Relapse of nephrotic syndrome during post-rituximab peripheral blood B-lymphocyte depletion (original) (raw)
Abstract
Background
Rituximab is effective against complicated childhood steroid-dependent nephrotic syndrome (SDNS). Peripheral blood B-lymphocyte (B-cell) depletion is strongly correlated with persistent remission, relapse rarely occurring during B-cell depletion; however, we have encountered several such patients.
Methods
We retrospectively analyzed the characteristics and clinical course of 82 patients with SDNS treated with rituximab from January 2007 to December 2012 in our institution.
Results
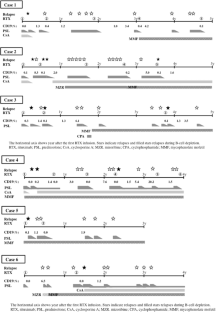
Six of 82 patients (7.3%) had relapses during B-cell depletion after receiving rituximab (relapsed group). The remaining 76 patients did not have relapses during B-cell depletion (non-relapsed group). The median time to initial relapse during B-cell depletion was 85 days after receiving rituximab, which is significantly shorter than in the non-relapsed group (410 days, p = 0.0003). The median annual numbers of relapses after receiving rituximab were 2.5 and 0.9 in the relapsed and non-relapsed groups, respectively (p < 0.0001). Five patients in the relapsed group also had a total of 10 relapses after B-cell recovery; their median time from B-cell recovery to initial relapse was significantly shorter than in the non-relapsed group (31 vs. 161 days, p = 0.014). Number of relapses before rituximab, history of steroid resistance, onset age, previous treatment, time to ceasing steroids after rituximab, and duration of B-cell depletion did not differ between the two groups.
Conclusion
Relapse during B-cell depletion after receiving rituximab suggests that various pathophysiological mechanisms play a part in childhood nephrotic syndrome.
Access this article
Subscribe and save
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime Subscribe now
Buy Now
Price excludes VAT (USA)
Tax calculation will be finalised during checkout.
Instant access to the full article PDF.
Fig. 1
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.
References
- Bagga A, Sinha A, Moudgil A. Rituximab in patients with the steroid resistant nephrotic syndrome. N Engl J Med. 2007;356(26):2751–2. doi:10.1056/NEJMc063706.
Article CAS PubMed Google Scholar - Guigonis V, Dallocchio A, Baudouin V, et al. Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol. 2008;23(8):1269–79. doi:10.1007/s00467-008-0814-1.
Article PubMed Google Scholar - Kamei K, Ito S, Nozu K, Fujinaga S, et al. Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr Nephrol. 2009;24(7):1321–8. doi:10.1007/s00467-009-1191-0.
Article PubMed Google Scholar - Kamei K, Ogura M, Sato M, Sako M, Iijima K, Ito S. Risk factors for relapse and long-term outcome in steroid-dependent nephrotic syndrome treated with rituximab. Pediatr Nephrol. 2016;31(1):89–95. doi:10.1007/s00467-015-3197-0.
Article PubMed Google Scholar - Sato M, Ito S, Ogura M, Kamei K. Impact of rituximab on height and weight in children with refractory steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2014;29(8):1373–9. doi:10.1007/s00467-014-2792-9.
Article PubMed Google Scholar - Iijima K, Sako M, Nozu K, Rituximab for Childhood-onset Refractory Nephrotic Syndrome (RCRNS) Study Group, et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2014;384(9950):1273–81. doi:10.1016/S0140-6736(14)60541-9.
Article CAS PubMed Google Scholar - Ravani P, Magnasco A, Edefonti A, et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6(6):1308–15. doi:10.2215/CJN.09421010.
Article CAS PubMed PubMed Central Google Scholar - Sellier-Leclerc AL, Baudouin V, Kwon T, et al. Rituximab in steroid-dependent idiopathic nephrotic syndrome in childhood-follow-up after CD19 recovery. Nephrol Dial Transplant. 2012;27(3):1083–9. doi:10.1093/ndt/gfr405.
