Impact of Metabolic Dysfunction-Associated Fatty Liver Disease of Varying Severity on Antiviral Treatment Outcomes and Clinical Prognosis in Patients with Chronic Hepatitis B: A Systematic Review and Meta-analysis (original) (raw)
Abstract
Introduction
The coexistence of hepatitis B virus (HBV) infection and metabolic dysfunction-associated fatty liver disease (MAFLD) is becoming increasingly common. The bidirectional interaction between persistent HBV infection and lipotoxicity may influence the progression of the disease. However, to date, there has been a lack of meta-analyses that stratify this dual-disease population based on the severity of MAFLD.
Methods
This study was conducted in accordance with the PRISMA guidelines. Relevant literature on chronic hepatitis B (CHB) coexisting with MAFLD, published in Chinese and English databases from inception to January 6, 2025, was systematically retrieved. A meta-analysis was performed to evaluate the impact of MAFLD of varying severity on the efficacy of antiviral therapy and clinical outcomes in patients with CHB.
Results
A total of 24 studies were included, among which seven investigated the impact of MAFLD severity on antiviral treatment efficacy, and 17 explored the influence of MAFLD on clinical outcomes in CHB. The meta-analysis revealed the following:
- HBeAg seroclearance rate: For the mild MAFLD group versus the CHB-only group, the odds ratio (OR) was 0.62; for the moderate-to-severe MAFLD group versus the CHB-only group, OR = 0.37, indicating a more pronounced negative impact of moderate-to-severe MAFLD on HBeAg seroconversion.
- HBsAg seroclearance rate: For the mild MAFLD group versus the CHB-only group, OR = 0.43; for the moderate-to-severe MAFLD group versus the CHB-only group, OR = 0.20, further supporting the greater adverse effect of more severe MAFLD on HBsAg seroclearance.
- Incidence of hepatocellular carcinoma (HCC): For the CHB combined with MAFLD group versus the CHB-only group, OR = 1.77, demonstrating a markedly increased risk of HCC development in the CHB combined with MAFLD group compared to the CHB-only group.
Conclusions
This study is the first to systematically examine the complex relationship between CHB and MAFLD from the perspective of hepatic steatosis severity stratification. CHB combined with moderate-to-severe MAFLD is associated with HBsAg/HBeAg seroclearance rate and the likelihood of achieving functional cure, suggesting that MAFLD may exert a potentially beneficial effect on antiviral therapy. However, MAFLD is also significantly associated with an increased risk of HCC, potentially accelerating hepatic carcinogenesis through lipotoxic pathways.
Similar content being viewed by others
FormalPara Key Summary Points
| The coexistence of chronic hepatitis B (CHB) and metabolic dysfunction-associated fatty liver disease (MAFLD) is increasingly observed in clinical settings, accompanied by significant economic burden and unmet clinical needs. However, systematic analyses stratified by the severity of MAFLD are currently lacking. |
|---|
| This study aimed to assess how varying severity of MAFLD affects antiviral treatment efficacy and clinical outcomes in patients with CHB, with a particular focus on how MAFLD severity influences therapeutic response and disease progression. |
| Coexistence of CHB and moderate-to-severe MAFLD is associated with reduced rates of HBsAg and HBeAg clearance but is also linked to a significantly higher risk of hepatocellular carcinoma (HCC). This suggests a dual-pathogenic mechanism of MAFLD in the context of CHB. |
| These results highlight the urgent need for stratified metabolic interventions, enhanced HCC surveillance, and personalized antiviral treatment strategies. Future research should aim to construct a “severity–effect” dynamic model linking hepatic steatosis to antiviral efficacy, in order to precisely define the metabolic–immune thresholds that determine MAFLD's impact on CHB therapy. This would ultimately improve prognoses for patients with CHB with coexisting MAFLD and guide future research directions. |
Introduction
Chronic Hepatitis B (CHB) is a long-term liver disease caused by the Hepatitis B Virus (HBV). As a hepatotropic DNA virus, HBV infection is globally widespread. Studies have shown [[1](/article/10.1007/s40121-025-01189-0#ref-CR1 "Cao G, Liu J, Liu M. Trends in mortality of liver disease due to hepatitis B in China from 1990 to 2019: findings from the Global Burden of Disease Study. Chin Med J (Engl). 2022;135(17):2049–55. https://doi.org/10.1097/cm9.0000000000002331
."), [2](/article/10.1007/s40121-025-01189-0#ref-CR2 "Hui Z, Yu W, Fuzhen W, Liping S, Guomin Z, Jianhua L, et al. New progress in HBV control and the cascade of health care for people living with HBV in China: evidence from the fourth national serological survey, 2020. Lancet Reg Health West Pac. 2024;51: 101193.
https://doi.org/10.1016/j.lanwpc.2024.101193
.")\] that approximately 296 million people worldwide are affected by CHB, and as of 2022, there were about 79.7 million chronic HBV infections in China, accounting for 31.5% of the global total \[[3](/article/10.1007/s40121-025-01189-0#ref-CR3 "Dan S, Jia J. Progress and challenges in the treatment of chronic hepatitis B in China. J Clin Hepatol. 2025;41(02):205–9.")\]. The virus possesses complex structural characteristics, the ability to persist via covalently closed circular DNA (cccDNA), multiple immune evasion strategies, and regulatory effects on host epigenetics \[[4](/article/10.1007/s40121-025-01189-0#ref-CR4 "Kar A, Samanta A, Mukherjee S, Barik S, Biswas A. The HBV web: An insight into molecular interactomes between the hepatitis B virus and its host en route to hepatocellular carcinoma. J Med Virol. 2023;95(1): e28436.
https://doi.org/10.1002/jmv.28436
.")\], forming the biological foundation for its high infectivity, chronicity, and carcinogenicity. These features not only contribute to the progressive pathogenesis of liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) but also present major obstacles to achieving complete cure with current antiviral treatments. Due to factors such as insulin resistance, genetic predisposition, and metabolic disorders, the prevalence of CHB combined with metabolic dysfunction-associated steatotic liver disease (MAFLD) continues to rise. In recent years, international organizations have proposed renaming non-alcoholic fatty liver disease (NAFLD) to MAFLD. Research has found \[[5](/article/10.1007/s40121-025-01189-0#ref-CR5 "Song SJ, Lai JC, Wong GL, Wong VW, Yip TC. Can we use old NAFLD data under the new MASLD definition? J Hepatol. 2024;80(2):e54–6.
https://doi.org/10.1016/j.jhep.2023.07.021
.")\] that the differences between MAFLD and NAFLD are minimal, and therefore, findings from studies conducted under the NAFLD definition remain valid for MAFLD. Reports indicate that the global incidence of CHB combined with MAFLD ranges from 14 to 70% \[[6](/article/10.1007/s40121-025-01189-0#ref-CR6 "Yao R, Lu S, Xue R, Wang J, Qiu Y, Chen Y, et al. NAFLD is associated with less severe liver fibrosis in chronic hepatitis B: a multi-center, retrospective study. Ann Hepatol. 2024;29(1): 101155.
https://doi.org/10.1016/j.aohep.2023.101155
.")\]. The interaction between CHB and MAFLD creates a unique pathophysiological environment in which persistent viral infection and lipotoxic injury mutually reinforce each other, resulting in a “double-edged sword” effect.As MAFLD progresses, the influence of viral factors in patients with CHB gradually weakens, while the role of metabolic factors increases. Studies have shown [7, [8](/article/10.1007/s40121-025-01189-0#ref-CR8 "Mak LY, Hui RW, Fung J, Liu F, Wong DK, Cheung KS, et al. Diverse effects of hepatic steatosis on fibrosis progression and functional cure in virologically quiescent chronic hepatitis B. J Hepatol. 2020;73(4):800–6. https://doi.org/10.1016/j.jhep.2020.05.040
.")\] that hepatic steatosis is significantly associated with the risk of liver fibrosis progression. On the other hand, it has been found \[[9](/article/10.1007/s40121-025-01189-0#ref-CR9 "Liu Q, Mu M, Chen H, Zhang G, Yang Y, Chu J, et al. Hepatocyte steatosis inhibits hepatitis B virus secretion via induction of endoplasmic reticulum stress. Mol Cell Biochem. 2022;477(11):2481–91.
https://doi.org/10.1007/s11010-021-04143-z
.")\] that cellular steatosis inhibits HBV secretion, promotes HBsAg seroclearance, and is associated with better outcomes, such as lower risks of HCC, cirrhosis, and mortality \[[10](/article/10.1007/s40121-025-01189-0#ref-CR10 "Wong YJ, Nguyen VH, Yang HI, Li J, Le MH, Wu WJ, et al. Impact of fatty liver on long-term outcomes in chronic hepatitis B: a systematic review and matched analysis of individual patient data meta-analysis. Clin Mol Hepatol. 2023;29(3):705–20.
https://doi.org/10.3350/cmh.2023.0004
.")\]. However, stratified meta-analyses based on the severity of MAFLD are still lacking, and current guidelines do not provide evidence-based recommendations on monitoring frequency or treatment adjustment for patients with this dual disease profile. Therefore, this study aims to conduct a systematic review and meta-analysis to evaluate the effects of varying degrees of MAFLD on antiviral efficacy and clinical outcomes in patients with CHB. The goal is to identify key thresholds at which MAFLD affects antiviral treatment, thereby providing an evidence-based basis for precision-stratified management of patients with CHB with coexisting MAFLD.Methods
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The study protocol has been registered in the PROSPERO database (registration number: CRD420250652601). This article is based on previously published studies and does not include any new research involving human participants or animals conducted by the authors.
Literature Search
A comprehensive computer-based search was conducted across the following databases: PubMed, Embase, Cochrane Library, Web of Science, CNKI, Wanfang, VIP, Chinese Medical Journal, and the Chinese Biomedical Literature Database (CBM), covering the period from the inception of each database to January 6, 2025. The search strategy was constructed using the PICOS framework and optimized using a combination of Medical Subject Headings (MeSH) and free-text terms to maximize sensitivity and specificity. English search terms included: “chronic hepatitis B virus,” “Hepatitis B, Chronic,” “fatty liver,” “Non-alcoholic Fatty Liver Disease,” “Clinical Outcomes,” “liver fibrosis,” “Liver Cirrhosis,” “Liver Neoplasms,” “Liver failure,” “antiviral therapy,” “Nucleoside Analogues,” “Interferon,” and “Therapeutic.” Chinese search terms included: “chronic viral hepatitis B,” “Chronic hepatitis B,” ‘Hepatitis B, chronic,” “Hepatic steatosis,” “Nonalcoholic fatty liver disease,” “Metabolic fatty liver disease,” “Antiviral therapy,” “Clinical outcome,” “Prognosis,” “Hepatocellular carcinoma,” “Liver cirrhosis,” and “Liver fibrosis,”
Inclusion and Exclusion Criteria
The specific inclusion and exclusion criteria for the literature are detailed in Table 1.
Table 1 Inclusion and exclusion criteria for literature selection
Literature Selection and Data Extraction
Two researchers (Qianqian Zhu and Chengde Su) independently performed literature screening and data extraction and cross-checked the results. Any disagreements were resolved through discussion with a third researcher (Mingdan Li). After removing duplicate records, titles and abstracts were reviewed to exclude irrelevant studies, reviews, meta-analyses, conference abstracts, and other non-eligible articles. Full texts of the remaining studies were then assessed strictly based on the predefined inclusion and exclusion criteria to determine final eligibility. A standardized data extraction form was developed using Excel. Extracted data included: first author, publication year, country, study design, sample size, diagnostic methods. Study quality was assessed using the Newcastle–Ottawa Scale (NOS) and the Agency for Healthcare Research and Quality (AHRQ) criteria for cross-sectional studies.
