Full ablation of C9orf72 in mice causes immune system-related pathology and neoplastic events but no motor neuron defects (original) (raw)
Non-coding hexanucleotide (GGGGCC) repeat expansions in C9ORF72 are the most common genetic cause of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD; C9ALS/FTD). Decreased C9orf72 protein levels in C9ALS/FTD patients [[4](/article/10.1007/s00401-016-1581-x#ref-CR4 "Waite AJ, Baumer D, East S et al (2014) Reduced C9orf72 protein levels in frontal cortex of amyotrophic lateral sclerosis and frontotemporal degeneration brain with the C9ORF72 hexanucleotide repeat expansion. Neurobiol Aging 35:1779.e5–1779.e13. doi: 10.1016/j.neurobiolaging.2014.01.016
")\] support the idea that C9ORF72 haploinsufficiency may contribute to disease pathogenesis. To test this hypothesis, we previously generated and analyzed neural-specific _C9orf72_ knockout mice. Our results showed that neural-specific ablation of C9orf72 (3110043O21Rik) in mice does not cause motor neuron degeneration or changes in motor function \[[3](/article/10.1007/s00401-016-1581-x#ref-CR3 "Koppers M, Blokhuis AM, Westeneng HJ et al (2015) C9orf72 ablation in mice does not cause motor neuron degeneration or motor deficits. Ann Neurol 78:426–438. doi:
10.1002/ana.24453
")\]. We therefore concluded that loss of C9ORF72 on its own is unlikely to cause ALS and that reducing C9ORF72 levels may comprise a promising strategy to treat C9-ALS patients. This therapeutic potential led us, and others \[[1](/article/10.1007/s00401-016-1581-x#ref-CR1 "Atanasio A, Decman V, White D et al (2016) C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci Rep 6:23204. doi:
10.1038/srep23204
"), [2](/article/10.1007/s00401-016-1581-x#ref-CR2 "O’Rourke JG, Bogdanik L, Yáñez A et al (2016) C9orf72 is required for proper macrophage and microglial function in mice. Science 351:1324–1329. doi:
10.1126/science.aaf1064
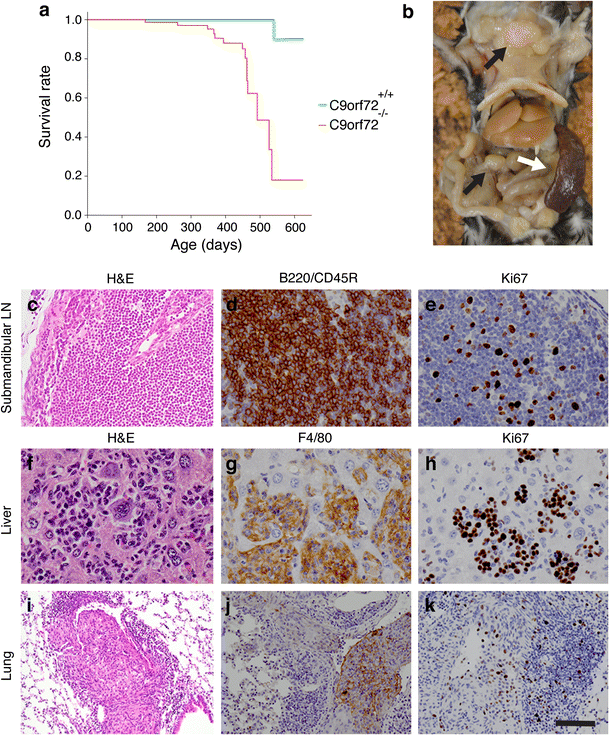
")\], to subsequently analyze knockout mice lacking C9orf72 in all tissues. Importantly, in contrast to our previous report, we find that full ablation of C9orf72 induces reduced survival (Fig. [1](/article/10.1007/s00401-016-1581-x#Fig1)a), which is in line with a recent study by Atanasio et al. \[[1](/article/10.1007/s00401-016-1581-x#ref-CR1 "Atanasio A, Decman V, White D et al (2016) C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci Rep 6:23204. doi:
10.1038/srep23204
")\] who report, but do not specify, decreased survival rates. In line with our previous observations \[[3](/article/10.1007/s00401-016-1581-x#ref-CR3 "Koppers M, Blokhuis AM, Westeneng HJ et al (2015) C9orf72 ablation in mice does not cause motor neuron degeneration or motor deficits. Ann Neurol 78:426–438. doi:
10.1002/ana.24453
")\], full C9orf72 ablation results in a 5.9 % decrease in body weight (_P_ \= 0.0056), without affecting motor function (accelerating rotarod and grip strength test) or inducing pathological hallmarks of ALS (see also \[[1](/article/10.1007/s00401-016-1581-x#ref-CR1 "Atanasio A, Decman V, White D et al (2016) C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci Rep 6:23204. doi:
10.1038/srep23204
"), [2](/article/10.1007/s00401-016-1581-x#ref-CR2 "O’Rourke JG, Bogdanik L, Yáñez A et al (2016) C9orf72 is required for proper macrophage and microglial function in mice. Science 351:1324–1329. doi:
10.1126/science.aaf1064
