariatric surgery are based on BMI alone or on BMI in combination with comorbidities [11, 12]. In the Swedish Obese Subjects (SOS) study (ClinicalTrials.gov registration NCT01479452), however, we have repeatedly found that baseline BMI is not a predictor of long-term bariatric surgery outcomes such as reduction of cardiovascular events, overall mortality and cancer or type 2 diabetes prevention and remission [3, 9, 13–15]. In this study, we analyse the SOS study data in more detail to reveal whether incidence and remission rates for type 2 diabetes differ among participants with different baseline BMI but similar weight loss over 2 years.
Methods
Study design
Between 1 September 1987 and 31 January 2001, a total of 4,047 obese persons were enrolled in the prospective, controlled SOS intervention trial [3, 4]. After a recruitment campaign in the mass media and at 480 primary healthcare centres, a matching examination was completed by 6,905 patients, 5,335 of whom were eligible for inclusion. Of these, 2,010 individuals electing surgery constituted the surgery group, and a contemporaneously matched control group of 2,037 individuals was created using 18 matching variables. The matching variables were sex, age, weight, height, waist and hip circumference, systolic blood pressure, serum cholesterol and triacylglycerol levels, smoking status, diabetes, menopausal status, four psychosocial variables having documented associations with the risk of death, and two personality traits related to treatment preferences. Although a surgery patient and his or her conventionally treated control always started the study on the day of surgery, the matching was not performed at an individual level. Instead the matching algorithm selected controls so that the current mean values of the matching variables in the control group became as similar as possible to the current mean values in the surgery group according to the method of sequential treatment assignment [16].
The two study groups had identical inclusion and exclusion criteria, and all controls were eligible for surgery. The inclusion criteria were as follows: age 37–60 years and BMI of 34 kg/m2 or more for men or 38 kg/m2 or more for women before or at the matching examination. The BMI cut-offs corresponded to an approximate doubling in the rate of death in men and women [17]. The exclusion criteria were as follows: earlier surgery for gastric or duodenal ulcer; earlier bariatric surgery; gastric ulcer during the past 6 months; ongoing malignancy or active malignancy during the past 5 years; myocardial infarction during the past 6 months; bulimic eating pattern; drug or alcohol abuse; psychiatric or cooperative problems contraindicating bariatric surgery or other contraindicating conditions (such as chronic glucocorticoid or anti-inflammatory treatment).
In the surgery group, 376 individuals underwent nonadjustable or adjustable gastric banding, 1,369 underwent vertical banded gastroplasty (VBG) and 265 underwent gastric bypass (GBP). The control group received the customary nonsurgical treatment for obesity at their primary healthcare centre.
Report population, examinations and data analysis
This report included individuals who had completed the 2 year follow-up. After excluding patients with type 1 diabetes (n = 4) and 21 patients with missing information on glucose level at baseline or at the 2 year follow-up, 3,485 participants were available for analysis.
Measurements of weight, height and waist circumference were obtained at baseline and at the 2 year follow-up. Blood samples, obtained in the morning after the participant had fasted overnight, were analysed at the Central Laboratory of Sahlgrenska University Hospital (accredited according to International Organization for Standardization/International Electrochemical Commission 15189:2007 standards).
Self-reported information about diabetes medication was obtained and glucose concentrations were measured at baseline and at the 2 year follow-up visit. From 1987 until the end of 2009, fasting glucose concentrations were measured in venous whole blood. After 2009, venous fasting plasma glucose was measured, and the concentrations were converted to those for blood glucose. The study was initiated before repeated measurements were routinely used for the diagnosis of type 2 diabetes; therefore, single fasting glucose determinations were used. Type 2 diabetes was defined as fasting blood glucose of 6.1 mmol/l or higher, corresponding to a fasting plasma glucose of 7.0 mmol/l or higher, or diabetes medication use (insulin, oral glucose-lowering drugs, or both). Diabetes remission was defined as blood glucose levels lower than 6.1 mmol/l and no diabetes medication [3, 9].