Quality Assessment of Included Studies
Two researchers (Qianqian Zhu and Chengde Su) assessed the quality of the included studies using the NOS [[11](/article/10.1007/s40121-025-01189-0#ref-CR11 "Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. https://doi.org/10.1007/s10654-010-9491-z
.")\] and AHRQ \[[12](/article/10.1007/s40121-025-01189-0#ref-CR12 "Zeng X, Liu H, Chen Xi, Leng W. Series on meta-analysis IV: quality assessment tools for observational studies. Chin J Evid Based Cardiovasc Med. 2012;4(04):297–9.")\] criteria. Discrepancies were resolved through consultation with a third researcher (Yali Xu). The NOS assesses three domains: study subject selection, intergroup comparability, and exposure factors, with a total score of 9\. Studies scoring ≥ 6 points were considered high quality. The AHRQ tool includes 11 items, each answered as “yes,” “no,” or “unclear.” Responses were scored as 0 points (no/unclear) or 1 point (yes). Based on the total score, studies were classified as: low quality (0–3 points), moderate quality (4–7 points), or high quality (8–11 points).Statistical Analysis
Meta-analysis was performed using Review Manager (RevMan) version 5.4, developed by the Cochrane Collaboration (UK). For all outcome indicators, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated as effect size measures. Heterogeneity was assessed using the _I_2 statistic. If P ≥ 0.1 and _I_2 ≤ 50%, no significant heterogeneity was assumed and a fixed-effect model was used. Otherwise, a random-effect model was applied. For indicators with significant heterogeneity, Egger’s test or Begg’s test was conducted using Stata version 18 (USA), with P < 0.05 considered statistically significant.
Results
Literature Screening Process and Basic Characteristics of Included Studies
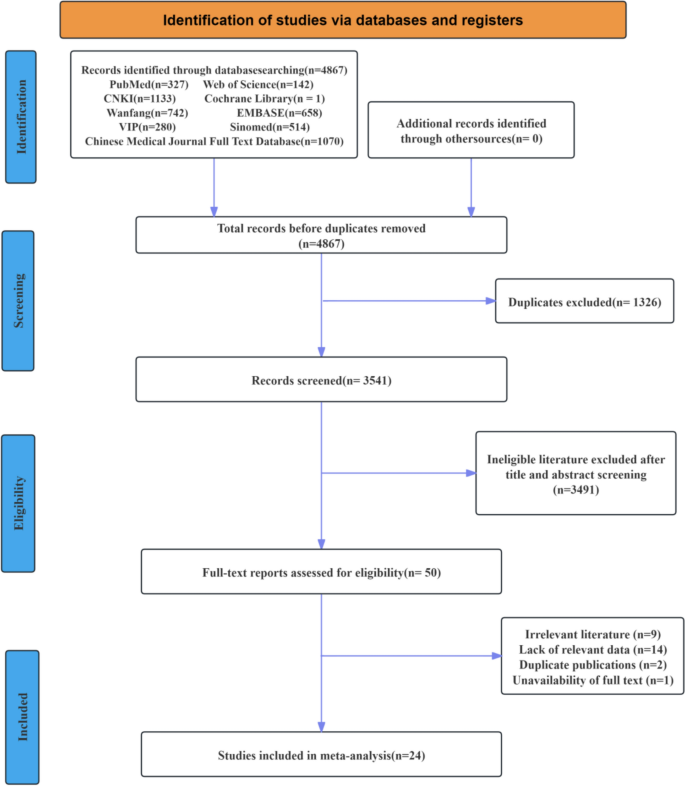
A total of 4867 articles were initially retrieved. After reviewing the titles, abstracts, and full texts, and applying the inclusion and exclusion criteria, 24 studies were finally included. Among them, 17 studies reported clinical outcome indicators [[13](#ref-CR13 "Lim CT, Goh GBB, Li H, Lim TK, Leow WQ, Wan WK, et al. Presence of hepatic steatosis does not increase the risk of hepatocellular carcinoma in patients with chronic hepatitis B over long follow-up. Microbiol Insights. 2020;13:1178636120918878. https://doi.org/10.1177/1178636120918878
."),[14](#ref-CR14 "Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, et al. Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology. 2020;71(2):539–48.
https://doi.org/10.1002/hep.30857
."),[15](#ref-CR15 "Ko HH, Patel NH, Haylock-Jacobs S, Doucette K, Ma MM, Cooper C, et al. Severe hepatic steatosis is associated with low-level viremia and advanced fibrosis in patients with chronic hepatitis B in North America. Gastro Hep Adv. 2022;1(1):106–16."),[16](#ref-CR16 "Yun B, Ahn SH, Oh J, Yoon JH, Kim BK. Effect of metabolic dysfunction-associated fatty liver disease on liver cancer risk in a population with chronic hepatitis B virus infection: a nationwide study. Hepatol Res. 2022;52(12):975–84.
https://doi.org/10.1111/hepr.13830
."),[17](#ref-CR17 "van Kleef LA, Choi HSJ, Brouwer WP, Hansen BE, Patel K, de Man RA, et al. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3(5): 100350.
https://doi.org/10.1016/j.jhepr.2021.100350
."),[18](#ref-CR18 "Yu MW, Lin CL, Liu CJ, Wu WJ, Hu JT, Huang YW. Metabolic-associated fatty liver disease, hepatitis B surface antigen seroclearance, and long-term risk of hepatocellular carcinoma in chronic hepatitis B. Cancers (Basel). 2022.
https://doi.org/10.3390/cancers14236012
."),[19](#ref-CR19 "Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Braun M, Leshno M, et al. Liver steatosis is a strong predictor of mortality and cancer in chronic hepatitis B regardless of viral load. JHEP Rep. 2019;1(1):9–16.
https://doi.org/10.1016/j.jhepr.2019.02.002
."),[20](#ref-CR20 "Huang SC, Su TH, Tseng TC, Chen CL, Hsu SJ, Liao SH, et al. Distinct effects of hepatic steatosis and metabolic dysfunction on the risk of hepatocellular carcinoma in chronic hepatitis B. Hepatol Int. 2023;17(5):1139–49.
https://doi.org/10.1007/s12072-023-10545-6
."),[21](#ref-CR21 "Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, et al. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25(1):52–64.
https://doi.org/10.3350/cmh.2018.0040
."),[22](#ref-CR22 "Boshi C. The impact of nonalcoholic fatty liver disease on liver-related adverse events in chronic hepatitis B [Master’s thesis]. 2023."),[23](#ref-CR23 "Chen F, Huang W, Li X, Qing L, Liu Y. Histological analysis of liver tissues in patients with chronic hepatitis B and nonalcoholic fatty liver disease. Chin J Viral Dis. 2021;11(04):261–5.
https://doi.org/10.16505/j.2095-0136.2021.0025
."),[24](#ref-CR24 "Yan J. Clinical characteristics and pathological changes in patients with chronic hepatitis B and hepatic steatosis [Master’s thesis]. 2017."),[25](#ref-CR25 "Na L. Impact of hepatic steatosis on the clinical prognosis of patients with chronic hepatitis B [Master’s thesis]. 2022."),[26](#ref-CR26 "Liu W, Liu H, Ding H, Li L. Clinical characteristics and prognostic factors in chronic hepatitis B with metabolic-associated fatty liver disease. J Clin Hepatol. 2022;38(10):2230–5."),[27](#ref-CR27 "Lü L, Li Qi, Ma W, Ding H, Liu H. Stratified analysis of virological features in patients with chronic hepatitis B and metabolic-associated fatty liver disease. J Clin Hepatol. 2024;40(07):1343–8."),[28](#ref-CR28 "Shuangyu Ou, Tang B, Li W, Zhong W. Predictive efficiency and correlation of serological virology and liver fibrosis staging in chronic hepatitis B patients with hepatic steatosis. J Liver Dis Integr Tradit Chin West Med. 2024;34(10):899–902."),[29](/article/10.1007/s40121-025-01189-0#ref-CR29 "Xie F, Meng Q, Hou W, Chen D. Clinical and pathological characteristics of HBeAg-positive chronic hepatitis B patients with nonalcoholic fatty liver disease. Chin J Exp Clin Infect Dis (Electronic Edition). 2018;12(03):256–61.")\], including 11 cohort studies \[[13](/article/10.1007/s40121-025-01189-0#ref-CR13 "Lim CT, Goh GBB, Li H, Lim TK, Leow WQ, Wan WK, et al. Presence of hepatic steatosis does not increase the risk of hepatocellular carcinoma in patients with chronic hepatitis B over long follow-up. Microbiol Insights. 2020;13:1178636120918878.
https://doi.org/10.1177/1178636120918878
."), [14](/article/10.1007/s40121-025-01189-0#ref-CR14 "Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, et al. Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology. 2020;71(2):539–48.
https://doi.org/10.1002/hep.30857
."), [16](#ref-CR16 "Yun B, Ahn SH, Oh J, Yoon JH, Kim BK. Effect of metabolic dysfunction-associated fatty liver disease on liver cancer risk in a population with chronic hepatitis B virus infection: a nationwide study. Hepatol Res. 2022;52(12):975–84.
https://doi.org/10.1111/hepr.13830
."),[17](#ref-CR17 "van Kleef LA, Choi HSJ, Brouwer WP, Hansen BE, Patel K, de Man RA, et al. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3(5): 100350.
https://doi.org/10.1016/j.jhepr.2021.100350
."),[18](#ref-CR18 "Yu MW, Lin CL, Liu CJ, Wu WJ, Hu JT, Huang YW. Metabolic-associated fatty liver disease, hepatitis B surface antigen seroclearance, and long-term risk of hepatocellular carcinoma in chronic hepatitis B. Cancers (Basel). 2022.
https://doi.org/10.3390/cancers14236012
."),[19](#ref-CR19 "Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Braun M, Leshno M, et al. Liver steatosis is a strong predictor of mortality and cancer in chronic hepatitis B regardless of viral load. JHEP Rep. 2019;1(1):9–16.
https://doi.org/10.1016/j.jhepr.2019.02.002
."),[20](#ref-CR20 "Huang SC, Su TH, Tseng TC, Chen CL, Hsu SJ, Liao SH, et al. Distinct effects of hepatic steatosis and metabolic dysfunction on the risk of hepatocellular carcinoma in chronic hepatitis B. Hepatol Int. 2023;17(5):1139–49.
https://doi.org/10.1007/s12072-023-10545-6
."),[21](/article/10.1007/s40121-025-01189-0#ref-CR21 "Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, et al. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25(1):52–64.
https://doi.org/10.3350/cmh.2018.0040
."), [25](/article/10.1007/s40121-025-01189-0#ref-CR25 "Na L. Impact of hepatic steatosis on the clinical prognosis of patients with chronic hepatitis B [Master’s thesis]. 2022."), [26](/article/10.1007/s40121-025-01189-0#ref-CR26 "Liu W, Liu H, Ding H, Li L. Clinical characteristics and prognostic factors in chronic hepatitis B with metabolic-associated fatty liver disease. J Clin Hepatol. 2022;38(10):2230–5."), [29](/article/10.1007/s40121-025-01189-0#ref-CR29 "Xie F, Meng Q, Hou W, Chen D. Clinical and pathological characteristics of HBeAg-positive chronic hepatitis B patients with nonalcoholic fatty liver disease. Chin J Exp Clin Infect Dis (Electronic Edition). 2018;12(03):256–61.")\], five case–control studies \[[22](#ref-CR22 "Boshi C. The impact of nonalcoholic fatty liver disease on liver-related adverse events in chronic hepatitis B [Master’s thesis]. 2023."),[23](#ref-CR23 "Chen F, Huang W, Li X, Qing L, Liu Y. Histological analysis of liver tissues in patients with chronic hepatitis B and nonalcoholic fatty liver disease. Chin J Viral Dis. 2021;11(04):261–5.