Article CAS PubMed Google Scholar - ISKDC: The primary nephrotic syndrome in children. Identification of patients with minimal change nephrontic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr. 1981;98(4):561–4.
- Hugle B, Solomon M, Harvey E, et al. Pneumocystis jiroveci pneumonia following rituximab treatment in Wegener’s granulomatosis. Arthritis Care Res. 2010;62(11):1661–4. doi:10.1002/acr.20279.
Article Google Scholar - Koyama A, Fujisaki M, Kobayashi M, Igarashi M, Narita M. A glomerular permeability factor produced by human T cell hybridomas. Kidney Int. 1991;40(3):453–60. doi:10.1038/ki.1991.232.
Article CAS PubMed Google Scholar - Vallerskog T, Gunnarsson I, Widhe M, et al. Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with S.L.E. Clin Immunol. 2007;122(1):62–74. doi:10.1016/j.clim.2006.08.016.
Article CAS PubMed Google Scholar - Hashimura Y, Nozu K, Kanda K, et al. Minimal change nephrotic syndrome associated with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Pediatr Nephrol. 2009;24(6):1181–6. doi:10.1007/s00467-009-1119-8.
Article PubMed Google Scholar - Prasad N, Jaiswal AK, Agarwal V, et al. Differential alteration in peripheral T-regulatory and T-effector cells with change in P-glycoprotein expression in childhood nephrotic syndrome: a longitudinal study. Cytokine. 2015;72(2):190–6. doi:10.1016/j.cyto.2014.12.028.
Article CAS PubMed Google Scholar - Bertelli R, Bonanni A, Di Donato A, Cioni M, Ravani P, Ghiggeri GM. Regulatory T cells and minimal change nephropathy: in the midst of a complex network. Clin Exp Immunol. 2016;183(2):166–74. doi:10.1111/cei.12675.
Article CAS PubMed Google Scholar - Stasi R, Cooper N, Del Poeta G, et al. Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B-cell depleting therapy with rituximab. Blood. 2008;112(4):1147–50. doi:10.1182/blood-2007-12-129262.
Article CAS PubMed Google Scholar - Vigna-Perez M, Hernández-Castro B, Paredes-Saharopulos O, et al. Clinical and immunological effects of rituximab in patients with lupus nephritis refractory to conventional therapy: a pilot study. Arthritis Res Ther. 2006;8:R83. doi:10.1186/ar1954.
Article PubMed PubMed Central Google Scholar - Sfikakis PP, Souliotis VL, Fragiadaki KG, Moutsopoulos HM, Boletis JN, Theofilopoulos AN. Increased expression of the FoxP3 functional marker of regulatory T cells following B-cell depletion with rituximab in patients with lupus nephritis. Clin Immunol. 2007;123(1):66–73. doi:10.1016/j.clim.2006.12.006.
Article CAS PubMed Google Scholar - Saadoun D, Rosenzwajg M, Landau D, Piette JC, Klatzmann D, Cacoub P. Restoration of peripheral immune hemostasis after rituximab in mixed cryoglobulinemia vasculitis. Blood. 2008;111(11):5334–41. doi:10.1182/blood-2007-11-122713.
Article CAS PubMed Google Scholar - Sfikakis PP, Boletis JN, Lionaki S, et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52(2):501–13. doi:10.1002/art.20858.
Article CAS PubMed Google Scholar - Tokunaga M, Fujii K, Saito K, et al. Down-regulation of CD40 and CD80 on B cells in patients with life-threatening systemic lupus erythematosus after successful treatment with rituximab. Rheumatology. 2005;44(2):176–82. doi:10.1093/rheumatology/keh443.
Article CAS PubMed Google Scholar - Ng CM, Bruno R, Combs D, Davies B. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol. 2005;45(7):792–801. doi:10.1177/0091270005277075.