")\]), such as motor neuron degeneration, gliosis, enhanced ubiquitination and TDP-43 mislocalization. However, post-mortem analysis of full _C9orf72_ knockout mice (_n_ \= 5; 11–15 months of age) revealed enlarged lymph nodes (LNs) (_n_ \= 4 mice) and splenomegaly (_n_ \= 5) (Fig. [1](/article/10.1007/s00401-016-1581-x#Fig1)b). Detailed histological evaluation detected massive infiltration of histiocytes/macrophages and lymphocytes in multiple organs, including LNs, spleen, bone marrow, liver, kidney and lung (Fig. [1](/article/10.1007/s00401-016-1581-x#Fig1)c–k). In addition to these immunological phenotypes, which are in part also reported by Atanasio et al. \[[1](/article/10.1007/s00401-016-1581-x#ref-CR1 "Atanasio A, Decman V, White D et al (2016) C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci Rep 6:23204. doi:
10.1038/srep23204
")\] and O’Rourke et al. \[[2](/article/10.1007/s00401-016-1581-x#ref-CR2 "O’Rourke JG, Bogdanik L, Yáñez A et al (2016) C9orf72 is required for proper macrophage and microglial function in mice. Science 351:1324–1329. doi:
10.1126/science.aaf1064
")\], we detect evidence of neoplastic events. LNs of several animals (_n_ \= 4) contained infiltrates of B220/CD45R-positive B-lymphocytes that disrupted tissue architecture and were accompanied by increased expression of the proliferation marker Ki67, suggesting the development of B-cell lymphomas (Fig. [1](/article/10.1007/s00401-016-1581-x#Fig1)c–e). Furthermore, disrupted tissue architecture and homogeneous populations of F4/80-positive macrophages expressing Ki67 were present in LNs (_n_ \= 3), spleen (_n_ \= 3), liver (_n_ \= 1) and lung (_n_ \= 1). Moreover, infiltrating cells in the liver and lung accumulated in intravascular spaces (Fig. [1](/article/10.1007/s00401-016-1581-x#Fig1)f–k), suggesting the occurrence of metastatic histiocytic sarcomas. These results indicate that the defects in immune cell function recently reported in _C9orf72_ knockout mice (e.g. changes in endosome/lysosomal trafficking, cytokine production) \[[1](/article/10.1007/s00401-016-1581-x#ref-CR1 "Atanasio A, Decman V, White D et al (2016) C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci Rep 6:23204. doi:
10.1038/srep23204
"), [2](/article/10.1007/s00401-016-1581-x#ref-CR2 "O’Rourke JG, Bogdanik L, Yáñez A et al (2016) C9orf72 is required for proper macrophage and microglial function in mice. Science 351:1324–1329. doi:
10.1126/science.aaf1064
")\] may ultimately lead to neoplastic events in multiple organs. These findings have important implications as they indicate that strategies aimed at lowering systemic C9ORF72 levels in C9ALS/FTD patients may have negative side effects and that emphasis should be on therapeutic approaches that selectively target the hexanucleotide repeat expansions or their downstream pathogenic effects.Fig. 1
C9orf72 knockout mice display reduced survival, immune system-related pathology and neoplastic events. a Kaplan–Meier curves show survival rates corrected for gender and body weight. C9orf72 knockout mice show reduced survival as compared to littermate controls (Hazard ratio = 19.0; 95 %, CI: 2.4–150.2, P = 0.005). Wild-type controls n = 24; C9orf72 knockout n = 29. b Gross image showing enlarged lymph nodes (LNs; black arrows) and splenomegaly (white arrow) in a C9orf72 knockout mouse (12 months of age). c–e B-cell lymphoma in the submandibular LNs of C9orf72 knockout mouse. Nodal tissue is effaced by a monotypic cell population composed of B220/CD45R-positive lymphocytes (B cells). Note the high proliferation rate of the neoplastic lymphocytes as indicated by immunostaining for Ki67 (proliferation marker). f–h Histiocytic sarcoma in the liver of C9orf72 knockout mouse. Hepatic sinusoids are filled with atypical histiocytes and multinucleated giant cells that stain positive for the macrophage lineage marker F4/80 and exhibit a high proliferation rate, as evidenced by Ki67 immunostaining. i–k Histiocytic sarcoma in lung vasculature in C9orf72 knockout mouse. Pulmonary blood vessels are filled with atypical and multinucleated giant cells that immunostain for F4/80 and Ki67. H&E hematoxylin and eosin. Scale bar 1.3 cm (b), 65 μm (c), 40 μm (d–h), 125 μm (i), and 90 μm (j, k)