For the analyses in the present report, surgery and control patients were pooled. Individuals were grouped into four BMI categories according to baseline BMI (<35, 35–40, 40–45 or ≥45 kg/m2) and five weight change categories depending on the resulting BMI at 2 years. The weight change categories were as follows: weight increase (≥1 BMI unit increase); no weight change (less than 1 BMI unit change); minor weight reduction (−1 to −9 BMI units); medium weight reduction (−10 to −14 BMI units) and major weight reduction (more than −15 BMI units). This resulted in 11 mutually exclusive BMI and weight change groups (Table 1).
Table 1 Baseline characteristics and changes over 2 years for SOS study participants grouped according to baseline BMI
Ethics
All the relevant ethics review boards in Sweden approved the study, and written or oral informed consent was obtained from all participants.
Statistical analysis
Baseline characteristics and changes in continuous variables were analysed with ANOVA, testing equality between BMI groups within weight-change groups. Diabetes incidence and remission rates were calculated among those with and without type 2 diabetes at baseline, respectively. Differences between the BMI categories were assessed with logistic regression models, adjusting for sex, age, fasting glucose and fasting insulin at baseline. A p value of less than 0.05 was considered significant. Statistical analyses were performed with Stata software (version 12.1; College Station, TX, USA).
Results
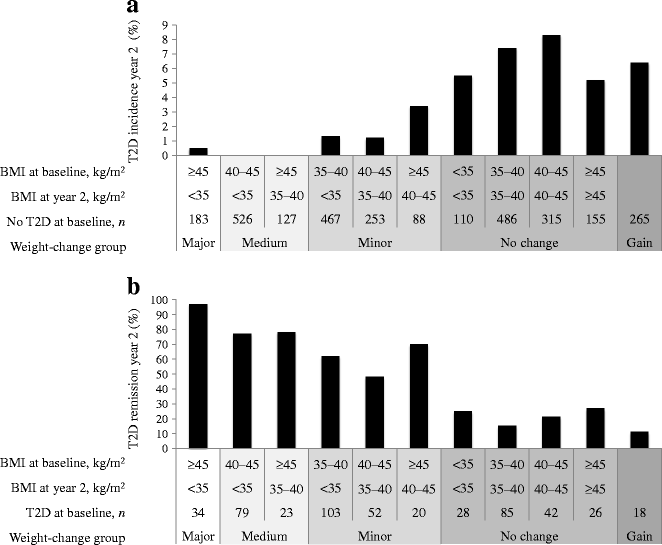
When comparing the different weight-change groups we found that the overall diabetes incidence was 7.1% in those with no weight change as compared with incidence rates of 1.5% (p < 0.001), 0.0% (p < 0.001) and 0.5% (p = 0.009) in the groups achieving minor, medium and major weight change, respectively. Patients who had gained weight at the 2 year follow-up displayed a 6.4% incidence rate (n = 17 out of 265 participants without diabetes at baseline; Fig. 1a). Similarly, failure to achieve remission was more common in those with no weight change than in those who lost weight. Remission rates were 20.0%, 59.9%, 77.5% and 97.1% in the groups with no weight change, minor, medium and major weight loss, respectively (p < 0.001 for all comparisons against the group with no weight change). Patients who had gained weight at the 2 year follow-up displayed an 11.1% remission rate (n = 2 out of 18 participants with diabetes at baseline; Fig. 1b).
