Changing epidemiology of Helicobacter pylori in Japan (original) (raw)
Abstract
Helicobacter pylori (H. Pylori) is known as the most important cause of gastric cancer. The prevalence of H. pylori infection varies widely by geographic area, age, and socioeconomic status. In Japan, H. pylori infection has been highly correlated with the incidence rate of gastric cancer, and a reduction in H. pylori infection is therefore crucial for decreasing the incidence of gastric cancer, especially at the population level. Infection occurs during childhood, commonly before 5 years of age. In Japan, where gastric cancer has ranked as the most common cancer by incidence and mortality for the last several decades, the prevalence of H. pylori infection has dramatically declined by birth cohort effect, mainly due to improvements in the general hygiene environment in childhood. Older generations born before around 1950 show a high prevalence of around 80–90 %, decreasing with age to reach around 10 % or less in those born around the 1990s, and less than 2 % for children born after the year 2000. This change will have generational effects on gastric cancer prevention strategies, both primary and secondary. The risk-stratified approach to gastric cancer prevention should be considered in Japan and other countries which have similarly experienced rapid economic development.
Similar content being viewed by others
Recent epidemiological trends in gastric cancer
A recent global estimate shows gastric cancer as the fifth-most common cancer worldwide, with 951,600 new cases in 2012 [1]. Occurrence was most frequent in the East Asian region. Globally, most developed countries and regions have observed a steady decline in this cancer with economic development and better sanitary conditions over the last century. A similar trend has also been observed in East Asia, including Japan.
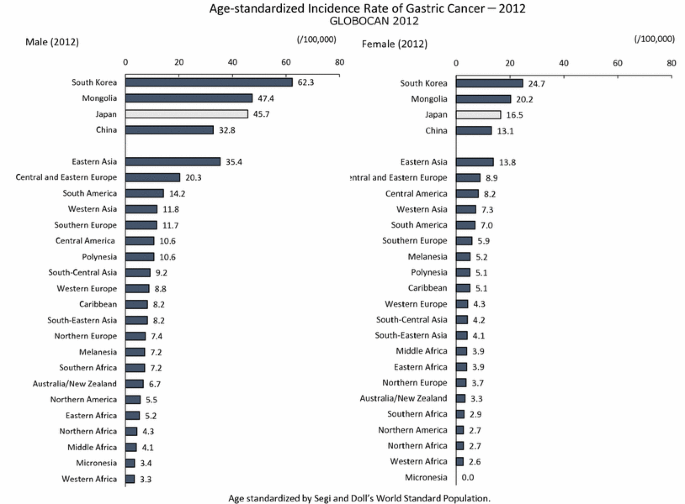
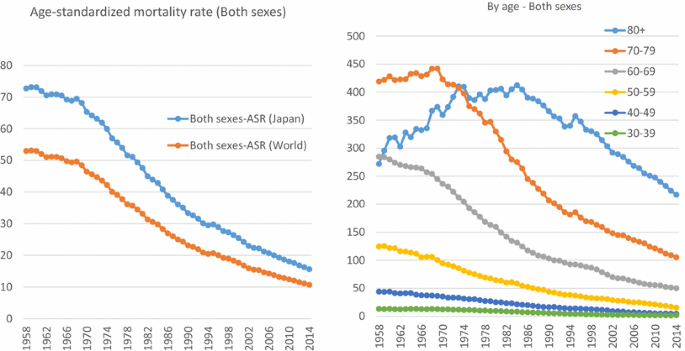
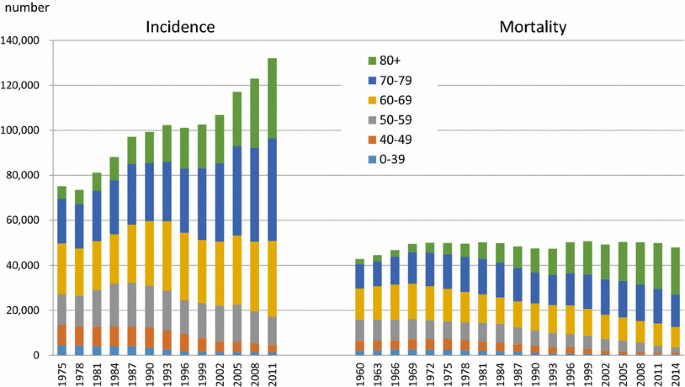
Japan no longer has the highest gastric cancer rate, as it once did, and the latest age-standardized incidence rate places Japan third, after South Korea and Mongolia [1] (Fig. 1). Gastric cancer rates in Japan have constantly declined over time in all age groups [2, 3] (Fig. 2). In spite of this decreasing trend, the number of incident cases has been increasing due to “aging”, and the number of deaths has remained unchanged. Both in incidence and mortality, we can clearly discern that most incident cases and deaths are in patients aged 60 years and over, and are prominent among those aged 70 years and over [2, 4] (Fig. 3). In short, gastric cancer in Japan has constantly declined, and has now became a “cancer of the elderly”. The question therefore arises as to whether this trend is caused merely by “aging” or by a birth-cohort effect; and if the latter, by what factor.