https://doi.org/10.16505/j.2095-0136.2021.0025
."),[24](/article/10.1007/s40121-025-01189-0#ref-CR24 "Yan J. Clinical characteristics and pathological changes in patients with chronic hepatitis B and hepatic steatosis [Master’s thesis]. 2017."), [27](/article/10.1007/s40121-025-01189-0#ref-CR27 "Lü L, Li Qi, Ma W, Ding H, Liu H. Stratified analysis of virological features in patients with chronic hepatitis B and metabolic-associated fatty liver disease. J Clin Hepatol. 2024;40(07):1343–8."), [28](/article/10.1007/s40121-025-01189-0#ref-CR28 "Shuangyu Ou, Tang B, Li W, Zhong W. Predictive efficiency and correlation of serological virology and liver fibrosis staging in chronic hepatitis B patients with hepatic steatosis. J Liver Dis Integr Tradit Chin West Med. 2024;34(10):899–902.")\], and one cross-sectional study \[[15](/article/10.1007/s40121-025-01189-0#ref-CR15 "Ko HH, Patel NH, Haylock-Jacobs S, Doucette K, Ma MM, Cooper C, et al. Severe hepatic steatosis is associated with low-level viremia and advanced fibrosis in patients with chronic hepatitis B in North America. Gastro Hep Adv. 2022;1(1):106–16.")\]. Seven studies reported antiviral efficacy indicators, all of which were cohort studies \[[30](#ref-CR30 "Hengyi D. Observation on the efficacy of entecavir in the treatment of HBeAg-positive chronic hepatitis B with nonalcoholic fatty liver disease [Master’s thesis]. 2023."),[31](#ref-CR31 "Han B, Zhang J, Liu W. Analysis of the effect of fatty liver on the antiviral efficacy of adefovir dipivoxil in chronic hepatitis B. Contemp Med. 2021;27(33):101–3."),[32](#ref-CR32 "Jianguo Lu. Clinical efficacy of pegylated interferon alpha-2a in the treatment of chronic hepatitis B with fatty liver. J Gastroenterol Hepatol. 2014;23(03):329–32."),[33](#ref-CR33 "Yanqin Wu, Shen L, Jihong Yu, Sun Y. Evaluation of the effect of entecavir in the treatment of chronic hepatitis B with hepatic steatosis. J Clin Hepatol. 2017;33(05):849–52."),[34](#ref-CR34 "Zhongsheng Yu, Zhou B, Tang D, Liu S. Study on the impact of hepatic steatosis on the antiviral effect in chronic hepatitis B. Chin Pract Med. 2019;14(10):48–9.
https://doi.org/10.14163/j.cnki.11-5547/r.2019.10.022
."),[35](#ref-CR35 "Bingbing Yu, Zhang L, Chen Z, Zhong Y. Effect of entecavir antiviral therapy in chronic hepatitis B patients with hepatic steatosis. Chin Med Sci. 2019;9(01):250–2."),[36](/article/10.1007/s40121-025-01189-0#ref-CR36 "Zhang W, Jianguo Yu, Zhu G, Zhao Z, Wang X. Effect of fatty liver and related factors on the efficacy of pegylated interferon alpha-2a in the treatment of chronic hepatitis B. Liver. 2014;19(09):692–4.
https://doi.org/10.14000/j.cnki.issn.1008-1704.2014.09.015
.")\]. The literature screening process is shown in Fig. [1](/article/10.1007/s40121-025-01189-0#Fig1), the basic characteristics of the included studies are shown in Table [2](/article/10.1007/s40121-025-01189-0#Tab2), and the criteria for classifying MASLD severity are presented in Table [3](/article/10.1007/s40121-025-01189-0#Tab3).Fig. 1
Literature screening flowchart
Table 2 Basic characteristics of included studies
Table 3 MASLD severity classification criteria
Quality Assessment of Included Studies
Among the 24 included studies, the cross-sectional study [15] received a score of 6 on the AHRQ scale. The remaining 23 studies [[13](/article/10.1007/s40121-025-01189-0#ref-CR13 "Lim CT, Goh GBB, Li H, Lim TK, Leow WQ, Wan WK, et al. Presence of hepatic steatosis does not increase the risk of hepatocellular carcinoma in patients with chronic hepatitis B over long follow-up. Microbiol Insights. 2020;13:1178636120918878. https://doi.org/10.1177/1178636120918878
."), [14](/article/10.1007/s40121-025-01189-0#ref-CR14 "Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, et al. Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology. 2020;71(2):539–48.
https://doi.org/10.1002/hep.30857
."), [16](#ref-CR16 "Yun B, Ahn SH, Oh J, Yoon JH, Kim BK. Effect of metabolic dysfunction-associated fatty liver disease on liver cancer risk in a population with chronic hepatitis B virus infection: a nationwide study. Hepatol Res. 2022;52(12):975–84.
https://doi.org/10.1111/hepr.13830
."),[17](#ref-CR17 "van Kleef LA, Choi HSJ, Brouwer WP, Hansen BE, Patel K, de Man RA, et al. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3(5): 100350.
https://doi.org/10.1016/j.jhepr.2021.100350
."),[18](#ref-CR18 "Yu MW, Lin CL, Liu CJ, Wu WJ, Hu JT, Huang YW. Metabolic-associated fatty liver disease, hepatitis B surface antigen seroclearance, and long-term risk of hepatocellular carcinoma in chronic hepatitis B. Cancers (Basel). 2022.
https://doi.org/10.3390/cancers14236012
."),[19](#ref-CR19 "Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Braun M, Leshno M, et al. Liver steatosis is a strong predictor of mortality and cancer in chronic hepatitis B regardless of viral load. JHEP Rep. 2019;1(1):9–16.
https://doi.org/10.1016/j.jhepr.2019.02.002
."),[20](#ref-CR20 "Huang SC, Su TH, Tseng TC, Chen CL, Hsu SJ, Liao SH, et al. Distinct effects of hepatic steatosis and metabolic dysfunction on the risk of hepatocellular carcinoma in chronic hepatitis B. Hepatol Int. 2023;17(5):1139–49.
https://doi.org/10.1007/s12072-023-10545-6
."),[21](#ref-CR21 "Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, et al. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25(1):52–64.
https://doi.org/10.3350/cmh.2018.0040
."),[22](#ref-CR22 "Boshi C. The impact of nonalcoholic fatty liver disease on liver-related adverse events in chronic hepatitis B [Master’s thesis]. 2023."),[23](#ref-CR23 "Chen F, Huang W, Li X, Qing L, Liu Y. Histological analysis of liver tissues in patients with chronic hepatitis B and nonalcoholic fatty liver disease. Chin J Viral Dis. 2021;11(04):261–5.
https://doi.org/10.16505/j.2095-0136.2021.0025
."),[24](#ref-CR24 "Yan J. Clinical characteristics and pathological changes in patients with chronic hepatitis B and hepatic steatosis [Master’s thesis]. 2017."),[25](#ref-CR25 "Na L. Impact of hepatic steatosis on the clinical prognosis of patients with chronic hepatitis B [Master’s thesis]. 2022."),[26](#ref-CR26 "Liu W, Liu H, Ding H, Li L. Clinical characteristics and prognostic factors in chronic hepatitis B with metabolic-associated fatty liver disease. J Clin Hepatol. 2022;38(10):2230–5."),[27](#ref-CR27 "Lü L, Li Qi, Ma W, Ding H, Liu H. Stratified analysis of virological features in patients with chronic hepatitis B and metabolic-associated fatty liver disease. J Clin Hepatol. 2024;40(07):1343–8."),[28](#ref-CR28 "Shuangyu Ou, Tang B, Li W, Zhong W. Predictive efficiency and correlation of serological virology and liver fibrosis staging in chronic hepatitis B patients with hepatic steatosis. J Liver Dis Integr Tradit Chin West Med. 2024;34(10):899–902."),[29](#ref-CR29 "Xie F, Meng Q, Hou W, Chen D. Clinical and pathological characteristics of HBeAg-positive chronic hepatitis B patients with nonalcoholic fatty liver disease. Chin J Exp Clin Infect Dis (Electronic Edition). 2018;12(03):256–61."),[30](#ref-CR30 "Hengyi D. Observation on the efficacy of entecavir in the treatment of HBeAg-positive chronic hepatitis B with nonalcoholic fatty liver disease [Master’s thesis]. 2023."),[31](#ref-CR31 "Han B, Zhang J, Liu W. Analysis of the effect of fatty liver on the antiviral efficacy of adefovir dipivoxil in chronic hepatitis B. Contemp Med. 2021;27(33):101–3."),[32](#ref-CR32 "Jianguo Lu. Clinical efficacy of pegylated interferon alpha-2a in the treatment of chronic hepatitis B with fatty liver. J Gastroenterol Hepatol. 2014;23(03):329–32."),[33](#ref-CR33 "Yanqin Wu, Shen L, Jihong Yu, Sun Y. Evaluation of the effect of entecavir in the treatment of chronic hepatitis B with hepatic steatosis. J Clin Hepatol. 2017;33(05):849–52."),[34](#ref-CR34 "Zhongsheng Yu, Zhou B, Tang D, Liu S. Study on the impact of hepatic steatosis on the antiviral effect in chronic hepatitis B. Chin Pract Med. 2019;14(10):48–9.
https://doi.org/10.14163/j.cnki.11-5547/r.2019.10.022
."),[35](#ref-CR35 "Bingbing Yu, Zhang L, Chen Z, Zhong Y. Effect of entecavir antiviral therapy in chronic hepatitis B patients with hepatic steatosis. Chin Med Sci. 2019;9(01):250–2."),[36](/article/10.1007/s40121-025-01189-0#ref-CR36 "Zhang W, Jianguo Yu, Zhu G, Zhao Z, Wang X. Effect of fatty liver and related factors on the efficacy of pegylated interferon alpha-2a in the treatment of chronic hepatitis B. Liver. 2014;19(09):692–4.
https://doi.org/10.14000/j.cnki.issn.1008-1704.2014.09.015
.")\] scored no less than 7 on the NOS scale, indicating high-quality literature, as shown in Table [2](/article/10.1007/s40121-025-01189-0#Tab2).Meta-analysis Results
Subgroup Analysis by MAFLD Severity: No Association Between HBV-DNA Suppression Rate or ALT Normalization Rate and MAFLD Severity
Six studies [30,31,32,33,[34](/article/10.1007/s40121-025-01189-0#ref-CR34 "Zhongsheng Yu, Zhou B, Tang D, Liu S. Study on the impact of hepatic steatosis on the antiviral effect in chronic hepatitis B. Chin Pract Med. 2019;14(10):48–9. https://doi.org/10.14163/j.cnki.11-5547/r.2019.10.022
."), [36](/article/10.1007/s40121-025-01189-0#ref-CR36 "Zhang W, Jianguo Yu, Zhu G, Zhao Z, Wang X. Effect of fatty liver and related factors on the efficacy of pegylated interferon alpha-2a in the treatment of chronic hepatitis B. Liver. 2014;19(09):692–4.