Article CAS PubMed Google Scholar - Breedveld F, Agarwal S, Yin M, et al. Rituximab pharmacokinetics in patients with rheumatoid arthritis: B-cell levels do not correlate with clinical response. J Clin Pharmacol. 2007;47(9):1119–28. doi:10.1177/0091270007305297.
Article CAS PubMed Google Scholar - Berinstein NL, Grillo-López AJ, White CA, et al. Association of serum rituximab concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin’s lymphoma. Ann Oncol. 1998;9(9):995–1001.
Article CAS PubMed Google Scholar - Fujinaga S, Hirano D, Nishizaki N et al. Single infusion of rituximab for persistent steroid-dependent minimal-change nephrotic syndrome after long-term cyclosporine. Pediatr Nephrol. 2010;25(39):539–4. doi:10.1007/s00467-009-1377-5.
- Ramos EJ, Pollinger HS, Stegall MD, Gloor JM, Dogan A, Grande JP. The effect of desensitization protocols on human splenic B-cell populations in vivo. Am J Transplant. 2007;7(2):402–7. doi:10.1111/j.1600-6143.2006.01632.x.
Article CAS PubMed Google Scholar - Boumans MJ, Tak PP. Rituximab treatment in rheumatoid arthritis: How does it work? Arthritis Res Ther. 2009;11(6):134. doi:10.1186/ar2852.
- McDonald V, Manns K, Mackie IJ, Machin SJ, Scully MA. Rituximab pharmacokinetics during the management of acute idiopathic thrombotic thrombocytopenic purpura. J Thromb Haemost. 2010;8(6):1201–8. doi:10.1111/j.1538-7836.2010.03818.x.
Article CAS PubMed Google Scholar
Acknowledgements
Edanz Group provided language editorial support in the preparation of the manuscript.
Author information
Authors and Affiliations
- Division of Nephrology and Rheumatology, National Center for Child Health and Development, 2-10-1 Okura, Setagaya-Ku, Tokyo, 157-8535, Japan
Mai Sato, Koichi Kamei, Masao Ogura & Kenji Ishikura - Department of Pediatrics, Yokohama City University Hospital, Graduate School of Medicine, 3-9 Fukuura, Kanazawa-Ku, Yokohama, 236-0004, Japan
Shuichi Ito
Authors
- Mai Sato
- Koichi Kamei
- Masao Ogura
- Kenji Ishikura
- Shuichi Ito
Contributions
MS prepared the manuscript; MO and KK collected the clinical data; KI revised the article; SI oversaw the work as the corresponding author and revised the article.
Corresponding author
Correspondence toShuichi Ito.
Ethics declarations
Funding statement
There is no funding to report for this submission.
Conflict of interest
MS and MO have no financial relationships relevant to this article to disclose. KK has received lecture fees from Chugai Pharma. KI has received lecture fees and travel expenses from Novartis Pharma and Asahi Kasei Pharma. SI has received lecture fees and travel expenses from Novartis Pharma, Asahi Kasei Pharma, Astellas Pharma and Chugai Pharma.
Research involving human participants and/or animals
This study was conducted in accordance with the principles of the Declaration of Helsinki and the ethical guidelines issued by the Ministry of Health, Labour and Welfare, Japan. The study was approved by a central ethics board at National Center for Child Health and Development (Approval number: 1316).
Informed consent
Because reported data were extracted from patients’ medical records, informed consent for participation in the study was not required.
About this article
Cite this article
Sato, M., Kamei, K., Ogura, M. et al. Relapse of nephrotic syndrome during post-rituximab peripheral blood B-lymphocyte depletion.Clin Exp Nephrol 22, 110–116 (2018). https://doi.org/10.1007/s10157-017-1415-8
- Received: 30 March 2017
- Accepted: 16 April 2017
- Published: 22 April 2017
- Issue Date: February 2018
- DOI: https://doi.org/10.1007/s10157-017-1415-8