International Group for Reducing Inappropriate Medication Use & Polypharmacy (IGRIMUP): Position Statement and 10 Recommendations for Action (original) (raw)
Abstract
Globally, the number of drug prescriptions is increasing causing more adverse drug events, which is now a significant cause of mortality, morbidity, and disability that has reached epidemic proportions. The risk of adverse drug events is correlated to very old age, multiple co-morbidities, dementia, frailty, and limited life expectancy, with the major contributor being polypharmacy. Each characteristic alters the risk–benefit balance of medications, typically reducing anticipated benefits and amplifying risk. Current clinical guidelines are based on evidence proven in younger/healthier adult populations using a single disease model and their application to older adults with multimorbidity, in whom testing has not been conducted, yields a different risk–benefit prospect and makes inappropriate medication use and polypharmacy inevitable. Applying inappropriate clinical practice guidelines to older adults is antithetical to good healthcare, is likely to increase health inequity, and is associated with substantial negative clinical, economic, and social implications for health systems. The casualties are on the scale of a war or epidemic, yet are usually invisible in measures of healthcare quality and formal recommendations. Radical and rapid action is required to achieve a better quality of life for older populations and to remain true to the principles of medical professionalism and evidence-based medicine that place patients’ interests and autonomy at the fore. This first International Group for Reducing Inappropriate Medication Use & Polypharmacy position statement briefly details the causes, consequences, and extent of inappropriate medication use and polypharmacy. This article outlines current strategies to reduce inappropriate medication use, provides evidence for their effect, and then proposes recommendations for moving forward with 10 recommendations for action and 12 recommendations for research. We conclude that an urgent integrated effort to reduce inappropriate medication use and polypharmacy should be a leading global target of the highest priority. The cornerstone of this position statement from the International Group for Reducing Inappropriate Medication Use & Polypharmacy is the understanding that without evidence of definite relevant benefit, when it comes to prescribing, for many older patients ‘less is more’. This approach differs from most other current recommendations and guidance in medical care, as the focus is on what, when, and how to stop, rather than on when to start medications/interventions. Disrupting the framework that indiscriminately applies standard guidelines to older adults requires a new approach that better serves patients with multimorbidity. This transition requires a shift in medical education, research, and diagnostic frameworks, and re-examination of the measures used as quality indicators. In achieving this objective, we promote a return to some of the original concepts of evidence-based medicine: which considers scientific data (where it exists), clinical judgment, patient/family preference, and context. A shift is needed: from the current model that focuses on single conditions to one that simultaneously considers multiple conditions and patient priorities. This approach reframes the clinician’s role as a professional providing care, rather than a disease technician.
Similar content being viewed by others
FormalPara Key Points
| Polypharmacy is an urgent issue that requires a co-ordinated global effort to provide medical care systems that better serve patients with multimorbidity. |
|---|
| This transition requires a shift in medical education, research, and diagnostic frameworks, and reexamination of the measures used as quality indicators. |
| This position statement from the International Group for Reducing Inappropriate Medication Use & Polypharmacy briefly summarizes the current situation and provides a call to action for moving forward, proposing 10 recommendations for action and 12 recommendations for research. |
1 Background and Objectives
Over recent decades, the number of drug prescriptions has increased globally. As a result of their increased number of chronic diseases and geriatric syndromes, older adults are the main drug users, consuming over one-third of medications used in the USA [1, 2]. Compared with a younger population, older adults are at higher risk of medication-related adverse events [3]. Vulnerability characteristics such as very old age, multiple co-morbidities, dementia, frailty, and limited life expectancy markedly alter the risk–benefit balance of medications. These characteristics are often accompanied by impaired medication clearance and reduced physiological reserve [4] and, in combination with polypharmacy (traditionally understood as taking five or more long-term medications), significantly reduce benefit and amplify the risk of most drug therapies, thus making adverse drug events (ADEs) and inappropriate medication use almost inevitable. There is a robust literature and reviews that already describe the negative medical, economic, and social consequences of inappropriate medication use and polypharmacy (IMUP), which are summarized in Table 1.