Fig. 1
Incidence and remission of type 2 diabetes (T2D) grouped according to weight change over 2 years: weight gain; no weight change; minor, medium and major weight reduction. Type 2 diabetes incidence is shown as the proportion of individuals with type 2 diabetes at year 2 among those without type 2 diabetes at baseline (a) and remission of type 2 diabetes is shown as the proportion of individuals without type 2 diabetes at year 2 among those with type 2 diabetes at baseline (b). Note that the weight-gain group is a mix of patients with different baseline BMIs. The p values for likelihood ratio tests of equality between the baseline BMI groups within weight-change groups were adjusted for sex, age, fasting glucose and fasting insulin at baseline and were all non-significant. However, it was not possible to calculate the p value for diabetes incidence in the medium-weight-reduction group as no patient in this group developed type 2 diabetes during follow-up
In the individuals displaying no weight change, i.e. who maintained a BMI of <35, 35–40, 40–45 or ≥45 kg/m2 over the 2 years, type 2 diabetes incidence rates were 5.5%, 7.4%, 8.3% and 5.2%, respectively. In those with an initial BMI of 35–40, 40–45 and ≥45 kg/m2, and who attained a minor weight reduction, the corresponding rates were 1.3%, 1.2% and 3.4%, respectively (Fig. 1a). The incidence rates in the groups with medium/major weight reduction were even lower, in the range 0–0.5% (Fig. 1a).
Similarly, the remission rates for type 2 diabetes at year 2 were lower in those with no weight change, as compared with those who lost weight, independent of initial BMI (Fig. 1b). The type 2 diabetes remission rates in patients with minor weight reduction were 62.1%, 48.1% and 70%, respectively in the different BMI baseline groups. In individuals with no weight change, the proportion in remission at year 2 was 25.0%, 15.3%, 21.4% and 26.9%, respectively (Fig. 1b). In line with the results for diabetes incidence, remission rates were even higher in groups with medium (77–78%) and major (97%) weight reduction (Fig. 1b).
The changes in fasting glucose and insulin did not vary between different baseline BMI groups with similar weight reduction over 2 years (Table 1). When analysing the total cohort (i.e. all weight-reduction and BMI categories in Table 1), the interaction term between baseline BMI and 2 year weight change (measured in kg) was non-significant for both incidence of type 2 diabetes (p = 0.479) and remission (p = 0.702). Furthermore, the results remained essentially unchanged when the analyses were performed separately for men and women, and also when individuals operated with GBP were excluded. Neither did the results change when adjusting for sex, age, fasting glucose and fasting insulin (Fig. 1).
Discussion
In the SOS study, which compared patients undergoing bariatric surgery with controls, we previously showed that the incidence of type 2 diabetes was markedly lower in the operated patients than in the control group [3]. Furthermore, we recently showed that diabetes remission is markedly higher in operated patients [9]. We now extend these findings and show that for a given degree of weight loss, from a minor weight reduction (1–9 BMI units) to a major weight reduction (over 15 BMI units), the effect of surgery on diabetes prevention and remission was independent of the initial BMI level. In other words, a certain magnitude of weight reduction appears to be equally effective for all degrees of obesity when it comes to favourable effects on diabetes.
The strength of this study is the large sample of individuals with various degrees of obesity and weight loss, and the prospective collection of data. A limitation is that, in all analyses, the surgery and control groups were combined to ensure reasonable number of patients for analyses of incidence and remission by weight change and baseline BMI. This was done under the rather strong assumption that the mechanism affecting diabetes risk was weight change and not surgery itself. However, most of the participants who lost weight had undergone bariatric surgery and the majority of those with no weight change belonged to the conventional treatment group. Furthermore, in line with other reports [18], GBP patients lost more weight than banding or VBG patients [3]. Several studies suggest that GBP may affect glucose metabolism by mechanisms other than weight loss and that early remission of type 2 diabetes after bariatric surgery is independent of weight loss and caused by mechanisms related to the surgery itself [19–23]. For long-term effects on glycaemic control, weight loss is probably a major determinant [18]. Unfortunately this study was underpowered to analyse each treatment group separately. Therefore, to address the uncertainty regarding differences between GBP and restrictive procedures discussed above, a sensitivity analysis excluding GBP patients was conducted. Ideally, the analyses should be repeated with a single surgical procedure, or in a larger study that would allow stratifying the analyses by surgery type. Another limitation is that the diagnosis of type 2 diabetes was based on fasting glucose levels and diabetes medication. Thus, we do not have any information on the association between weight change and post-load glucose levels. Incidence and remission rates in individuals who gained weight were similar to the rates in those whose weight was stable. It should be noted that remission was observed in some patients in the group with no weight change and two patients in the weight gain group. However, it is difficult to interpret this, since this observation is confounded by the regression-to-the-mean phenomenon [24].