Fig. 1
Age-standardized Incidence Rate of Gastric Cancer-2012 (GLOBOCAN 2012, International Agency for Research on Cancer) [1]
Fig. 2
Data source: Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan http://ganjoho.jp/reg_stat/statistics/dl/index.html
Time trend of gastric cancer mortality in Japan. ASR (Japan): Age standardized by Japanese model population in 1985. ASR (World): Age standardized by Segi and Doll’s world standard population
Fig. 3
Data source: Cancer Registry and Statistics. Cancer Information Service, National Cancer Center, Japan http://ganjoho.jp/reg_stat/statistics/dl/index.html
Number of incidence and mortality of gastric cancer in Japan
Helicobacter pylori (H. pylori) infection
Needless to say, H. pylori is known as the most important cause of gastric cancer. H. pylori has been identified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC) [5, 6], and is typically associated with non-cardia gastric carcinoma. Approximately 90 % of non-cardia gastric cancer worldwide is estimated to be explained by this infection [7]. The pathogenic relationship between H. pylori and gastric cancer has been reported in various studies, and these have shown that _H. pylori_-associated carcinogenesis is primarily due to the gastric inflammatory response and resulting oxidative stress [6, 8, 9].
The prevalence of H. pylori infection varies widely by geographic area, age, and socioeconomic status. Prevalence in less developed regions may reach 70 % or higher, compared with 40 % or less in more developed regions. In Japan, as well as Eastern Asia in general, H. pylori infection has been highly correlated with incidence rate of gastric cancer [10], and a reduction in H. pylori infection is therefore crucial for decreasing the incidence of gastric cancer, especially at the population level. The incidence rate is generally more reflective of the prevalence of H. pylori in older age groups in Japan, which are prone to gastric cancer, and so the high prevalence of H. pylori in Japan in the elderly corresponds to a high incidence of gastric cancer [10]. In contrast, countries with low gastric cancer rates, such as Australia and the US, have a very low prevalence in younger age groups. Most countries with decreased gastric cancer rates show a lower prevalence of H. pylori in younger age groups [10].
A comparison of the prevalence of H. pylori infection in various Asian countries revealed that the prevalence in Japan shows a mixed pattern of developed and developing countries, namely a developed country pattern (low prevalence) for those aged under 29 years and a developing country pattern (high prevalence) for those aged over 40 [11]. Korea shows a similar pattern although the prevalence of H. pylori in younger age groups is slightly higher than in Japan [12]. Prevalence of H. pylori infection and gastric cancer rates do not necessarily correlate in other parts of the world, due to differences in the virulence factors of the bacteria [13]. Despite a high prevalence in young as well as old age groups in other countries in Eastern Asia (China, Mongolia), south-eastern Asia (Vietnam, Malaysia, Singapore, Brunei, Thailand, Myanmar), southern Asia (Bhutan, Bangladesh, Nepal, India), Central Asia (Kazakhstan, Iran, Iraq, Pakistan) and western Asia (Saudi Arabia, Turkey) [11], rates of gastric cancer are not high. Economic development and an improved hygienic environment may contribute to a decline in the prevalence of H. pylori infection in infancy and childhood. Furthermore, the prevalence trend of H. pylori in younger age groups is the key to predicting the future course of gastric cancer incidence in high-risk countries.
Effect of birth cohort in Japan
It is known that the acquisition of H. pylori infection occurs mostly under the age of 5 years. Infection status is suggested to be dependent on sanitary conditions during early childhood and lifestyle eating behavior (mouth to mouth transmission). Therefore, a sanitary environment around infancy greatly impacts the prevalence of H. pylori infection in adulthood [14]. Many reports on prevalence have reported their results by age group. For the reasons above, however, data by birth cohort or birth year rather than age group may facilitate understanding, and even help in predicting the future of gastric cancer.