Table 1 Negative outcomes and hazards of inappropriate medication use and polypharmacy
Many risk factors, such as elevated cholesterol, glucose, and blood pressure, lose much of their negative portent in older age [37,38,39] and some risk factors reverse to become predictors of better outcomes. Therefore, treating marker-defined conditions to target goals developed for younger people may be ineffective or, worse, damaging [26, 40,41,42].
While noting the importance and risks of under-prescribing in some settings, the focus of this position paper is over-prescribing. Medical errors, including ADEs are now leading causes of death, with many events yet to be recognized [24, 43, 44]. These casualties are on the scale of a war or epidemic; yet, this iatrogenic ‘epidemic’ is nearly invisible in guideline recommendations and quality measures. Just as with a war or epidemic, radical and rapid action is required to place the interests and autonomy of older populations at the fore, to achieve the best quality of life possible. This radical action is not against drugs or their prescription, nor against drug companies, but against the many forces that, in aggregate, can lead to damaging or unwanted overmedication of the elderly.
As the portion of older subpopulations with complex morbidity expands, many warn that we are ill equipped intellectually, economically, and professionally to face this global challenge, in general, and the IMUP epidemic, in particular [36, 45,46,47,48,49,50]. We therefore believe that current global trends in healthcare require assertive and coordinated action against IMUP. We present the first position statement of IGRIMUP (International Group for Reducing Inappropriate Medication Use & Polypharmacy) on the international co-operative effort, and recommendations for actions needed to prevent and counter IMUP and its drivers globally.
2 Main Approaches for Reducing Inappropriate Medication Use and Polypharmacy and Their Clinical Efficacies
Many studies report on tools and strategies that have attempted to reduce IMUP. However, the global increase in the IMUP epidemic indicates that their efficacy is limited. There is inadequate information about the beneficial effect of these approaches on health outcomes, such as mortality, morbidity, function, cognition, patient well-being, healthcare services’ utilization, and cost. A 2010 cohort study of multiple medication discontinuation using a ‘consecutive patient’ sampling frame [50] is often referenced in the literature on polypharmacy as supporting the benefical health outcomes of using a general approach to drug discontinuation in community-dwelling patients. A further cohort study suggests long-term health benefits from drug discontinuation, compared with controls who declined deprescribing [51]. These prospective studies achieved medication reductions and apparent benefits to health outcomes while measuring potential adverse effects, but were not randomized controlled trials. Studies in long-term residential care settings show that medications can be reduced but have yet to demonstrate beneficial outcomes, aside from a reduction in falls in those who have already had a fall [52, 53]. Other evidence of potential benefit is based on retrospective assessments of explicit lists of ‘drugs to avoid’, comparing these with admissions for ADEs to determine the sensitivity in predicting such events. One review concluded that evidence for health outcomes from pharmacist and physician based interventions to reduce polypharmacy is limited and conflicting, and recommended randomized controlled trials evaluating multidisciplinary interventions and clinical outcomes across different settings [54].
We present the main methods suggested to improve IMUP and briefly note where successful clinical outcomes are achieved by some of them. These methods are usually categorized as explicit criteria-based tools (some computer assisted) and implicit judgment-based tools.
2.1 Computer-Assisted Digital Tools
A variety of computer programs have been developed in many countries to ease application of the explicit criteria to detect IMUP. In countries with full medication information in an electronic medical record, these systems may provide alarms that alert clinicians to a variety of potential drug interactions as they turn on their computers and try to prescribe new drugs [55]. However, a systematic review of 10 studies of computerized physician order entry with clinical decision support showed a mixed effect on the reduction of ADEs [56]. There are suggestions that automatic messages, particularly where there are many alerts and more repeated alerts, lead to reminder fatigue and insensitivity [57]. Others warn that relying too much on computers, where decision support is single-disease oriented, may be misleading or harmful to older patients [50, 58].