https://doi.org/10.14000/j.cnki.issn.1008-1704.2014.09.015
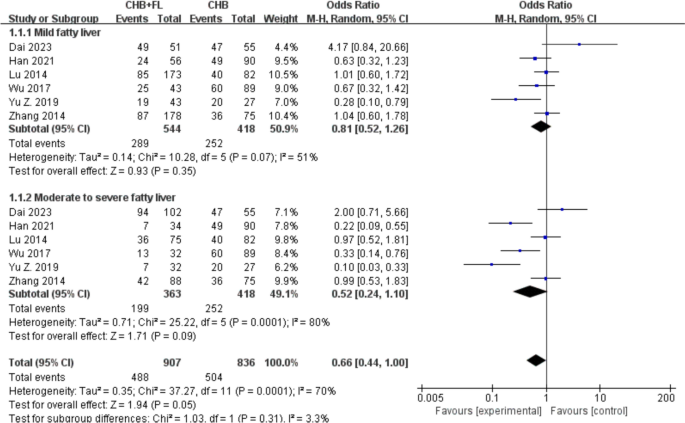
.")\] reported HBV-DNA suppression rates. A subgroup analysis based on MAFLD severity (mild vs. moderate-to-severe) included a total of 1325 patients—907 in the CHB combined with MAFLD group and 418 in the CHB-only group. The results are shown in Fig. [2](/article/10.1007/s40121-025-01189-0#Fig2). The pooled OR for HBV-DNA suppression rate was 0.66 (95% CI 0.44–1.00, _I_2 \= 70%, _P_ \= 0.05), suggesting that the suppression rate in the CHB combined with MAFLD group was 0.52 times that of the CHB-only group. A random-effects model was used due to significant heterogeneity (_I_2 \> 50%). There were no statistically significant differences between the CHB combined with mild MAFLD subgroup and CHB combined with moderate-to-severe MAFLD subgroup (_P_ \= 0.35 and _P_ \= 0.09, respectively).Fig. 2
Subgroup analysis of HBV DNA suppression rate based on MASLD severity. CHB + FL CHB combined with MAFLD group (intervention group); CHB CHB-only group (control group)
Six studies [30,31,32,33] reported ALT normalization rates. Subgroup analysis by MAFLD severity (mild vs. moderate-to-severe) included 1305
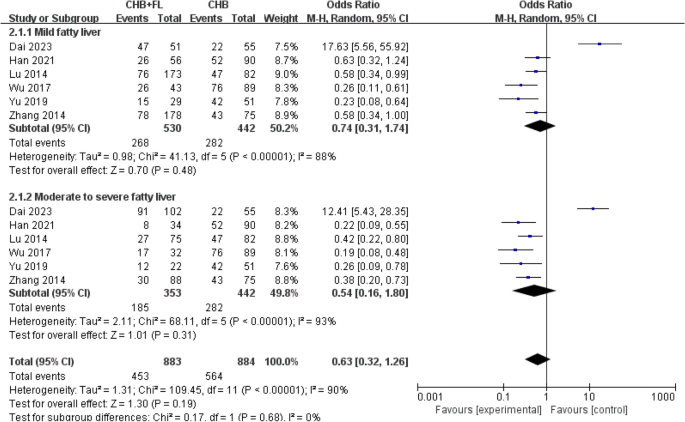
patients—883 in the CHB combined with MAFLD group and 442 in the CHB-only group. The results are shown in Fig. 3. A random-effects model was used due to significant heterogeneity (_I_2 > 50%). No statistically significant differences were found between the CHB combined with mild MAFLD subgroup versus the CHB-only group (P = 0.48), or between the CHB combined with moderate-to-severe MAFLD subgroup versus the CHB-only group (P = 0.31).
Fig. 3
Subgroup analysis of ALT normalization rate based on MASLD severity. CHB + FL CHB combined with MAFLD group (intervention group), CHB CHB-only group (control group)
Subgroup Analysis by MAFLD Severity: Significantly Higher HBeAg Seroclearance Rate in CHB Combined with Moderate-to-Severe MAFLD Group
Six studies [31,32,33,[34](#ref-CR34 "Zhongsheng Yu, Zhou B, Tang D, Liu S. Study on the impact of hepatic steatosis on the antiviral effect in chronic hepatitis B. Chin Pract Med. 2019;14(10):48–9. https://doi.org/10.14163/j.cnki.11-5547/r.2019.10.022
."),[35](#ref-CR35 "Bingbing Yu, Zhang L, Chen Z, Zhong Y. Effect of entecavir antiviral therapy in chronic hepatitis B patients with hepatic steatosis. Chin Med Sci. 2019;9(01):250–2."),[36](/article/10.1007/s40121-025-01189-0#ref-CR36 "Zhang W, Jianguo Yu, Zhu G, Zhao Z, Wang X. Effect of fatty liver and related factors on the efficacy of pegylated interferon alpha-2a in the treatment of chronic hepatitis B. Liver. 2014;19(09):692–4.
https://doi.org/10.14000/j.cnki.issn.1008-1704.2014.09.015
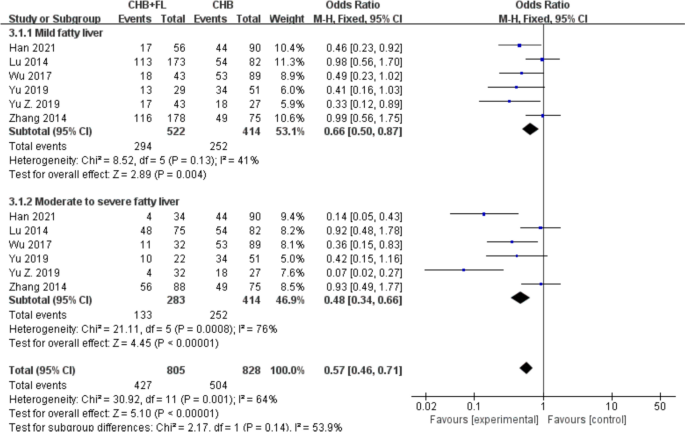
.")\] reported HBeAg seroclearance rates. A subgroup analysis based on MAFLD severity (mild vs. moderate-to-severe) included a total of 1219 patients—805 in the CHB combined with MAFLD group and 414 in the CHB-only group. The results are shown in Fig. [4](/article/10.1007/s40121-025-01189-0#Fig4). Since a lower OR value indicates better improvement in the experimental group versus controls, the analysis revealed: CHB combined with moderate-to-severe MAFLD showed significantly superior HBeAg seroclearance (OR 0.48, 95% CI 0.34–0.66, _P_ < 0.00001) compared to CHB combined with mild MAFLD (OR 0.66, 95% CI 0.50–0.87, _P_ \= 0.004). A random-effects model was applied due to substantial heterogeneity (_I_2 \> 50%). The pooled analysis demonstrated that, despite moderate heterogeneity (_I_2 \= 64%), the CHB combined with MAFLD group had a significantly higher HBeAg seroclearance rate than the CHB-only group (OR 0.57, 95% CI 0.46–0.71, _P_ < 0.00001).Fig. 4
Subgroup analysis of HBeAg seroclearance rate based on MASLD severity. CHB + FL CHB combined with MAFLD group (intervention group); CHB CHB-only group (control group)
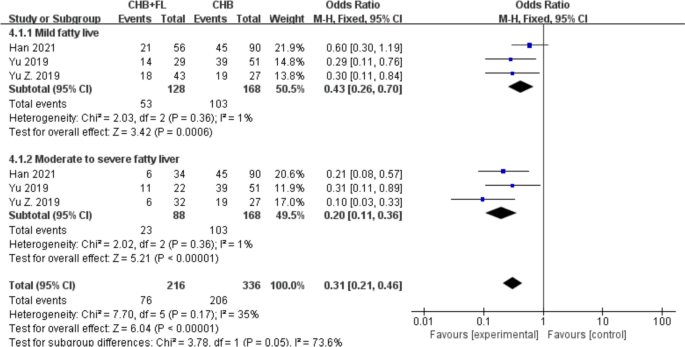
Subgroup Analysis by MAFLD Severity: Significantly Higher HBsAg Seroclearance Rate in CHB Combined with Moderate-to-Severe MAFLD Group
Three studies [31, [34](/article/10.1007/s40121-025-01189-0#ref-CR34 "Zhongsheng Yu, Zhou B, Tang D, Liu S. Study on the impact of hepatic steatosis on the antiviral effect in chronic hepatitis B. Chin Pract Med. 2019;14(10):48–9. https://doi.org/10.14163/j.cnki.11-5547/r.2019.10.022
."), [35](/article/10.1007/s40121-025-01189-0#ref-CR35 "Bingbing Yu, Zhang L, Chen Z, Zhong Y. Effect of entecavir antiviral therapy in chronic hepatitis B patients with hepatic steatosis. Chin Med Sci. 2019;9(01):250–2.")\] reported HBsAg seroclearance rates. A subgroup analysis based on MAFLD severity (mild vs. moderate-to-severe) included a total of 384 patients—216 in the CHB combined with MAFLD group and 168 in the CHB-only group. The results are shown in Fig. [5](/article/10.1007/s40121-025-01189-0#Fig5). A fixed-effect model was employed due to low heterogeneity (_I_2 < 50%). The HBsAg seroclearance rate in the CHB combined with moderate-to-severe MAFLD subgroup (OR 0.20, 95% CI 0.11–0.36, _P_ < 0.00001) was significantly better than that in the CHB combined with mild MAFLD subgroup (OR 0.43, 95% CI 0.26–0.70, _P_ \= 0.0006). Overall, the CHB combined with MAFLD group exhibited a significantly higher HBsAg seroclearance rate than the CHB-only group (OR 0.31, 95% CI 0.21–0.46, _P_ < 0.00001).Fig. 5
Subgroup analysis of HBsAg seroclearance rate based on MASLD severity. CHB + FL CHB combined with MAFLD group (intervention group); CHB CHB-only group (control group)
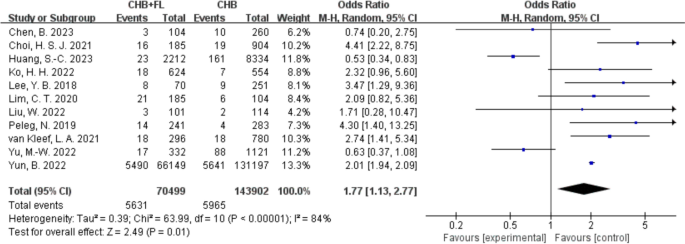
Increased Risk of HCC in CHB Combined with MAFLD Group
Eleven studies [[13](#ref-CR13 "Lim CT, Goh GBB, Li H, Lim TK, Leow WQ, Wan WK, et al. Presence of hepatic steatosis does not increase the risk of hepatocellular carcinoma in patients with chronic hepatitis B over long follow-up. Microbiol Insights. 2020;13:1178636120918878. https://doi.org/10.1177/1178636120918878
."),[14](#ref-CR14 "Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, et al. Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology. 2020;71(2):539–48.
https://doi.org/10.1002/hep.30857
."),[15](#ref-CR15 "Ko HH, Patel NH, Haylock-Jacobs S, Doucette K, Ma MM, Cooper C, et al. Severe hepatic steatosis is associated with low-level viremia and advanced fibrosis in patients with chronic hepatitis B in North America. Gastro Hep Adv. 2022;1(1):106–16."),[16](#ref-CR16 "Yun B, Ahn SH, Oh J, Yoon JH, Kim BK. Effect of metabolic dysfunction-associated fatty liver disease on liver cancer risk in a population with chronic hepatitis B virus infection: a nationwide study. Hepatol Res. 2022;52(12):975–84.