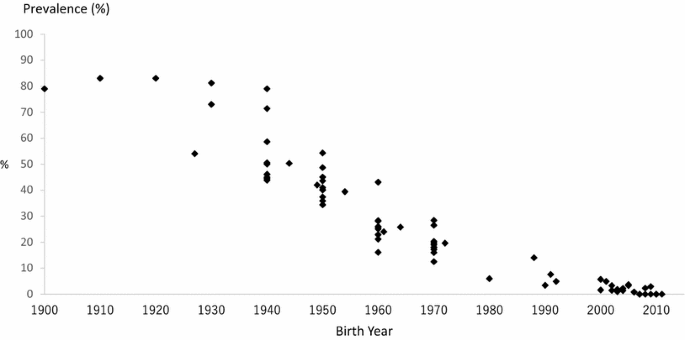
The earliest study on the H. pylori prevalence in Japanese asymptomatic population was reported by Asaka et al. [15], which showed ≈80 % for those born before 1950s,≈40 % for those born in 1950s, ≈25 % for those born in 1960s, ≈20 % for those born in 1970s and ≈5 % for those born in 1980s. Ueda et al. reported the prevalence of H. pylori infection based on health checkups in a multi-institutional study across Japan in 1997–2013, which broadly included Hokkaido, Aomori, Yamagata, Gunma, Aichi, Saga and Kagawa [16]. The study showed a clear decline by birth cohort, particularly in those born after 1950–1959. Similar declining trends in prevalence by birth-year were also reported by the HERPACC III Study (2005–2013) in Nagoya using joinpoint regression analysis [17]: the prevalence of H. pylori infection was 50 % for those born in 1920s, 40 % for those born in 1950s, 20 % for those born in 1960s, and 15 % for those born in 1980s. Tamura et al. reported that prevalence in an urban area of Japan (Nagoya) in 2008–2010 also showed clear decline, from 50 % for those born in 1940s to 20 % for those born in 1970s [18]. Hirayama et al. reported a similar prevalence using annual health check-up data from a major company in Japan (28 % overall; 18 % for those born in 1970s, 23 % for those born in 1960s, 37 % for those born in 1950s, and 46 % for those born in 1940s), with no clear difference by sex [19].
Akamatsu et al. examined the prevalence of H. pylori in Japanese high school students [20] and found the low prevalence of H. pylori, 5 % for those born around 1990. Okuda et al. targeted Japanese children born after 2000 [21] and reported a very low prevalence for those born between 2000 and 2010. This study reported that H. pylori prevalence in Japanese children is less than 2 %; parent-to-child infection is thought to be the main infection route; and that the possibility of new infection other than by vertical infection in Japanese children is low.
When taken together, older generations born before around 1950 show a high prevalence of around 80–90 %. Prevalence decreases with age to reach around 10–20 % in those born during the 1980s, 5 % in those born around 1990 and less than 2 % in children born after 2000 (Fig. 4) [15–21].
Fig. 4
Prevalence of Helicobacter pylori infection in Japan by birth-year
Conclusion
This accumulating evidence indicates that the prevalence of H. pylori infection in Japan will undoubtedly decline substantially in future in all age groups, to the extent that it will be recognized as a rare event. This change will have generational effects on gastric cancer prevention strategies, both primary and secondary. The risk-stratified approach to gastric cancer prevention should be considered in Japan [22] and other countries which have similarly experienced rapid economic development.
References
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F, GLOBOCAN 2012 v1.0. Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet]. Lyon: International Agency for Research on Cancer; 2013.
Google Scholar - Vital Statistics. The Minsitry of Health, Labour, Welfare, Japan.
- Katanoda K, Hori M, Matsuda T, Shibata A, Nishino Y, Hattori M, Soda M, Ioka A, Sobue T, Nishimoto H. An updated report on the trends in cancer incidence and mortality in Japan, 1958–2013. Jpn J Clin Oncol. 2015;45:390–401.
Article PubMed Google Scholar - Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, Japan Cancer Surveillance Research G. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884–91.
Article PubMed Google Scholar - Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V, Group WHOIAfRoCMW. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009;10:321–2.
Article PubMed Google Scholar - IARC. A Review of Human Carcinogens. B. Biological Agents. Lyon: WHO Press; 2012.
Google Scholar - Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Article PubMed Google Scholar - Suzuki H, Hibi T, Marshall BJ. Helicobacter pylori: present status and future prospects in Japan. J Gastroenterol. 2007;42:1–15.
Article PubMed PubMed Central Google Scholar - IARC. IARC Helicobacter pylori Working Group. Helicobacter pylori Eradication as a strategy for preventing gastric cancer. In: IARC Working Group reports volume 8. Lyon: international Agency for Research on Cancer; 2014. pp. 1–181.
- Peleteiro B, Bastos A, Ferro A, Lunet N. Prevalence of Helicobacter pylori infection worldwide: a systematic review of studies with national coverage. Dig Dis Sci. 2014;59:1698–709.
Article PubMed Google Scholar - Matsuhisa T. The trend of Helicobacter pylori infection rate: time, region, race and strain. Helicobacter Res. 2015;19:469–80.