References
- Atanasio A, Decman V, White D et al (2016) C9orf72 ablation causes immune dysregulation characterized by leukocyte expansion, autoantibody production, and glomerulonephropathy in mice. Sci Rep 6:23204. doi:10.1038/srep23204
Article CAS PubMed PubMed Central Google Scholar - O’Rourke JG, Bogdanik L, Yáñez A et al (2016) C9orf72 is required for proper macrophage and microglial function in mice. Science 351:1324–1329. doi:10.1126/science.aaf1064
Article PubMed Google Scholar - Koppers M, Blokhuis AM, Westeneng HJ et al (2015) C9orf72 ablation in mice does not cause motor neuron degeneration or motor deficits. Ann Neurol 78:426–438. doi:10.1002/ana.24453
Article CAS PubMed PubMed Central Google Scholar - Waite AJ, Baumer D, East S et al (2014) Reduced C9orf72 protein levels in frontal cortex of amyotrophic lateral sclerosis and frontotemporal degeneration brain with the C9ORF72 hexanucleotide repeat expansion. Neurobiol Aging 35:1779.e5–1779.e13. doi:10.1016/j.neurobiolaging.2014.01.016
Article CAS Google Scholar
Acknowledgments
This study was supported by funding from the Netherlands Organization for Health Research and Development (NWO-VICI; LvdB and RJP); Thierry Latran Foundation (JHV and RJP), Prinses Beatrix Spierfonds (LvdB and RJP), Van Meer Stichting, and Netherlands ALS foundation (TOTALS; LvdB and RJP).
Author information
Authors and Affiliations
- Department of Translational Neuroscience, Brain Center Rudolf Magnus, University Medical Center Utrecht, Universiteitsweg 100, 3584 CG, Utrecht, The Netherlands
Emma Sudria-Lopez, Max Koppers, Marina de Wit, Christiaan van der Meer, Caroline A. C. Zundel & R. Jeroen Pasterkamp - Department of Neurology and Neurosurgery, Brain Center Rudolf Magnus, University Medical Center Utrecht, 3584 CX, Utrecht, The Netherlands
Max Koppers, Henk-Jan Westeneng, Jan H. Veldink & Leonard H. van den Berg - Department of Pathobiology, Faculty of Veterinary Medicine, Dutch Molecular Pathology Center, Utrecht University, 3584 CL, Utrecht, The Netherlands
Sameh A. Youssef, Liesbeth Harkema & Alain de Bruin - Division of Molecular Genetics, Department of Pediatrics, University Medical Center Groningen, 9713 AV, Groningen, The Netherlands
Alain de Bruin
Authors
- Emma Sudria-Lopez
- Max Koppers
- Marina de Wit
- Christiaan van der Meer
- Henk-Jan Westeneng
- Caroline A. C. Zundel
- Sameh A. Youssef
- Liesbeth Harkema
- Alain de Bruin
- Jan H. Veldink
- Leonard H. van den Berg
- R. Jeroen Pasterkamp
Corresponding author
Correspondence toR. Jeroen Pasterkamp.
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
L. H. van den Berg and R. J. Pasterkamp are joint senior authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sudria-Lopez, E., Koppers, M., de Wit, M. et al. Full ablation of C9orf72 in mice causes immune system-related pathology and neoplastic events but no motor neuron defects.Acta Neuropathol 132, 145–147 (2016). https://doi.org/10.1007/s00401-016-1581-x
- Received: 22 April 2016
- Revised: 11 May 2016
- Accepted: 11 May 2016
- Published: 20 May 2016
- Issue Date: July 2016
- DOI: https://doi.org/10.1007/s00401-016-1581-x