Characterization of polyethylene synthesized by zirconium single site catalysts (original) (raw)
Abstract
New non-metallocene, bridged-zirconium center catalysts were prepared by Schiff base condensations reaction of two equivalents of appropriate 2-hydroxybenzaldehyde with one equivalent of 4,4′-methylene bis(2-methylcyclohexylamine), and subsequent metathesis reaction with ZrCl4(THF)2, and tested for ethylene polymerization reaction at different conditions. Catalyst productivities were found to be high, in the range of 29.5 × 103 kg/mole·Zr·h to 2.3 × 103 kg/mole·Zr·h, dependents on the polymerization condition. The molecular weight of the product, i.e. polyethylene, varied from 397,000 to 988,000. 13C NMR study of the polymers indicate that fraction of branches and end groups percentage range from 0.01 to 0.55. X-ray diffraction analysis showed that polyethylene samples had highly crystalline structure within the orthorhombic space group (Pnam) and carbon–carbon interatomic distance of 0.154061 nm and the C–C–C angle of 112.192°.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.
Introduction
The area of olefin polymerization catalysis has endured a remarkable increase in research outcome over the past 20 years, with many academic and industrial research centers involving in the design of new organometallic precatalysts for the controlled synthesis of polyolefin products. During that period, studies on the Group 4 metallocenes afforded much needed insight into the nature of the activated species. During the first half of the 1990s, interest grew in developing new generation “non-metallocene” catalysts, partly to avoid the growing patent minefield in Group 4 cyclopentadienyl systems, but also because late transition metal systems also offered the commercially attractive possibility of being able to incorporate polar comonomers into polyolefin materials, to give modified surface properties at low levels of comonomer incorporation, to change the bulk properties of the polyolefin at higher levels of incorporation.
The potential for this approach was realized with the discovery in the late 1990s of highly active ethylene polymerization catalysts based on iron, a metal with no previous track record in olefin polymerization. Another significant development over the past years or so has been the introduction of systems capable of catalyzing the living polymerization of olefinic monomers. The absence of chain-transfer or chain-termination processes allows access to polyolefinic materials with very high molecular weight (>600,000) and narrow molecular weight distributions (typically <1.1), block copolymers, and polymers with novel topologies [1, 2]. Ultrahigh molecular weight polyethylene is an important material for production of high performance fibers where the polymer chains are extended and aligned as in the crystalline material. The performance of polymers is improved as the chain length and crystallinity are improved [3].
Polyethylene is the simplest possible polymer; however, the simplicity of the molecular formula (–CH2–) n does not reflect the real complexity of the material and its properties. Polyethylene is generally synthesized as a mixture of crystalline and amorphous components. The physical properties of the polyethylene are usually described using at least a two-phase model consisting of crystalline blocks in an amorphous matrix. [4] The crystalline phase ranges from around 50 to >98 % and its concentration generally increases with increasing molecular weight and decreases with increasing chain branching. As the temperature of a polymer is raised, the percentage of crystallinity diminishes and above the melting point the structure is entirely amorphous, and consists of a viscous liquid.
Polycrystalline polyethylene forms chained molecules that are distributed on lattice points with a rigorous periodicity, thus forming a long-range order crystalline structure. For the first time the basic structural features of polyethylene were determined, by means of powder diffraction, in 1939 by Bunn [5] who found that polyethylene crystallizes in the orthorhombic structure (space group Pnam) with two polymeric chains per unit cell. The orthorhombic unit cell, which is identical to the crystal structure of straight-chain alkanes, contains four CH2 groups. Paraffin’s are identical with polyethylene in regard to their molecular structure except for their molecular length; they crystallize in the same crystal form as the polyethylene analogues.
13C NMR and X-ray powder diffraction (XRD) techniques are widely used in polymer science. Solution and melt 13C NMR has been established as a useful tool for a quantitative determination of branch content of short- and long-chain branched polyethylenes while XRD can reveal important information on the morphology, structure and the degree of crystallinity of polythene [6–8]. In the present paper we report the synthesis of high molecular weight polyethylene using Zr-based single site catalyst and results of the use of melt-state 13C MAS NMR and X-ray diffraction techniques for the determination of branch content of long-chain polyethylene’s and their crystal structure.