https://doi.org/10.1111/hepr.13830
."),[17](#ref-CR17 "van Kleef LA, Choi HSJ, Brouwer WP, Hansen BE, Patel K, de Man RA, et al. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3(5): 100350.
https://doi.org/10.1016/j.jhepr.2021.100350
."),[18](/article/10.1007/s40121-025-01189-0#ref-CR18 "Yu MW, Lin CL, Liu CJ, Wu WJ, Hu JT, Huang YW. Metabolic-associated fatty liver disease, hepatitis B surface antigen seroclearance, and long-term risk of hepatocellular carcinoma in chronic hepatitis B. Cancers (Basel). 2022.
https://doi.org/10.3390/cancers14236012
."), [20](#ref-CR20 "Huang SC, Su TH, Tseng TC, Chen CL, Hsu SJ, Liao SH, et al. Distinct effects of hepatic steatosis and metabolic dysfunction on the risk of hepatocellular carcinoma in chronic hepatitis B. Hepatol Int. 2023;17(5):1139–49.
https://doi.org/10.1007/s12072-023-10545-6
."),[21](#ref-CR21 "Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, et al. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25(1):52–64.
https://doi.org/10.3350/cmh.2018.0040
."),[22](/article/10.1007/s40121-025-01189-0#ref-CR22 "Boshi C. The impact of nonalcoholic fatty liver disease on liver-related adverse events in chronic hepatitis B [Master’s thesis]. 2023."), [26](/article/10.1007/s40121-025-01189-0#ref-CR26 "Liu W, Liu H, Ding H, Li L. Clinical characteristics and prognostic factors in chronic hepatitis B with metabolic-associated fatty liver disease. J Clin Hepatol. 2022;38(10):2230–5.")\] reported on the incidence of hepatocellular carcinoma (HCC), involving a total of 213,870 patients—70,499 in the CHB combined with MAFLD group and 143,902 in the CHB-only group. The results are shown in Fig. [6](/article/10.1007/s40121-025-01189-0#Fig6). A random-effects model was applied due to high heterogeneity (_I_2 \= 84%). The analysis indicated that patients with CHB with MAFLD had a 1.77-fold higher risk of developing HCC compared to CHB-only patients (OR 1.77, 95% CI 1.13–2.77, _P_ \= 0.01), suggesting that MAFLD may serve as a pro-carcinogenic factor in patients with CHB, significantly increasing HCC risk.Fig. 6
Clinical outcome—incidence of hepatocellular carcinoma (HCC). CHB + FL CHB combined with MAFLD group (intervention group); CHB CHB-only group (control group)
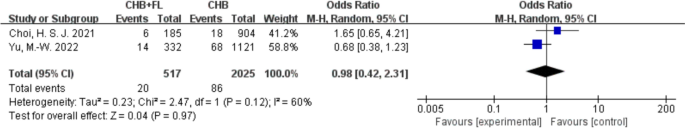
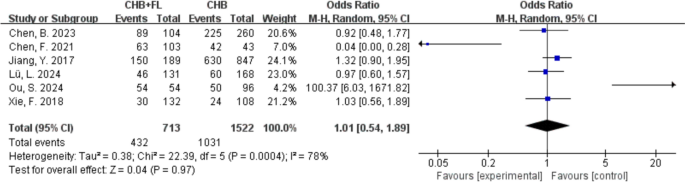
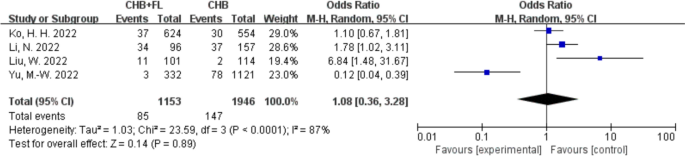
No Significant Increase in Mortality, Liver Fibrosis Incidence, or Liver Cirrhosis Incidence in Patients with CHB and MAFLD
Two studies [[14](/article/10.1007/s40121-025-01189-0#ref-CR14 "Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, et al. Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology. 2020;71(2):539–48. https://doi.org/10.1002/hep.30857
."), [18](/article/10.1007/s40121-025-01189-0#ref-CR18 "Yu MW, Lin CL, Liu CJ, Wu WJ, Hu JT, Huang YW. Metabolic-associated fatty liver disease, hepatitis B surface antigen seroclearance, and long-term risk of hepatocellular carcinoma in chronic hepatitis B. Cancers (Basel). 2022.
https://doi.org/10.3390/cancers14236012
.")\] reported mortality rates, as shown in Fig. [7](/article/10.1007/s40121-025-01189-0#Fig7). There was no significant difference in mortality between the CHB combined with MAFLD group and the CHB-only group (OR 0.98, 95% CI 0.42–2.31, _P_ \> 0.05). Six studies \[[22](#ref-CR22 "Boshi C. The impact of nonalcoholic fatty liver disease on liver-related adverse events in chronic hepatitis B [Master’s thesis]. 2023."),[23](#ref-CR23 "Chen F, Huang W, Li X, Qing L, Liu Y. Histological analysis of liver tissues in patients with chronic hepatitis B and nonalcoholic fatty liver disease. Chin J Viral Dis. 2021;11(04):261–5.
https://doi.org/10.16505/j.2095-0136.2021.0025
."),[24](/article/10.1007/s40121-025-01189-0#ref-CR24 "Yan J. Clinical characteristics and pathological changes in patients with chronic hepatitis B and hepatic steatosis [Master’s thesis]. 2017."), [27](#ref-CR27 "Lü L, Li Qi, Ma W, Ding H, Liu H. Stratified analysis of virological features in patients with chronic hepatitis B and metabolic-associated fatty liver disease. J Clin Hepatol. 2024;40(07):1343–8."),[28](#ref-CR28 "Shuangyu Ou, Tang B, Li W, Zhong W. Predictive efficiency and correlation of serological virology and liver fibrosis staging in chronic hepatitis B patients with hepatic steatosis. J Liver Dis Integr Tradit Chin West Med. 2024;34(10):899–902."),[29](/article/10.1007/s40121-025-01189-0#ref-CR29 "Xie F, Meng Q, Hou W, Chen D. Clinical and pathological characteristics of HBeAg-positive chronic hepatitis B patients with nonalcoholic fatty liver disease. Chin J Exp Clin Infect Dis (Electronic Edition). 2018;12(03):256–61.")\] reported liver fibrosis incidence, as shown in Fig. [8](/article/10.1007/s40121-025-01189-0#Fig8), with no significant difference between the CHB combined with MAFLD group and the CHB-only group (OR 1.01, 95% CI 0.54–1.89, _P_ \> 0.05). Four studies \[[15](/article/10.1007/s40121-025-01189-0#ref-CR15 "Ko HH, Patel NH, Haylock-Jacobs S, Doucette K, Ma MM, Cooper C, et al. Severe hepatic steatosis is associated with low-level viremia and advanced fibrosis in patients with chronic hepatitis B in North America. Gastro Hep Adv. 2022;1(1):106–16."), [18](/article/10.1007/s40121-025-01189-0#ref-CR18 "Yu MW, Lin CL, Liu CJ, Wu WJ, Hu JT, Huang YW. Metabolic-associated fatty liver disease, hepatitis B surface antigen seroclearance, and long-term risk of hepatocellular carcinoma in chronic hepatitis B. Cancers (Basel). 2022.
https://doi.org/10.3390/cancers14236012
."), [25](/article/10.1007/s40121-025-01189-0#ref-CR25 "Na L. Impact of hepatic steatosis on the clinical prognosis of patients with chronic hepatitis B [Master’s thesis]. 2022."), [26](/article/10.1007/s40121-025-01189-0#ref-CR26 "Liu W, Liu H, Ding H, Li L. Clinical characteristics and prognostic factors in chronic hepatitis B with metabolic-associated fatty liver disease. J Clin Hepatol. 2022;38(10):2230–5.")\] reported liver cirrhosis incidence, as shown in Fig. [9](/article/10.1007/s40121-025-01189-0#Fig9), with no significant difference between the CHB combined with MAFLD group and the CHB-only group (OR 1.08, 95% CI 0.36–3.28, _P_ \> 0.05). In summary, the presence of MAFLD did not significantly alter mortality, liver fibrosis progression, or cirrhosis risk in patients with CHB.Fig. 7
Clinical outcome—mortality rate. CHB + FL CHB combined with MAFLD group (intervention group), CHB CHB-only group (control group)
Fig. 8
Clinical outcome—incidence of liver fibrosis. CHB + FL CHB combined with MAFLD group (intervention group), CHB CHB-only group (control group)
Fig. 9
Clinical outcome—incidence of cirrhosis. CHB + FL CHB combined with MAFLD group (intervention group); CHB CHB-only group (control group)
Publication Bias
Egger’s test was performed using HCC incidence (with > 10 included studies) as the outcome indicator. The results showed a P value of 0.53 for HCC incidence, suggesting a low probability of publication bias. Funnel plots for HBeAg and HBsAg seroclearance rates showed roughly symmetrical distributions on both sides of the vertical line, and Begg’s test yielded P > 0.05, again indicating low likelihood of publication bias. For HBV-DNA suppression rate, ALT normalization rate, and liver fibrosis incidence, Begg’s test also showed P > 0.05, suggesting minimal publication bias. For other outcome indicators, due to the small number of studies, publication bias could not be formally assessed. Future research should expand sample sizes to enable more comprehensive evaluation of publication bias.
Discussion
This study, through a meta-analysis, reveals the complex interaction patterns between CHB and the stratified severity of MAFLD, filling a gap in previous research regarding the grading of MAFLD. The results show that patients with CHB combined with MAFLD exhibit both beneficial and adverse clinical outcomes in terms of antiviral efficacy and adverse clinical outcome indicators. While moderate-to-severe MAFLD may enhance the HBsAg seroclearance rate, it also significantly increases the risk of HCC, indicating a paradoxical “immune activation-carcinogenic transformation” effect. This suggests that MAFLD may positively influence certain aspects of antiviral therapy but does not necessarily improve all therapeutic indicators. Therefore, MAFLD may exert dual influences during the treatment of patients with CHB, warranting further in-depth research to elucidate its specific roles and underlying mechanisms.