In conclusion, our findings suggest that the positive effect of weight reduction in obese individuals, for both prevention and remission of type 2 diabetes, is independent of baseline BMI.
Abbreviations
GBP:
Gastric bypass
SOS:
Swedish Obese Subjects
VBG:
Vertical banded gastroplasty
References
- Naser KA, Gruber A, Thomson GA (2006) The emerging pandemic of obesity and diabetes: are we doing enough to prevent a disaster? Int J Clin Pract 60:1093–1097
Article CAS PubMed Google Scholar - Buchwald H, Avidor Y, Braunwald E et al (2004) Bariatric surgery: a systematic review and meta-analysis. JAMA 292:1724–1737
Article CAS PubMed Google Scholar - Carlsson LM, Peltonen M, Ahlin S et al (2012) Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 367:695–704
Article CAS PubMed Google Scholar - Sjöström L, Lindroos AK, Peltonen M et al (2004) Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351:2683–2693
Article PubMed Google Scholar - Tuomilehto J, Lindstrom J, Eriksson JG et al (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344:1343–1350
Article CAS PubMed Google Scholar - Knowler WC, Barrett-Connor E, Fowler SE et al (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403
Article CAS PubMed Google Scholar - Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L (2004) XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 27:155–161
Article CAS PubMed Google Scholar - Li G, Zhang P, Wang J et al (2008) The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 371:1783–1789
Article PubMed Google Scholar - Sjöstrom L, Peltonen M, Jacobson P et al (2014) Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 311:2297–2304
Article PubMed Google Scholar - Gloy VL, Briel M, Bhatt DL et al (2013) Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 347:f5934
Article PubMed Central PubMed Google Scholar - NIH Consensus Development Conference Panel (1992) Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. March 25–27, 1991. Am J Clin Nutr 55(2Suppl):615S–619S
Google Scholar - National Clinical Guideline Centre (UK) (2014) Obesity: identification, assessment and management of overweight and obesity in children, young people and adults. National Institute for Health and Care Excellence, London
Google Scholar - Sjöström L, Peltonen M, Jacobson P et al (2012) Bariatric surgery and long-term cardiovascular events. JAMA 307:56–65
Article PubMed Google Scholar - Sjöström L, Narbro K, Sjöstrom CD et al (2007) Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 357:741–752
Article PubMed Google Scholar - Sjöström L, Gummesson A, Sjöstrom CD et al (2009) Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol 10:653–662
Article PubMed Google Scholar - Pocock SJ, Simon R (1975) Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 31:103–115
Article CAS PubMed Google Scholar - Waaler HT (1984) Height, weight and mortality. The Norwegian experience. Acta Med Scand Suppl 679:1–56
CAS PubMed Google Scholar - Buchwald H, Estok R, Fahrbach K et al (2009) Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 122:248–256
Article PubMed Google Scholar - Rubino F, Schauer PR, Kaplan LM, Cummings DE (2010) Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med 61:393–411
Article CAS PubMed Google Scholar - Sweeney TE, Morton JM (2014) Metabolic surgery: action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract Res Clin Gastroenterol 28:727–740
Article CAS PubMed Google Scholar - Steinert RE, Peterli R, Keller S et al (2013) Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity 21:E660–E668
Article CAS PubMed Google Scholar - Woelnerhanssen B, Peterli R, Steinert RE, Peters T, Borbely Y, Beglinger C (2011) Effects of postbariatric surgery weight loss on adipokines and metabolic parameters: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy—a prospective randomized trial. Surg Obes Relat Dis 7:561–568
Article PubMed Google Scholar - Bojsen-Moller KN, Dirksen C, Jorgensen NB et al (2014) Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes 63:1725–1737
Article PubMed Google Scholar - Barnett AG, van der Pols JC, Dobson AJ (2005) Regression to the mean: what it is and how to deal with it. Int J Epidemiol 34:215–220
Article PubMed Google Scholar
Acknowledgements
We thank the staff members at the 480 primary healthcare centres and 25 surgical departments in Sweden that participated in the SOS study. Some of the data in this paper were presented as a poster at the International Congress of Endocrinology/European Congress of Endocrinology in 2012 and will be presented at the European Congress on Obesity in 2015.