Google Scholar - Lim SH, Kwon JW, Kim N, Kim GH, Kang JM, Park MJ, Yim JY, Kim HU, Baik GH, Seo GS, Shin JE, Joo YE, Kim JS, Jung HC. Prevalence and risk factors of Helicobacter pylori infection in Korea: nationwide multicenter study over 13 years. BMC Gastroenterol. 2013;13:104.
Article PubMed PubMed Central Google Scholar - Wu CY, Lin JT. The changing epidemiology of Asian digestive cancers: from etiologies and incidences to preventive strategies. Best Pract Res Clin Gastroenterol. 2015;29:843–53.
Article PubMed Google Scholar - Drumm B, Rowland M. The epidemiology of Helicobacter pylori: where to from here? J Pediatr Gastroenterol Nutr. 2003;36:7–8.
Article PubMed Google Scholar - Asaka M, Kimura T, Kudo M, Takeda H, Mitani S, Miyazaki T, Miki K, Graham DY. Relationship of Helicobacter pylori to serum pepsinogens in an asymptomatic Japanese population. Gastroenterology. 1992;102:760–6.
Article CAS PubMed Google Scholar - Ueda J, Gosho M, Inui Y, Matsuda T, Sakakibara M, Mabe K, Nakajima S, Shimoyama T, Yasuda M, Kawai T, Murakami K, Kamada T, Mizuno M, Kikuchi S, Lin Y, Kato M. Prevalence of Helicobacter pylori infection by birth-year and geographic area in Japan. Helicobacter. 2014;19:105–10.
Article PubMed Google Scholar - Watanabe M, Ito H, Hosono S, Oze I, Ashida C, Tajima K, Katoh H, Matsuo K, Tanaka H. Declining trends in prevalence of Helicobacter pylori infection by birth-year in a Japanese population. Cancer Sci. 2015;106:1738–43.
Article CAS PubMed PubMed Central Google Scholar - Tamura T, Morita E, Kondo T, Ueyama J, Tanaka T, Kida Y, Hori Y, Inoue S, Tomita K, Okada R, Kawai S, Hishida A, Naito M, Wakai K, Hamajima N. Prevalence of Helicobacter pylori infection measured with urinary antibody in an urban area of Japan, 2008–2010. Nagoya J Med Sci. 2012;74:63–70.
PubMed PubMed Central Google Scholar - Hirayama Y, Kawai T, Otaki J, Kawakami K, Harada Y. Prevalence of Helicobacter pylori infection with healthy subjects in Japan. J Gastroenterol Hepatol. 2014;29(Suppl 4):16–9.
Article PubMed Google Scholar - Akamatsu T, Ichikawa S, Okudaira S, Yokosawa S, Iwaya Y, Suga T, Ota H, Tanaka E. Introduction of an examination and treatment for Helicobacter pylori infection in high school health screening. J Gastroenterol. 2011;46:1353–60.
Article PubMed Google Scholar - Okuda M, Osaki T, Lin Y, Yonezawa H, Maekawa K, Kamiya S, Fukuda Y, Kikuchi S. Low prevalence and incidence of Helicobacter pylori infection in children: a population-based study in Japan. Helicobacter. 2015;20:133–8.
Article PubMed Google Scholar - Inoue S. Stratification of gastric cancer risk by H. pylori infection. In: Suzuki H, Warren R, Marshall B, editors. Helicobacter pylori. Tokyo: Springer Japan; 2016. p.169–179.
Author information
Authors and Affiliations
- AXA Department of Health and Human Security, Graduate School of Medicine, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan
Manami Inoue - Prevention Division, Center for Public Health Sciences, National Cancer Center, 5-1-1 Tsukiji, Chuo-ku, Tokyo, 104-0045, Japan
Manami Inoue
Corresponding author
Correspondence toManami Inoue.
Ethics declarations
Compliance with ethical standards
This is a review article of published studies and the article does not contain any studies with human or animal subjects performed by the author.
Conflict of interest
Manami Inoue is the beneficiary of a financial contribution from the AXA Research fund as chair holder of the AXA Department of Health and Human Security, Graduate School of Medicine, The University of Tokyo, from November 1, 2012. The AXA Research Fund has no role in this work.
Rights and permissions
About this article
Cite this article
Inoue, M. Changing epidemiology of Helicobacter pylori in Japan.Gastric Cancer 20 (Suppl 1), 3–7 (2017). https://doi.org/10.1007/s10120-016-0658-5
- Received: 30 August 2016
- Accepted: 05 October 2016
- Published: 18 October 2016
- Issue date: March 2017
- DOI: https://doi.org/10.1007/s10120-016-0658-5