The relationship between HBV infection and MAFLD remains an actively explored and debated area. Several studies [[44](/article/10.1007/s40121-025-01189-0#ref-CR44 "Zhou R, Yang L, Zhang B, Gu Y, Kong T, Zhang W, et al. Clinical impact of hepatic steatosis on chronic hepatitis B patients in Asia: a systematic review and meta-analysis. J Viral Hepat. 2023;30(10):793–802. https://doi.org/10.1111/jvh.13872
."), [45](/article/10.1007/s40121-025-01189-0#ref-CR45 "Mao X, Cheung KS, Peng C, Mak LY, Cheng HM, Fung J, et al. Steatosis, HBV-related HCC, cirrhosis, and HBsAg seroclearance: a systematic review and meta-analysis. Hepatology. 2023;77(5):1735–45.
https://doi.org/10.1002/hep.32792
.")\] have reported a significant inverse correlation between hepatic steatosis and viral load; however, the severity of steatosis was not clearly defined in these studies, thereby limiting a comprehensive understanding of MAFLD’s impact on antiviral efficacy in HBV infection. Against this backdrop, we stratified patients based on the severity of steatosis into the CHB combined with mild MAFLD subgroup and the CHB combined with moderate-to-severe MAFLD subgroup, to further investigate the effect of MAFLD on antiviral treatment outcomes in patients with CHB. Our findings revealed that patients in the CHB combined with moderate-to-severe MAFLD subgroup had better antiviral responses (HBeAg and HBsAg seroclearance rates) than those in the CHB combined with mild MAFLD subgroup. A case–control study \[[46](/article/10.1007/s40121-025-01189-0#ref-CR46 "Chu CM, Lin DY, Liaw YF. Does increased body mass index with hepatic steatosis contribute to seroclearance of hepatitis B virus (HBV) surface antigen in chronic HBV infection? Int J Obes (Lond). 2007;31(5):871–5.
https://doi.org/10.1038/sj.ijo.0803479
.")\] similarly reported that the prevalence of moderate-to-severe hepatic steatosis was significantly higher in patients who achieved HBsAg clearance (the HBsAg seroclearance group) compared to chronic HBV carriers (the CHB group), with moderate and severe steatosis increasing the likelihood of HBsAg clearance by 3.2-fold and 3.9-fold, respectively. This may be due to hepatic steatosis inducing oxidative stress and remodeling the hepatic immune microenvironment, thereby activating immune function via stimulation of envelope-specific CD4 + T cells and promoting B-cell activation for anti-HBs production, which indirectly enhances antiviral efficacy \[[47](/article/10.1007/s40121-025-01189-0#ref-CR47 "Joo EJ, Chang Y, Yeom JS, Ryu S. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: a cohort study. Hepatology. 2017;65(3):828–35.
https://doi.org/10.1002/hep.28917
.")\]. Alternatively, fatty acids may activate the Toll-like receptor 4 (TLR4) signaling pathway, enhancing hepatic immune responses. Upregulation of TLR4 can promote the restoration of innate immunity and activate adaptive immune cells, thereby accelerating HBsAg/HBV DNA clearance \[[48](/article/10.1007/s40121-025-01189-0#ref-CR48 "Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15(6):349–64.
https://doi.org/10.1038/s41575-018-0009-6
."), [49](/article/10.1007/s40121-025-01189-0#ref-CR49 "Zhang RN, Pan Q, Zhang Z, Cao HX, Shen F, Fan JG. Saturated fatty acid inhibits viral replication in chronic hepatitis B virus infection with nonalcoholic Fatty liver disease by toll-like receptor 4-mediated innate immune response. Hepat Mon. 2015;15(5): e27909.
https://doi.org/10.5812/hepatmon.15(5)2015.27909
.")\]. However, a meta-analysis by Li Jie et al. \[[50](/article/10.1007/s40121-025-01189-0#ref-CR50 "Rui F, Garcia E, Hu X, Ni W, Xue Q, Xu Y, et al. Antiviral therapy response in patients with chronic hepatitis B and fatty liver: a systematic review and meta-analysis. J Viral Hepat. 2024;31(7):372–82.
https://doi.org/10.1111/jvh.13942
.")\] evaluating antiviral treatment duration showed no statistically significant differences in complete viral suppression rate or HBeAg seroclearance rate at 48 and 96 weeks between patients with CHB with and without fatty liver disease.Although these studies have shed light on some of the mechanisms underlying the interaction between CHB and MAFLD, the two diseases involve multiple pathophysiological pathways, and the current body of evidence has yet to fully elucidate the essential characteristics and causal relationships of their interaction. Future research should aim to establish a dynamic “severity-effect” correlation model between hepatic steatosis and antiviral efficacy to precisely define the metabolic-immune thresholds at which MAFLD impacts CHB treatment. To achieve this, multi-modal systems biology approaches may be employed, including mass spectrometry imaging-based analysis of spatial lipid subtypes in hepatocytes [[51](/article/10.1007/s40121-025-01189-0#ref-CR51 "Kurabe N, Igarashi H, Ohnishi I, Tajima S, Inoue Y, Takahashi Y, et al. Visualization of sphingolipids and phospholipids in the fundic gland mucosa of human stomach using imaging mass spectrometry. World J Gastrointest Pathophysiol. 2016;7(2):235–41. https://doi.org/10.4291/wjgp.v7.i2.235
.")\], single-cell transcriptomics to profile dynamic immune cell subpopulations associated with the TLR4 pathway \[[52](/article/10.1007/s40121-025-01189-0#ref-CR52 "Sun F, Li H, Sun D, Fu S, Gu L, Shao X, et al. Single-cell omics: experimental workflow, data analyses and applications. Sci China Life Sci. 2025;68(1):5–102.
https://doi.org/10.1007/s11427-023-2561-0
.")\], and chromatin conformation capture techniques to evaluate epigenetic modifications of HBV cccDNA \[[53](/article/10.1007/s40121-025-01189-0#ref-CR53 "Tian H, Yang Z, Xu X, Liu L. Three-dimensional chromosome conformation capture and its derived technologies. Sheng Wu Gong Cheng Xue Bao. 2020;36(10):2040–50.
https://doi.org/10.13345/j.cjb.200112
.")\]. Additionally, feature fusion using machine learning-driven graph neural networks, combined with longitudinal antiviral efficacy data, could support the construction of multi-parameter predictive models to identify critical thresholds of MAFLD's impact on antiviral efficacy. Ultimately, an adaptive clinical trial design, incorporating causal forest algorithms to quantify the synergistic effects of metabolic interventions, may drive the clinical translation of precision medicine research.Although studies suggest that MAFLD may have positive effects on patients with CHB, long-term follow-up indicates a possible increase in HCC risk, which poses a serious threat to patient health and increases the medical burden, highlighting the need for further research and proactive intervention [[54](/article/10.1007/s40121-025-01189-0#ref-CR54 "Diao Y, Tang J, Wang X, Deng W, Tang J, You C. Metabolic syndrome, nonalcoholic fatty liver disease, and chronic hepatitis B: a narrative review. Infect Dis Ther. 2023;12(1):53–66. https://doi.org/10.1007/s40121-022-00725-6
."), [55](/article/10.1007/s40121-025-01189-0#ref-CR55 "Khalili M, Kleiner DE, King WC, Sterling RK, Ghany MG, Chung RT, et al. Hepatic steatosis and steatohepatitis in a large North American cohort of adults with chronic hepatitis B. Am J Gastroenterol. 2021;116(8):1686–97.
https://doi.org/10.14309/ajg.0000000000001257
.")\]. Our study, by integrating data from multiple regions, found that the presence of MAFLD significantly increases the risk of HCC in patients with CHB. Currently, the mechanisms by which MAFLD contributes to CHB-related HCC remain unclear. A potential explanation involves the synergistic effects of inflammatory responses, oxidative stress, and lipid metabolism disorders under comorbid conditions. A study \[[56](/article/10.1007/s40121-025-01189-0#ref-CR56 "Lu Y, Yang X, Kuang Q, Wu Y, Tan X, Lan J, et al. HBx induced upregulation of FATP2 promotes the development of hepatic lipid accumulation. Exp Cell Res. 2023;430(1): 113721.
https://doi.org/10.1016/j.yexcr.2023.113721
.")\] showed that the HBV-encoded X protein (HBx) can upregulate fatty acid transport protein 2 (FATP2), activating the FATP2-ACSL1 and FATP2-PPARγ signaling axes, thereby promoting excessive lipid accumulation in the liver. Further studies \[[57](/article/10.1007/s40121-025-01189-0#ref-CR57 "Zhang J, Ling N, Lei Y, Peng M, Hu P, Chen M. Multifaceted interaction between hepatitis B virus infection and lipid metabolism in hepatocytes: a potential target of antiviral therapy for chronic hepatitis B. Front Microbiol. 2021;12: 636897.
https://doi.org/10.3389/fmicb.2021.636897
.")\] have shown that the high-lipid microenvironment in the liver exerts a dual effect on CHB. On one hand, lipid accumulation induces the expansion of regulatory T cells (Tregs) and activates oncogenic pathways such as PI3K/AKT/mTOR, thereby indirectly accelerating the development of HCC. On the other hand, lipid metabolic products directly interfere with the HBV life cycle: saturated fatty acids stabilize the HBx protein by inhibiting its proteasomal degradation, thereby enhancing viral transcriptional activity; cholesterol, as an essential component of the viral envelope, promotes viral particle assembly and secretion; and lipid metabolic reprogramming mediated by ACSL1 suppresses HBsAg expression, weakening host immune surveillance and ultimately exacerbating HBV immune evasion and persistent infection. These mechanisms suggest that targeting key regulators of lipid metabolism (e.g., FAS, ACSL1) may enable dual “metabolic-viral” intervention, providing a novel approach for the combined treatment of CHB and MAFLD.In the context of CHB combined with MAFLD, MAFLD may promote HBV integration through oxidative stress, metabolic dysregulation, remodeling of the inflammatory microenvironment, and alterations in virus-host interactions [[4](/article/10.1007/s40121-025-01189-0#ref-CR4 "Kar A, Samanta A, Mukherjee S, Barik S, Biswas A. The HBV web: An insight into molecular interactomes between the hepatitis B virus and its host en route to hepatocellular carcinoma. J Med Virol. 2023;95(1): e28436. https://doi.org/10.1002/jmv.28436
.")\], thereby independently increasing the risk of HCC via integration-driven carcinogenic pathways, without significantly elevating cirrhosis incidence. Although direct studies on how MAFLD regulates HBV integration are currently lacking, this finding offers a conceptual basis for the precise management of HBV infection associated with MAFLD. Future research should further explore synergistic strategies involving metabolic intervention and antiviral therapy to block the integration-carcinogenesis cascade and improve patient prognosis. Evidence suggests \[[58](/article/10.1007/s40121-025-01189-0#ref-CR58 "Mukherjee S, Kar A, Khatun N, Datta P, Biswas A, Barik S. Familiarity breeds strategy: in silico untangling of the molecular complexity on course of autoimmune liver disease-to-hepatocellular carcinoma transition predicts novel transcriptional signatures. Cells. 2021.
https://doi.org/10.3390/cells10081917
.")\] that liver fibrosis plays a critical role in the transition from autoimmune liver disease to HCC, with dynamic changes in gene regulatory networks across progression stages. Similar molecular mechanisms may also exist in the progression of CHB combined with MAFLD. Therefore, mapping the CHB phenotype based on MAFLD severity could not only help clarify phenotypic changes such as serologic conversion but also reveal, at a multi-omics level, how MAFLD influences HBV infection and HCC development, offering new perspectives and potential targets for clinical treatment. In clinical practice, enhanced HCC surveillance is recommended for patients with CHB with concurrent MAFLD, including shortening the intervals for ultrasound and Alpha-Fetoprotein (AFP) testing. Although some studies have proposed combination strategies involving antiviral therapy and metabolic management \[[59](/article/10.1007/s40121-025-01189-0#ref-CR59 "Shi YW, Yang RX, Fan JG. Chronic hepatitis B infection with concomitant hepatic steatosis: current evidence and opinion. World J Gastroenterol. 2021;27(26):3971–83.
https://doi.org/10.3748/wjg.v27.i26.3971
.")\], and certain clinical trials have shown promise in improving liver function and reducing fat accumulation \[[60](/article/10.1007/s40121-025-01189-0#ref-CR60 "Younossi ZM, Corey KE, Lim JK. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2021;160(3):912–8.
https://doi.org/10.1053/j.gastro.2020.11.051
.")\], there is still no consensus on personalized treatment plans, and a lack of precision treatment guidelines based on patients' genetic backgrounds and disease states. Additionally, it is necessary to further explore the potential role of metabolic interventions (e.g., weight loss and improved insulin sensitivity) in reducing liver cancer risk.One of the strengths of this study lies in its large sample size and multi-regional design, which incorporates a substantial number of cases and considers data from multiple regions, thereby enhancing the generalizability and reliability of the conclusions. Uniquely, although many studies have examined the impact of disease duration on CHB outcomes, studies stratifying by MAFLD severity remain rare. This study innovatively stratified patients by the severity of MAFLD, which allows for a more precise evaluation of how different degrees of MAFLD affect antiviral efficacy and other outcomes in patients with CHB, offering more nuanced clinical insights.