Experimental
All operations involving air sensitive reagents and materials during catalysts precursor synthesis were carried out under nitrogen atmosphere using standard Schlenk, vacuum or dry box techniques. Solvents were dried and distilled under nitrogen prior to use of by passing through a column of activated alumina and a supported copper (Q5) catalyst. Imino-ligands were synthesized by condensation of two equivalents of appropriate 2-hydroxybenzaldehyde with one equivalent of 4, 4′-methylene bis(2-methylcyclohexylamine), and subsequent metathesis with ZrCl4(THF)2 [9].
The polymerization reactions were performed in stainless steel 1 L reactor, Polyclave Buchi glass uster. Solvent (Toluene) and MAO were loaded in the reactor in a glove box and removed after sealed. The reactor then was perched with ethylene. After reaching the reaction temperatures and pressure, catalyst was injected into the reactor. The polymerization product was transferred to 1 L flask with 100 mL methanol (5 % HCl). Polymer was isolated by filtration and washed with acidic methanol and dried under vacuum [10].
13C MAS NMR measurements of melted polymers were performed using Varian Unity Inova 300 NMR Spectrometer operated at 75 MHz frequency and 2.58 kHz spin rate. Powdered polyethylene samples were placed into 7.5 mm ceramic rotors and heated to 170 °C during measurements. 13C spectra were recorded with acquisition time of 100.0 ms, 90° pulse angle, 5 s recycle delay and 2,070 scans per spectra. Branch content was determined by numerical peak integration of branching 13C NMR signals.
X-ray powder diffraction patterns of polymers were recorded using a Philips PW3040/60 X’pert PRO Console diffractometer equipped with a focus graphite monochromator and two Soller slits, 1 mm divergence slit and a 0.2 receiving slit. The spectra were recorded using Cu_K_α1 (1.5406 Å) incident radiation; the tube voltage was 45 mV and tube current was 40 mA. The scan diffraction angle varied from 5° to 100° 2_θ_ angular range and the samples were scanned at a rate of 0.008°/min in 2_θ_; the collection time was 3 h. The spectra were recorded in air at 25 °C using sample holders with a 1 mm deep depression filled with polymer powders. The sample holder was rotated at a rate of 0.5 Hz to enhance scattering signal.
Results and discussions
The ethylene polymerization activity of the zirconium dihalide complexes was investigated using MAO as co-catalyst. The activity of imino- of bridged-zirconium catalysts was found to be 29.5 × 103 to 2.3 × 103 kg mmol−1 h−1 Zr−1, which is considerably higher than the activities seen from (alpha-dimines) ZrCl2 mononuclear catalysts [11]. The molecular weight of the polyethylene product is also significantly larger ranging between 397,000 and 988,000 g/mol (Table 1).
Table 1 Polymerization conditions, molecular weight and fraction of branches and end groups in several polyethylene samples
Results of 13C MAS NMR of a series of molten polymers performed at 170 °C are shown in the last column of the Table 1. The total fraction of branches and end groups of main chains was determined by calculating the ratio between the area of peak at around 14.2 ppm (corresponding to CH3 group shown as 1 in the scheme below) to the area of main carbon peak at 30 ppm, which corresponds to long-chain CH2 groups (δ and further carbons in the scheme below). Peaks corresponding to carbons 2 and 3 (23 and 32.3 ppm, respectively) are visible in all spectra and their observed intensities are the same as those for terminal carbon labeled as 1.
As seen from Table 1, the fraction of branches and end groups correlates well with molecular weight of polymers. Samples for tests 1, 5 and 6 have smallest fraction of branches and the largest molecular weight.
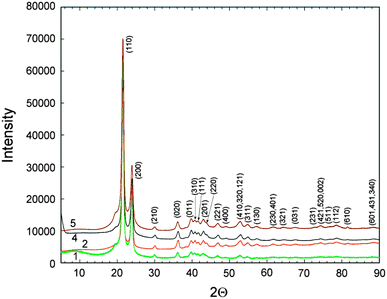
Typical results of X-ray diffraction measurements of polyethylene samples are presented in Fig. 1. All samples exhibit very similar diffraction patterns with sharp high-intensity peaks at 21.5° and 23.9° in 2_θ_ and several lower intensity peaks in the range of 30°–55° and 74°–82° in 2_θ_. The spectra in the range of 90°–100° are not shown in Fig. 1 because they do not contain any well-defined diffraction peaks. For all the samples studied it was found that there are no appreciable changes in the position and the width of crystalline peaks.