Funding
This study was supported by grants from the Swedish Research Council (K2012-55X-22082-01, K2013-54X-11285-19, K2013-99X-22279-01), the Swedish Foundation for Strategic Research (to Sahlgrenska Center for Cardiovascular and Metabolic Research), the Swedish federal government under the LUA/ALF agreement concerning research and education of doctors, Diabetesfonden and the VINNOVA-VINNMER program. The SOS study has previously been supported by grants to authors from Hoffmann–La Roche, AstraZeneca, Cederroth, Sanofi-Aventis and Johnson & Johnson.
Duality of interest
LS has received lecture fees from AstraZeneca and Johnson & Johnson and provided an expert statement on drug effects and weight-loss effects on obesity for AstraZeneca. KS owns stock in Pfizer. LMSC has received consulting fees from AstraZeneca and lecture fees from Johnson & Johnson. CDS is an employee of AstraZeneca. The other authors declare that there is no duality of interest associated with their contribution to this manuscript.
Contribution statement
All authors had full access to all data and take responsibility for the integrity of the data and accuracy of analyses. All authors provided input to the analytical approach, interpretation of the data, preparation, revision and final approval of the manuscript. LS and MP are the guarantors of this work.
Author information
Authors and Affiliations
- Department of Molecular and Clinical Medicine, Institute of Medicine, The Sahlgrenska Academy at the University of Gothenburg, SOS-sekr., Vita Stråket 15, SE-41345, Gothenburg, Sweden
Kajsa Sjöholm, Peter Jacobson, Kristjan Karason, Lena M. S. Carlsson & Lars Sjöström - Department of Chronic Disease Prevention, National Institute for Health and Welfare, Helsinki, Finland
Pia Pajunen & Markku Peltonen - Global Medicines Development, Cardiovascular and Metabolic Disease, AstraZeneca, Mölndal, Sweden
C. David Sjöström - Department of Health Care, Västra Götaland Region, Gothenburg, Sweden
Jarl Torgerson
Authors
- Kajsa Sjöholm
You can also search for this author inPubMed Google Scholar - Pia Pajunen
You can also search for this author inPubMed Google Scholar - Peter Jacobson
You can also search for this author inPubMed Google Scholar - Kristjan Karason
You can also search for this author inPubMed Google Scholar - C. David Sjöström
You can also search for this author inPubMed Google Scholar - Jarl Torgerson
You can also search for this author inPubMed Google Scholar - Lena M. S. Carlsson
You can also search for this author inPubMed Google Scholar - Lars Sjöström
You can also search for this author inPubMed Google Scholar - Markku Peltonen
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toKajsa Sjöholm.
Rights and permissions
About this article
Cite this article
Sjöholm, K., Pajunen, P., Jacobson, P. et al. Incidence and remission of type 2 diabetes in relation to degree of obesity at baseline and 2 year weight change: the Swedish Obese Subjects (SOS) study.Diabetologia 58, 1448–1453 (2015). https://doi.org/10.1007/s00125-015-3591-y
- Received: 13 November 2014
- Accepted: 26 March 2015
- Published: 30 April 2015
- Issue Date: July 2015
- DOI: https://doi.org/10.1007/s00125-015-3591-y