This study also has several limitations. First, because China has the largest HBV-infected population globally [[1](/article/10.1007/s40121-025-01189-0#ref-CR1 "Cao G, Liu J, Liu M. Trends in mortality of liver disease due to hepatitis B in China from 1990 to 2019: findings from the Global Burden of Disease Study. Chin Med J (Engl). 2022;135(17):2049–55. https://doi.org/10.1097/cm9.0000000000002331
.")\], the proportion of Chinese samples is relatively high, and the genetic background \[[61](/article/10.1007/s40121-025-01189-0#ref-CR61 "Kim DH, Choi YM, Jang J, Kim BJ. Global prevalence and molecular characteristics of three clades within hepatitis B virus subgenotype C2: predominance of the C2(3) clade in South Korea. Front Microbiol. 2023;14:1137084.
https://doi.org/10.3389/fmicb.2023.1137084
.")\] (e.g., HBV genotype distribution) of the Chinese population may differ from that of other regions, potentially influencing the relationship between fatty liver and CHB. In addition, the included studies span a wide time period (2014–2024), during which diagnostic criteria, treatment guidelines, and clinical practices for CHB and fatty liver have undergone significant changes. Such temporal heterogeneity may affect the comparability of outcome indicators and increase heterogeneity in conclusions. Finally, the absence of individual-level data (e.g., baseline BMI, comorbid diabetes, etc.) limited our ability to control for metabolic factors and assess confounding effects, which may have increased variability in the results.Conclusions
This study is the first to systematically reveal the complex association between CHB and MAFLD from the perspective of stratified severity of hepatic steatosis. CHB combined with moderate-to-severe MAFLD is associated with HBsAg/HBeAg seroclearance rate and the likelihood of achieving functional cure, suggesting that MAFLD may exert a potentially beneficial effect on antiviral therapy. However, MAFLD is also significantly associated with an increased risk of HCC, potentially accelerating hepatic carcinogenesis through lipotoxic pathways. This paradox underscores the complexity of metabolic–viral interactions and highlights the clinical need for an integrated “graded intervention-dynamic monitoring” management strategy. Future studies should incorporate multi-omics data and artificial intelligence technologies to develop precision treatment models based on dynamic changes in hepatic steatosis and genetic features, ultimately achieving personalized management of CHB-MAFLD comorbidity.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- Cao G, Liu J, Liu M. Trends in mortality of liver disease due to hepatitis B in China from 1990 to 2019: findings from the Global Burden of Disease Study. Chin Med J (Engl). 2022;135(17):2049–55. https://doi.org/10.1097/cm9.0000000000002331.
Article PubMed Google Scholar - Hui Z, Yu W, Fuzhen W, Liping S, Guomin Z, Jianhua L, et al. New progress in HBV control and the cascade of health care for people living with HBV in China: evidence from the fourth national serological survey, 2020. Lancet Reg Health West Pac. 2024;51: 101193. https://doi.org/10.1016/j.lanwpc.2024.101193.
Article PubMed PubMed Central Google Scholar - Dan S, Jia J. Progress and challenges in the treatment of chronic hepatitis B in China. J Clin Hepatol. 2025;41(02):205–9.
Google Scholar - Kar A, Samanta A, Mukherjee S, Barik S, Biswas A. The HBV web: An insight into molecular interactomes between the hepatitis B virus and its host en route to hepatocellular carcinoma. J Med Virol. 2023;95(1): e28436. https://doi.org/10.1002/jmv.28436.
Article CAS PubMed Google Scholar - Song SJ, Lai JC, Wong GL, Wong VW, Yip TC. Can we use old NAFLD data under the new MASLD definition? J Hepatol. 2024;80(2):e54–6. https://doi.org/10.1016/j.jhep.2023.07.021.
Article PubMed Google Scholar - Yao R, Lu S, Xue R, Wang J, Qiu Y, Chen Y, et al. NAFLD is associated with less severe liver fibrosis in chronic hepatitis B: a multi-center, retrospective study. Ann Hepatol. 2024;29(1): 101155. https://doi.org/10.1016/j.aohep.2023.101155.
Article CAS PubMed Google Scholar - Fu MM, Sun R, Tian ZG, Wei HM. Increased susceptibility to experimental steatohepatitis induced by methionine-choline deficiency in HBs-Tg mice. Hepatobiliary Pancreat Dis Int. 2010;9(5):513–9.
PubMed Google Scholar - Mak LY, Hui RW, Fung J, Liu F, Wong DK, Cheung KS, et al. Diverse effects of hepatic steatosis on fibrosis progression and functional cure in virologically quiescent chronic hepatitis B. J Hepatol. 2020;73(4):800–6. https://doi.org/10.1016/j.jhep.2020.05.040.
Article CAS PubMed Google Scholar - Liu Q, Mu M, Chen H, Zhang G, Yang Y, Chu J, et al. Hepatocyte steatosis inhibits hepatitis B virus secretion via induction of endoplasmic reticulum stress. Mol Cell Biochem. 2022;477(11):2481–91. https://doi.org/10.1007/s11010-021-04143-z.
Article CAS PubMed Google Scholar - Wong YJ, Nguyen VH, Yang HI, Li J, Le MH, Wu WJ, et al. Impact of fatty liver on long-term outcomes in chronic hepatitis B: a systematic review and matched analysis of individual patient data meta-analysis. Clin Mol Hepatol. 2023;29(3):705–20. https://doi.org/10.3350/cmh.2023.0004.
Article PubMed PubMed Central Google Scholar - Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. https://doi.org/10.1007/s10654-010-9491-z.
Article PubMed Google Scholar - Zeng X, Liu H, Chen Xi, Leng W. Series on meta-analysis IV: quality assessment tools for observational studies. Chin J Evid Based Cardiovasc Med. 2012;4(04):297–9.
Google Scholar - Lim CT, Goh GBB, Li H, Lim TK, Leow WQ, Wan WK, et al. Presence of hepatic steatosis does not increase the risk of hepatocellular carcinoma in patients with chronic hepatitis B over long follow-up. Microbiol Insights. 2020;13:1178636120918878. https://doi.org/10.1177/1178636120918878.
Article PubMed PubMed Central Google Scholar - Choi HSJ, Brouwer WP, Zanjir WMR, de Man RA, Feld JJ, Hansen BE, et al. Nonalcoholic steatohepatitis is associated with liver-related outcomes and all-cause mortality in chronic hepatitis B. Hepatology. 2020;71(2):539–48. https://doi.org/10.1002/hep.30857.
Article CAS PubMed Google Scholar - Ko HH, Patel NH, Haylock-Jacobs S, Doucette K, Ma MM, Cooper C, et al. Severe hepatic steatosis is associated with low-level viremia and advanced fibrosis in patients with chronic hepatitis B in North America. Gastro Hep Adv. 2022;1(1):106–16.
Article PubMed PubMed Central Google Scholar - Yun B, Ahn SH, Oh J, Yoon JH, Kim BK. Effect of metabolic dysfunction-associated fatty liver disease on liver cancer risk in a population with chronic hepatitis B virus infection: a nationwide study. Hepatol Res. 2022;52(12):975–84. https://doi.org/10.1111/hepr.13830.
Article CAS PubMed Google Scholar - van Kleef LA, Choi HSJ, Brouwer WP, Hansen BE, Patel K, de Man RA, et al. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021;3(5): 100350. https://doi.org/10.1016/j.jhepr.2021.100350.
Article PubMed PubMed Central Google Scholar - Yu MW, Lin CL, Liu CJ, Wu WJ, Hu JT, Huang YW. Metabolic-associated fatty liver disease, hepatitis B surface antigen seroclearance, and long-term risk of hepatocellular carcinoma in chronic hepatitis B. Cancers (Basel). 2022. https://doi.org/10.3390/cancers14236012.
Article PubMed PubMed Central Google Scholar - Peleg N, Issachar A, Sneh Arbib O, Cohen-Naftaly M, Braun M, Leshno M, et al. Liver steatosis is a strong predictor of mortality and cancer in chronic hepatitis B regardless of viral load. JHEP Rep. 2019;1(1):9–16. https://doi.org/10.1016/j.jhepr.2019.02.002.
Article PubMed PubMed Central Google Scholar - Huang SC, Su TH, Tseng TC, Chen CL, Hsu SJ, Liao SH, et al. Distinct effects of hepatic steatosis and metabolic dysfunction on the risk of hepatocellular carcinoma in chronic hepatitis B. Hepatol Int. 2023;17(5):1139–49. https://doi.org/10.1007/s12072-023-10545-6.
Article PubMed Google Scholar - Lee YB, Ha Y, Chon YE, Kim MN, Lee JH, Park H, et al. Association between hepatic steatosis and the development of hepatocellular carcinoma in patients with chronic hepatitis B. Clin Mol Hepatol. 2019;25(1):52–64. https://doi.org/10.3350/cmh.2018.0040.
Article PubMed Google Scholar - Boshi C. The impact of nonalcoholic fatty liver disease on liver-related adverse events in chronic hepatitis B [Master’s thesis]. 2023.
- Chen F, Huang W, Li X, Qing L, Liu Y. Histological analysis of liver tissues in patients with chronic hepatitis B and nonalcoholic fatty liver disease. Chin J Viral Dis. 2021;11(04):261–5. https://doi.org/10.16505/j.2095-0136.2021.0025.
Article CAS Google Scholar - Yan J. Clinical characteristics and pathological changes in patients with chronic hepatitis B and hepatic steatosis [Master’s thesis]. 2017.
- Na L. Impact of hepatic steatosis on the clinical prognosis of patients with chronic hepatitis B [Master’s thesis]. 2022.
- Liu W, Liu H, Ding H, Li L. Clinical characteristics and prognostic factors in chronic hepatitis B with metabolic-associated fatty liver disease. J Clin Hepatol. 2022;38(10):2230–5.
CAS Google Scholar - Lü L, Li Qi, Ma W, Ding H, Liu H. Stratified analysis of virological features in patients with chronic hepatitis B and metabolic-associated fatty liver disease. J Clin Hepatol. 2024;40(07):1343–8.
Google Scholar - Shuangyu Ou, Tang B, Li W, Zhong W. Predictive efficiency and correlation of serological virology and liver fibrosis staging in chronic hepatitis B patients with hepatic steatosis. J Liver Dis Integr Tradit Chin West Med. 2024;34(10):899–902.
Google Scholar - Xie F, Meng Q, Hou W, Chen D. Clinical and pathological characteristics of HBeAg-positive chronic hepatitis B patients with nonalcoholic fatty liver disease. Chin J Exp Clin Infect Dis (Electronic Edition). 2018;12(03):256–61.
Google Scholar - Hengyi D. Observation on the efficacy of entecavir in the treatment of HBeAg-positive chronic hepatitis B with nonalcoholic fatty liver disease [Master’s thesis]. 2023.
- Han B, Zhang J, Liu W. Analysis of the effect of fatty liver on the antiviral efficacy of adefovir dipivoxil in chronic hepatitis B. Contemp Med. 2021;27(33):101–3.