Fig. 1
XRD spectra of long-chain polyethylene samples 1, 2, 4 and 5 with all the diffraction peaks indexed within the Pnam space group
The indexing of polyethylene XRD pattern is presented in Fig. 1 and agrees well with literature data [12]. All well defined peaks are indexed within the Pnam space group with the only exception of a weak shoulder at around 19° in 2_θ_ which cannot be assigned to any hkl index within this space group. Some authors attribute the peaked feature of the maximum at about 19° in 2_θ_ to the presence of a small amount of monoclinic polyethylene [13]. However, according to Fontana et al. [14] the monoclinic polyethylene phase can be formed only at high pressures (around 6 GPa) and the formation of this crystallographic modification during polyethylene synthesis (10–40 bar) is very unlikely.
It should be noted that both crystalline orthorhombic polyethylene and amorphous polyethylene exhibit a diffraction peak at 19° in 2_θ_ [15] and from our point of view, the weak shoulder at 19° corresponds to a broad peak of amorphous or semi-amorphous polyethylene phase.
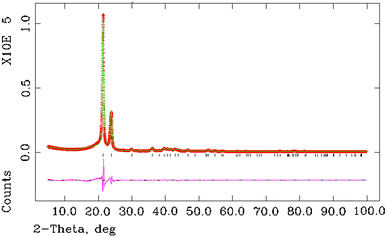
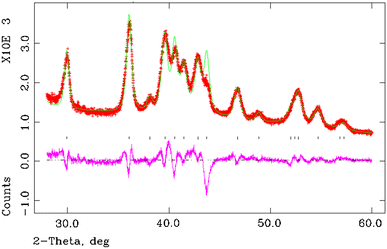
Rietveld refinement of XRD spectra was performed using the structure refinement program GSAS [16], with the orthorhombic space group (Pnam) of crystalline polyethylene reported by Bunn [17] as the structural model. The peak shape was fitted with a convoluted pseudo-Voigt function to allow for broadening induced though finite crystallites. The preferred orientation of the films was not accounted for at this stage and it was assumed that the crystallite orientation in the polyethylene powders is completely random. The results of the Rietveld refinement of sample 5 are shown in Fig. 2 over the range of 5° to 100° in 2_θ_. Figure 3 displays refinement results over an enlarged area between 28° and 60° in 2_θ_. It is seen that there is a very good agreement between experimental and calculates intensities and positions of X-ray peaks with the exception of one peak at 43.5° in 2_θ_ (Fig. 3).
Fig. 2
Rietveld refinement of XRD spectrum of polyethylene sample 5. Red crosses experimental X-ray diffraction points, green line Rietveld refinement results, pink line difference line, black ticks calculated position of X-ray peaks
Fig. 3
Rietveld refinement of XRD spectrum of polyethylene sample 5. Enlarged area between 28° and 60° in 2_θ_ is shown
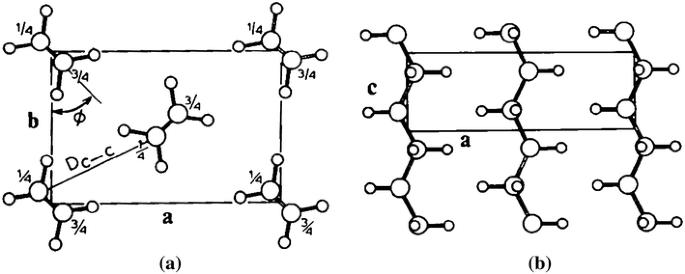
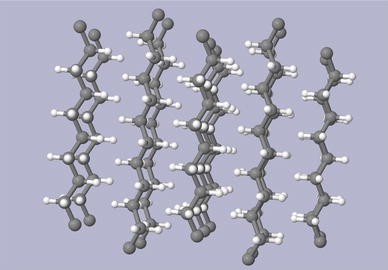
Figures 4a and b depict the projections of packing of orthorhombic polyethylene chains along the c and b axes, respectively. The two planes containing the skeletal carbon chains within the unit cell are almost orthogonal to each other while all-trans chains are parallel, as seen in Fig. 4a. It has been shown that the setting angle of the molecular packing φ, i.e. the angle between the plane containing the ···C–C–C··· zigzag molecular chains and the b axis of the unit cell (see Fig. 3a), is in the range 41°–46° [5, 17]. Such differences may be attributed to the presence of an amorphous component that may significantly distort the diffraction profile in both peak position and intensity [18]. While the crystalline constituent gives sharp diffraction peaks, the amorphous phase produces three broad diffuse peaks at around 19°, 41° and 80° in 2_Θ_ for Cu_K_α radiation.
