Potential drug-drug interactions and adverse drug reactions in patients with liver cirrhosis (original) (raw)
Abstract
Background and aims
Patients with liver cirrhosis may be at risk for potential drug-drug interactions (pDDIs) and/or adverse drug reactions (ADRs) due to the severity of their disease and comorbidities associated with polypharmacy.
Methods
We performed a cross-sectional retrospective study including 400 cirrhotic patients and assessed diagnoses, medication patterns, pDDIs, and ADRs at hospital admission.
Results
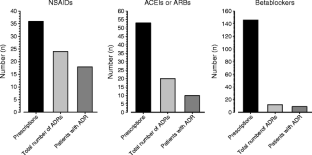
The median (range) age of the patients was 60 (21–88) years; 68.5% were male. They had a total of 2,415 diagnoses, resulting in 6 (1–10) diagnoses per patient. Frequent were diagnoses of the digestive system (28.4%), circulatory system (14.2%), blood and blood-forming organs (8.7%), and psychiatric disorders (7.5%); 60.7% of the diagnoses were not liver-associated. The median number of drugs per patient was 5 (0–18), whereof 3 (0–16) were predominantly hepatically eliminated. Drugs were primarily indicated for gastrointestinal, cardiovascular, or nervous system disorders, reflecting the prevalent diagnoses. In 112 (28%) patients, 200 ADRs were detected, mainly associated with spironolactone, torasemide, furosemide, and ibuprofen. In 86 (21.5%) patients, 132 pDDIs were detected. Seven of these pDDIs were the direct cause of 15 ADRs, whereof 3 resulted in hospital admission. Patients with ADRs were older, had more comorbidities, were treated with more drugs, and had a worse renal function and more pDDIs than patients without ADRs.
Conclusions
Pharmacotherapy is complex in cirrhotic patients. Hepatologists should know the principles of dose adjustment in cirrhosis and renal failure, but also the most important pDDIs of the drugs used to treat liver disease and comorbidities in this population.
Access this article
Subscribe and save
- Starting from 10 chapters or articles per month
- Access and download chapters and articles from more than 300k books and 2,500 journals
- Cancel anytime View plans
Buy Now
Price excludes VAT (USA)
Tax calculation will be finalised during checkout.
Instant access to the full article PDF.
Fig. 1
Similar content being viewed by others
Abbreviations
pDDIs:
Potential drug-drug interactions
ADRs:
Adverse drug reactions
NSAIDs:
Nonsteroidal anti-inflammatory drugs
Q0 :
Extrarenal elimination fraction
ATC code:
Anatomical Therapeutic Chemical Classification System
ACE:
Angiotensin-converting enzyme
HSCT:
Hematopoietic stem cell transplantation
RAAS:
Renin angiotensin aldosterone system
SSRI:
Selective serotonine reuptake inhibitor
COX:
Cyclooxygenase
References
- Leon DA, McCambridge J (2006) Liver cirrhosis mortality rates in Britain from 1950 to 2002: an analysis of routine data. Lancet 367:52–56
Article PubMed Google Scholar - Delco F, Tchambaz L, Schlienger R, Drewe J, Krahenbuhl S (2005) Dose adjustment in patients with liver disease. Drug Saf 28:529–545
Article PubMed CAS Google Scholar - Zuckerman MJ, Menzies IS, Ho H, Gregory GG, Casner NA, Crane RS et al (2004) Assessment of intestinal permeability and absorption in cirrhotic patients with ascites using combined sugar probes. Dig Dis Sci 49:621–626
Article PubMed Google Scholar - Blaschke TF, Rubin PC (1979) Hepatic first-pass metabolism in liver disease. Clin Pharmacokinet 4:423–432
Article PubMed CAS Google Scholar - Vyas K, Gala B, Sawant P, Das HS, Kulhalli PM, Mahajan SS (2002) Assessment of portal hemodynamics by ultrasound color Doppler and laser Doppler velocimetry in liver cirrhosis. Indian J Gastroenterol 21:176–178
PubMed Google Scholar - Adedoyin A, Arns PA, Richards WO, Wilkinson GR, Branch RA (1998) Selective effect of liver disease on the activities of specific metabolizing enzymes: investigation of cytochromes P450 2C19 and 2D6. Clin Pharmacol Ther 64:8–17
Article PubMed CAS Google Scholar - George J, Murray M, Byth K, Farrell GC (1995) Differential alterations of cytochrome P450 proteins in livers from patients with severe chronic liver disease. Hepatology 21:120–128
PubMed CAS Google Scholar - Tegeder I, Lotsch J, Geisslinger G (1999) Pharmacokinetics of opioids in liver disease. Clin Pharmacokinet 37:17–40
Article PubMed CAS Google Scholar - MacGilchrist AJ, Birnie GG, Cook A, Scobie G, Murray T, Watkinson G et al (1986) Pharmacokinetics and pharmacodynamics of intravenous midazolam in patients with severe alcoholic cirrhosis. Gut 27:190–195
Article PubMed CAS Google Scholar - Gines P, Arrovo V, Rodes J (1992) Pharmacotherapy of ascites associated with cirrhosis. Drugs 43:316–332
Article PubMed CAS Google Scholar - Krahenbuhl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krahenbuhl S (2007) Drug-related problems in hospitals: a review of the recent literature. Drug Saf 30:379–407