Google Scholar - Jianguo Lu. Clinical efficacy of pegylated interferon alpha-2a in the treatment of chronic hepatitis B with fatty liver. J Gastroenterol Hepatol. 2014;23(03):329–32.
Google Scholar - Yanqin Wu, Shen L, Jihong Yu, Sun Y. Evaluation of the effect of entecavir in the treatment of chronic hepatitis B with hepatic steatosis. J Clin Hepatol. 2017;33(05):849–52.
Google Scholar - Zhongsheng Yu, Zhou B, Tang D, Liu S. Study on the impact of hepatic steatosis on the antiviral effect in chronic hepatitis B. Chin Pract Med. 2019;14(10):48–9. https://doi.org/10.14163/j.cnki.11-5547/r.2019.10.022.
Article Google Scholar - Bingbing Yu, Zhang L, Chen Z, Zhong Y. Effect of entecavir antiviral therapy in chronic hepatitis B patients with hepatic steatosis. Chin Med Sci. 2019;9(01):250–2.
Google Scholar - Zhang W, Jianguo Yu, Zhu G, Zhao Z, Wang X. Effect of fatty liver and related factors on the efficacy of pegylated interferon alpha-2a in the treatment of chronic hepatitis B. Liver. 2014;19(09):692–4. https://doi.org/10.14000/j.cnki.issn.1008-1704.2014.09.015.
Article Google Scholar - Fang X, Qinghua M, Wei H, Dexi C, Qing HM, Wei H, et al. Clinical and pathological features of HBeAg-positive chronic hepatitis B patients with nonalcoholic fatty liver disease. Chin J Exp Clin Infect Dis (Electronic Edition). 2018;12(03):256–61.
Google Scholar - Guidelines for the prevention and treatment of chronic hepatitis B (2010 Edition). Chin J Hepatol. 2011(01):13–24
- Fatty Liver and Alcoholic Liver Disease Group, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for diagnosis and treatment of nonalcoholic fatty liver disease (2010 Revision). Chin J Hepatol. 2010;18(03):163–166
- Chinese Society of Infectious Diseases, Chinese Society of Hepatology. Guidelines for the prevention and treatment of chronic hepatitis B. Chin J Hepatol. 2005(12):881–891
- Fatty Liver and Alcoholic Liver Disease Group, Chinese Society of Hepatology. Guidelines for diagnosis and treatment of nonalcoholic fatty liver disease. Chin J Hepatol. 2006(03):161–163
- Chinese Society of Infectious Diseases, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (2019 Edition). Chin J Hepatol. 2019(12):938–961
- Fatty Liver and Alcoholic Liver Disease Group, Chinese Society of Hepatology, Fatty Liver Disease Expert Committee of Chinese Medical Doctor Association. Guidelines for the prevention and treatment of nonalcoholic fatty liver disease (2018 Update). Chin J Hepatol. 2018(03):195–203
- Zhou R, Yang L, Zhang B, Gu Y, Kong T, Zhang W, et al. Clinical impact of hepatic steatosis on chronic hepatitis B patients in Asia: a systematic review and meta-analysis. J Viral Hepat. 2023;30(10):793–802. https://doi.org/10.1111/jvh.13872.
Article CAS PubMed Google Scholar - Mao X, Cheung KS, Peng C, Mak LY, Cheng HM, Fung J, et al. Steatosis, HBV-related HCC, cirrhosis, and HBsAg seroclearance: a systematic review and meta-analysis. Hepatology. 2023;77(5):1735–45. https://doi.org/10.1002/hep.32792.
Article CAS PubMed Google Scholar - Chu CM, Lin DY, Liaw YF. Does increased body mass index with hepatic steatosis contribute to seroclearance of hepatitis B virus (HBV) surface antigen in chronic HBV infection? Int J Obes (Lond). 2007;31(5):871–5. https://doi.org/10.1038/sj.ijo.0803479.
Article PubMed Google Scholar - Joo EJ, Chang Y, Yeom JS, Ryu S. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: a cohort study. Hepatology. 2017;65(3):828–35. https://doi.org/10.1002/hep.28917.
Article CAS PubMed Google Scholar - Schuster S, Cabrera D, Arrese M, Feldstein AE. Triggering and resolution of inflammation in NASH. Nat Rev Gastroenterol Hepatol. 2018;15(6):349–64. https://doi.org/10.1038/s41575-018-0009-6.
Article CAS PubMed Google Scholar - Zhang RN, Pan Q, Zhang Z, Cao HX, Shen F, Fan JG. Saturated fatty acid inhibits viral replication in chronic hepatitis B virus infection with nonalcoholic Fatty liver disease by toll-like receptor 4-mediated innate immune response. Hepat Mon. 2015;15(5): e27909. https://doi.org/10.5812/hepatmon.15(5)2015.27909.
Article PubMed PubMed Central Google Scholar - Rui F, Garcia E, Hu X, Ni W, Xue Q, Xu Y, et al. Antiviral therapy response in patients with chronic hepatitis B and fatty liver: a systematic review and meta-analysis. J Viral Hepat. 2024;31(7):372–82. https://doi.org/10.1111/jvh.13942.
Article CAS PubMed Google Scholar - Kurabe N, Igarashi H, Ohnishi I, Tajima S, Inoue Y, Takahashi Y, et al. Visualization of sphingolipids and phospholipids in the fundic gland mucosa of human stomach using imaging mass spectrometry. World J Gastrointest Pathophysiol. 2016;7(2):235–41. https://doi.org/10.4291/wjgp.v7.i2.235.
Article PubMed PubMed Central Google Scholar - Sun F, Li H, Sun D, Fu S, Gu L, Shao X, et al. Single-cell omics: experimental workflow, data analyses and applications. Sci China Life Sci. 2025;68(1):5–102. https://doi.org/10.1007/s11427-023-2561-0.
Article PubMed Google Scholar - Tian H, Yang Z, Xu X, Liu L. Three-dimensional chromosome conformation capture and its derived technologies. Sheng Wu Gong Cheng Xue Bao. 2020;36(10):2040–50. https://doi.org/10.13345/j.cjb.200112.
Article PubMed Google Scholar - Diao Y, Tang J, Wang X, Deng W, Tang J, You C. Metabolic syndrome, nonalcoholic fatty liver disease, and chronic hepatitis B: a narrative review. Infect Dis Ther. 2023;12(1):53–66. https://doi.org/10.1007/s40121-022-00725-6.
Article PubMed Google Scholar - Khalili M, Kleiner DE, King WC, Sterling RK, Ghany MG, Chung RT, et al. Hepatic steatosis and steatohepatitis in a large North American cohort of adults with chronic hepatitis B. Am J Gastroenterol. 2021;116(8):1686–97. https://doi.org/10.14309/ajg.0000000000001257.
Article PubMed PubMed Central Google Scholar - Lu Y, Yang X, Kuang Q, Wu Y, Tan X, Lan J, et al. HBx induced upregulation of FATP2 promotes the development of hepatic lipid accumulation. Exp Cell Res. 2023;430(1): 113721. https://doi.org/10.1016/j.yexcr.2023.113721.
Article CAS PubMed Google Scholar - Zhang J, Ling N, Lei Y, Peng M, Hu P, Chen M. Multifaceted interaction between hepatitis B virus infection and lipid metabolism in hepatocytes: a potential target of antiviral therapy for chronic hepatitis B. Front Microbiol. 2021;12: 636897. https://doi.org/10.3389/fmicb.2021.636897.
Article PubMed PubMed Central Google Scholar - Mukherjee S, Kar A, Khatun N, Datta P, Biswas A, Barik S. Familiarity breeds strategy: in silico untangling of the molecular complexity on course of autoimmune liver disease-to-hepatocellular carcinoma transition predicts novel transcriptional signatures. Cells. 2021. https://doi.org/10.3390/cells10081917.
Article PubMed PubMed Central Google Scholar - Shi YW, Yang RX, Fan JG. Chronic hepatitis B infection with concomitant hepatic steatosis: current evidence and opinion. World J Gastroenterol. 2021;27(26):3971–83. https://doi.org/10.3748/wjg.v27.i26.3971.
Article CAS PubMed PubMed Central Google Scholar - Younossi ZM, Corey KE, Lim JK. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: expert review. Gastroenterology. 2021;160(3):912–8. https://doi.org/10.1053/j.gastro.2020.11.051.
Article PubMed Google Scholar - Kim DH, Choi YM, Jang J, Kim BJ. Global prevalence and molecular characteristics of three clades within hepatitis B virus subgenotype C2: predominance of the C2(3) clade in South Korea. Front Microbiol. 2023;14:1137084. https://doi.org/10.3389/fmicb.2023.1137084.
Article PubMed PubMed Central Google Scholar
Funding
Guizhou Provincial Science and Technology Department Fund (Qiankehe Support [2021] General 049) and Guizhou Provincial Science and Technology Department Fund (Qiankehe Platform Talent-CXTD[2023]028) funded the study. The authors provided funding for the journal’s publication fees.
Author information
Authors and Affiliations
- Department of Nursing, Affiliated Hospital of Zunyi Medical University, Zunyi, 563000, China
Qianqian Zhu, Chengde Su, Mingdan Li, Yali Xu, Qian Liu, Ying Zhang, Xinyi Zhang, Qiuxiang Li & Ping Yang - Department of Infectious Diseases, Affiliated Hospital of Zunyi Medical University, Zunyi, 563000, China
Qianqian Zhu, Chengde Su, Qiuxiang Li, Yawen Luo & Ping Yang - School of Nursing, Zunyi Medical University, Zunyi, 563000, China
Qianqian Zhu, Chengde Su, Mingdan Li, Yali Xu, Qian Liu, Ying Zhang, Xinyi Zhang, Huajun Wang & Ping Yang
Authors
- Qianqian Zhu
- Chengde Su
- Mingdan Li
- Yali Xu
- Qian Liu
- Ying Zhang
- Xinyi Zhang
- Qiuxiang Li
- Huajun Wang
- Yawen Luo
- Ping Yang
Contributions
Qianqian Zhu: Writing-original draft, designed figures, Methodology, Formal analysis, Conceptualization. Chengde Su: Software application, Formal analysis, Data curation. Mingdan Li: Writing-review & editing, designed figures, Data curation. Yali Xu: Writing-review & editing, designed figures, Methodology, Formal analysis. Qian Liu: Data curation. Ying Zhang: designed figures. Xinyi Zhang: Visualization, Provision of materials. Qiuxiang Li, Huajun Wang: performed the quality assessment. Yawen Luo: Writing-review & editing. Ping Yang: critically reviewed and revised the manuscript. All authors read and approved of the final manuscript.
Corresponding author
Correspondence toPing Yang.
Ethics declarations
Conflict of Interest
Qianqian Zhu: Chengde Su: Mingdan Li: Yali Xu, Qian Liu, Ying Zhang, Xinyi Zhang, Qiuxiang Li, Huajun Wang, Yawen Luo, Yawen Luo, Ping Yang declare that they have no conflicts of interest.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhu, Q., Su, C., Li, M. et al. Impact of Metabolic Dysfunction-Associated Fatty Liver Disease of Varying Severity on Antiviral Treatment Outcomes and Clinical Prognosis in Patients with Chronic Hepatitis B: A Systematic Review and Meta-analysis.Infect Dis Ther 14, 1599–1617 (2025). https://doi.org/10.1007/s40121-025-01189-0
- Received: 15 April 2025
- Accepted: 23 June 2025
- Published: 10 July 2025
- Version of record: 10 July 2025
- Issue date: August 2025
- DOI: https://doi.org/10.1007/s40121-025-01189-0