Fig. 4
Projection of polyethylene packing along the c axis a and along the b axis b. Polymer chain axes are parallel to axis c
The orthorhombic unit cell of branched polyethylene is expanded along axis a and, to a smaller degree, along axis b. At room temperature the unit cell parameters or linear polyethylene are a = 0.74069 nm, b = 0.49491 nm and c = 0.25511 nm [19]. The difference in a unit cell parameters might be due to a different degree of branching in polyethylene samples used by different authors.
With rising temperature the dimensions of the a and b axes change, while the c dimension does not change appreciably since it is determined by the strong intra-chain covalent C–C and C–H bonds unlike weak inter-chain van der Waals interactions which determine lattice parameters along the a and b axes. Due to a significant difference in the attractive bond forces between atoms parallel and perpendicular to the chain axis c the orthorhombic crystal phase of polyethylene, like most other crystalline polymers, has pronounced anisotropy of mechanical, optical and dielectric properties.
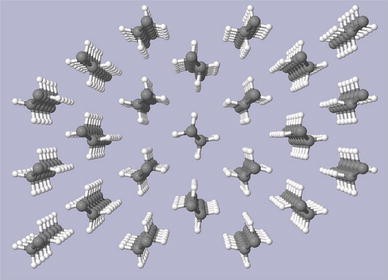
The simulated crystal structure of polyethylene sample 5 along and perpendicular to the c axis is presented in Figs. 5 and 6.
Fig. 5
Simulated crystal structure of polyethylene sample 5 along the c axis
Fig. 6
Simulated crystal structure of polyethylene sample 5 perpendicular to axis c
The calculated lattice parameters of sample 5 are as follows: a = 0.74510 ± 0.00003 nm, b = 0.49724 ± 0.00002 nm and c = 0.25573 ± 0.00004 nm. The lattice parameters a and b are strongly affected by nonbonded interactions between the chains and, potentially, by the degree of branching. The c lattice parameter of polyethylene sample 5 which is mainly determined by the length of ···C–C–C··· zigzag chains is very close to the literature data for polyethylene samples [19] while a and b lattice parameters in sample 5 are significantly larger. On the basis of our results on 13C MAS NMR investigation which shoved very small degree of branching (~0.01 %) in this sample it is unlikely that branching can explain larger values of both a and b lattice parameters in sample 5 comparing to those reported in the literature, for example a = 0.74241(7) nm and b = 0.49491(5) nm; c = 0.25534(1) nm [13]. One of the possible reasons could be the fact that literature X-ray diffraction data were obtained on re-melted polyethylene samples while we used as-synthesised polyethylene powder.
The calculated carbon–carbon interatomic distance in polyethylene sample 5 is 0.154061(1) nm and the C–C–C angle is 112.191(7)°. The calculated unit cell volume is 0.09474(7) nm3 and calculated density of sample 5 is 0.9836 g/cm3 which corresponds to a very high density polyethylene. Lattice parameters and densities of other samples in Table 1 are expected to be very similar to those of sample 5 as the positions of XRD diffraction peaks are almost identical.
Conclusion
In the polymerization of ethylene, the activity of the catalyst and the molecular weight of the polymer are highly dependent on the nature of the metal and the sizes of the group (R′′) attached to the imino nitrogen atoms and the ortho constituent R. Developed Zr-based single site catalysts and optimized reaction condition enabled the synthesis of very high density polyethylene samples with very low branching content and high crystallinity.