Article PubMed Google Scholar - Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Article PubMed CAS Google Scholar - Child CG, Turcotte JG (1964) Surgery and portal hypertension. In: Child C (ed) The liver and portal hypertension. Saunders, Philadelphia, pp 50–64
Google Scholar - Egger SS, Meier S, Leu C, Christen S, Gratwohl A, Krahenbuhl S et al (2010) Drug interactions and adverse events associated with antimycotic drugs used for invasive aspergillosis in hematopoietic SCT. Bone Marrow Transplant 45:1197–1203
Article PubMed CAS Google Scholar - Lucena MI, Andrade RJ, Tognoni G, Hidalgo R, De La Cuesta FS (2002) Multicenter hospital study on prescribing patterns for prophylaxis and treatment of complications of cirrhosis. Eur J Clin Pharmacol 58:435–440
Article PubMed CAS Google Scholar - Lucena MI, Andrade RJ, Tognoni G, Hidalgo R, Sanchez de la Cuesta F (2003) Drug use for non-hepatic associated conditions in patients with liver cirrhosis. Eur J Clin Pharmacol 59:71–76
PubMed Google Scholar - Lazarou J, Pomeranz BH, Corey PN (1998) Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 279:1200–1205
Article PubMed CAS Google Scholar - van der Hooft CS, Dieleman JP, Siemes C, Aarnoudse AJ, Verhamme KM, Stricker BH et al (2008) Adverse drug reaction-related hospitalisations: a population-based cohort study. Pharmacoepidemiol Drug Saf 17:365–371
Article PubMed Google Scholar - Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ et al (2004) Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ 329:15–19
Article PubMed Google Scholar - Fattinger K, Roos M, Vergeres P, Holenstein C, Kind B, Masche U et al (2000) Epidemiology of drug exposure and adverse drug reactions in two Swiss departments of internal medicine. Br J Clin Pharmacol 49:158–167
Article PubMed CAS Google Scholar - Classen DC, Pestotnik SL, Evans RS, Burke JP (1991) Computerized surveillance of adverse drug events in hospital patients. JAMA 266:2847–2851
Article PubMed CAS Google Scholar - Herr RD, Caravati EM, Tyler LS, Iorg E, Linscott MS (1992) Prospective evaluation of adverse drug interactions in the emergency department. Ann Emerg Med 21:1331–1336
Article PubMed CAS Google Scholar - Ratz Bravo AE, Tchambaz L, Krahenbuhl-Melcher A, Hess L, Schlienger RG, Krahenbuhl S (2005) Prevalence of potentially severe drug-drug interactions in ambulatory patients with dyslipidaemia receiving HMG-CoA reductase inhibitor therapy. Drug Saf 28:263–275
Article PubMed Google Scholar - Corsonello A, Pedone C, Corica F, Mussi C, Carbonin P, Antonelli Incalzi R et al (2005) Concealed renal insufficiency and adverse drug reactions in elderly hospitalized patients. Arch Intern Med 165:790–795
Article PubMed Google Scholar - Papadakis MA, Arieff AI (1987) Unpredictability of clinical evaluation of renal function in cirrhosis. Prospective study. Am J Med 82:945–952
Article PubMed CAS Google Scholar - Angeli P, Gatta A, Caregaro L, Menon F, Sacerdoti D, Merkel C et al (1990) Tubular site of renal sodium retention in ascitic liver cirrhosis evaluated by lithium clearance. Eur J Clin Invest 20:111–117
Article PubMed CAS Google Scholar - Amir O, Hassan Y, Sarriff A, Awaisu A, Abd Aziz N, Ismail O (2009) Incidence of risk factors for developing hyperkalemia when using ACE inhibitors in cardiovascular diseases. Pharm World Sci 31:387–393
Article PubMed CAS Google Scholar - Sacerdoti D, Bolognesi M, Merkel C, Angeli P, Gatta A (1993) Renal vasoconstriction in cirrhosis evaluated by duplex Doppler ultrasonography. Hepatology 17:219–224
PubMed CAS Google Scholar - Wensing G, Lotterer E, Link I, Hahn EG, Fleig WE (1997) Urinary sodium balance in patients with cirrhosis: relationship to quantitative parameters of liver function. Hepatology 26:1149–1155
PubMed CAS Google Scholar - De Ledinghen V, Heresbach D, Fourdan O, Bernard P, Liebaert-Bories MP, Nousbaum JB et al (1999) Anti-inflammatory drugs and variceal bleeding: a case-control study. Gut 44:270–273
Article PubMed Google Scholar
Conflict of interest
None of the authors indicates a conflict of interest with this work.
Financial support
S.K. is supported by the Swiss National Science Foundation (31003A_132992/1).
Author information
Authors and Affiliations
- Division of Clinical Pharmacology and Toxicology, University Hospital, 4031, Basel, Switzerland
Carmen C. Franz, Sabin Egger, Christa Born, Alexandra E. Rätz Bravo & Stephan Krähenbühl - Regional Pharmacovigilance Center, University Hospital, Basel, Switzerland
Alexandra E. Rätz Bravo
Authors
- Carmen C. Franz
- Sabin Egger
- Christa Born
- Alexandra E. Rätz Bravo
- Stephan Krähenbühl
Corresponding author
Correspondence toStephan Krähenbühl.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Franz, C.C., Egger, S., Born, C. et al. Potential drug-drug interactions and adverse drug reactions in patients with liver cirrhosis.Eur J Clin Pharmacol 68, 179–188 (2012). https://doi.org/10.1007/s00228-011-1105-5
- Received: 04 May 2011
- Accepted: 21 July 2011
- Published: 13 August 2011
- Issue date: February 2012
- DOI: https://doi.org/10.1007/s00228-011-1105-5