References
- Alsaygh A (2013) Distinct new catalysts for utilization of ethylene gas to polymer and oligomer. Appl Petrochem Res 3:101–105
Article Google Scholar - Gibson VC, Spitzmesser SK (2003) Advances in non-metallocene olefin polymerization catalysis. Chem Rev 103:283–315
Article Google Scholar - Crist B (1995) The ultimate strength and stiffness of polymers. Annu Rev Mater Sci 25:295–323
Google Scholar - Young RJ, Lovell PA (1991) Introduction to polymers. Chapman, London
Book Google Scholar - Bunn CW (1939) The crystal structure of long-chain normal paraffin hydrocarbons. Trans Faraday Soc 35:482–491
Google Scholar - Hatfield GR, Killinge WE, Zeigler RC (1995) MeltlState 13C MAS NMR determination of comonomer type and content in ethylene/α-Olefin copolymers. Anal Chem 67:3082–3085
Article Google Scholar - Hay JN, Kemmish DJ, Langford JL, Rae AIM (1984) Polymer Commun 25:175
Google Scholar - Ballirano P, Caminiti R, D’Ilario L, Martinelli A, Piozzi A, Maras A (1998) J Mater Sci 33:3519
Article Google Scholar - Luo H-K, Schumann H (2005) New bi-nuclear and multi-nuclear α-diimine/nickel catalysts for ethylene polymerization. J Mol Catal A Chem 227(1–2):153–161 (And references therein)
Article Google Scholar - Hsieh K-C, Chang C Jr, Lee M-T, Hu C-H, Hung C-H, Lee HM, Huang J-H, Wang M-H, Lee T-Y (2004) Hafnium chloride and hafnium methyl complexes bearing substituted pyrrolyl ligands: synthesis, characterization, and ethylene polymerization. Inorg Chim Acta 357:3517–3524
Article Google Scholar - Britovsek GJP, Baugh SPD, Hoarau O, Gibson VC, Wass DF, White AJP, Williams DJ (2003) The role of bulky substituents in the polymerization of ethylene using late transition metal catalysts: a comparative study of nickel and iron catalyst systems. Inorg Chim Acta 345:279–291
Article Google Scholar - Laridjani M, Leboucher P (2009) The structural dilemma of bulk polyethylene: an intermediary structure. PLoS ONE 4:e6228
Article Google Scholar - Caminiti R, Pandolfi L, Ballirano P (2000) Structure of polyethylene from x-ray powder diffraction: influence of the amorphous fraction on data analysis. J Macromol Sci Phys B39:481–492
Article Google Scholar - Fontana L, Vinh DQ, Santoro M, Scandolo S, Gorelli FA, Bini R, Hanfland M (2007) High-pressure crystalline polyethylene studied by x-ray diffraction and ab initio simulations. Phys Rev B 75:174112
Article Google Scholar - VanderHart DL, Khoury F (1984) Polymer 25:1589
Article Google Scholar - Larson AC, Von Dreele RB (2004) General structure analysis system (GSAS). Los Alamos National Laboratory Report LAUR, pp 86–748
- Dorset DL, Moss B (1983) Polymer 24:291–294
Article Google Scholar - Schwartz KB, Von Dreele RB (1997) Adv X-Ray Anal 39:515
Google Scholar - Clark ES (2007) Chapter 38. Physical properties of polymers handbook. In: Mark JE (ed) Unit cell information on some important polymers, 2nd edn. Springer, New York
Google Scholar
Acknowledgments
Authors would like to thank King Abdulaziz City for Science and Technology (KACST) for founding and supporting our collaborations with Oxford University. Solid-state NMR spectra were obtained at the EPSRC UK National Solid-state NMR Service at Durham.
Author information
Authors and Affiliations
- Petroleum and Petrochemicals Research Institute, King Abdulaziz City for Science and Technology (KACST), P.O. Box 6086, Riyadh, 11442, Saudi Arabia
Abdulhamid A. Alsaygh, Fares D. Alsewailem & Ibrahim M. Al-Najjar - Chemistry Department, College of Science, Princess Nora Bint Abdulrahman University, Riyadh, Saudi Arabia
Jehan Al-hamidi - Department of Chemistry, University of Oxford, South Parks Road, Oxford, OX1 3QR, UK
Vladimir L. Kuznetsov
Authors
- Abdulhamid A. Alsaygh
You can also search for this author inPubMed Google Scholar - Jehan Al-hamidi
You can also search for this author inPubMed Google Scholar - Fares D. Alsewailem
You can also search for this author inPubMed Google Scholar - Ibrahim M. Al-Najjar
You can also search for this author inPubMed Google Scholar - Vladimir L. Kuznetsov
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toAbdulhamid A. Alsaygh.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Alsaygh, A.A., Al-hamidi, J., Alsewailem, F.D. et al. Characterization of polyethylene synthesized by zirconium single site catalysts.Appl Petrochem Res 4, 79–84 (2014). https://doi.org/10.1007/s13203-014-0053-2
- Received: 07 February 2014
- Accepted: 12 March 2014
- Published: 26 March 2014
- Issue Date: May 2014
- DOI: https://doi.org/10.1007/s13203-014-0053-2