2.2 Explicit Tools
Explicit tools include lists of drugs to avoid or specific indicators of inappropriate medication use. A comparison of seven tools failed to show close similarities [59]. The most widely used are the Beers criteria and the Screening Tool of Older Persons’ potentially inappropriate Prescriptions/Screening Tool to Alert to Right Treatment (STOPP/START) [60,61,62,63,64,65,66]. Both were updated in 2015. The criteria divide potentially inappropriate medications into: medications to avoid prescribing; to avoid in certain circumstances; and to be used with caution. Following the Beers initiative in 1991, other country-specific lists were established: in Germany, PRISCUS (Latin for ‘old and venerable’) [67] and the Fit for the Aged (FORTA); in France, a consensus panel list [68]; in Norway, the Norwegian General Practice (NORGEP) list [69]; in Canada, the Improving Prescribing in the Elderly Tool (IPET) [70]; and others [71, 72], including in Australia [73, 74] and Thailand [75]. In the USA, Assessing Care of Vulnerable Elders (ACOVE) [76, 77], the Health Care Financing Administration expert consensus panel drug utilization review criteria [78], and Geriatric Risk Assessment MedGuide (GRAM) [79] have also been developed.
Other scales specifically identify the anticholinergic burden of medications. Among the multiple scales developed to assess anticholinergic burden, the Drug Burden Index seemed to best predict adverse health outcomes [80].
The Beers criteria has not yet been evaluated in a randomized controlled trial and, therefore, the extent of the lists effect in decreasing ADEs, morbidity, mortality, hospitalization and/or costs is uncertain. Analysis in a prospective cohort study shows modest sensitivity in predicting hospital admissions as a result of ADEs [65]. Similarly, while applying STOPP/START criteria in older inpatients significantly improves the appropriateness of prescribing [64] and could potentially reduce avoidable ADEs that may cause urgent hospitalization [65, 81], the tool has not yet been prospectively tested in the primary care setting and its effect on long-term outcomes is yet to be described. These lists are limited by their single drug/disease-oriented approach, and require regular updating.
Applying the Geriatric Risk Assessment MedGuide (GRAM) tool in long-term care was proven efficacious in reducing the rate of delirium, hospitalizations, and mortality resulting from ADEs in newly admitted residents [79]. However, other clinical outcomes were not assessed and it has not yet been prospectively tested in the primary care setting.
‘Fit for the Aged Criteria’ (FORTA) combines both negative and positive labeling based on individual indications. It ranks drugs into four groups depending on evidence for safety, efficacy, and overall age appropriateness: (A) indispensable with obvious benefit; (B) proven efficacy but limited effects or possible safety concerns; (C) questionable efficacy or safety; (D) avoid [82]. A randomized trial to validate FORTA in hospitalized geriatric patients was associated with an improvement in medication quality and a reduction in ADEs [83]. Its effect on overall patient outcomes is not yet tested beyond feasibility studies [84].
Other evidence of the potential benefit of explicit tools is based on retrospective assessments that use lists of ‘drugs to avoid’ and compare these with admissions for ADEs to determine the sensitivity in predicting (and therefore their potential to prevent) such events. These studies provide retrospective epidemiological data, but again, do not supply adequate unbiased proof that is required to understand the benefits of prospective application of these lists. There are other ongoing positive efforts to develop frameworks that (1) guide drug review; (2) reduce the harms of polypharmacy; and (3) advance single drug class deprescribing guidelines [50, 63, 85,86,87].
2.3 Implicit Approaches
To judge medication appropriateness, implicit approaches take into consideration research data, clinical circumstances, and patient/family preferences [88, 89]. Implicit approaches are less algorithmic and require much more time, knowledge, and judgment. This more complex approach is better suited to multimorbidity and to a shared decision-making model. However, the complexity of this approach creates problems for study design and relatively few studies have been carried out to assess efficacy and safety.
2.3.1 Comprehensive Geriatric Assessment
Comprehensive Geriatric Assessment (CGA) is a thorough evaluation of the older patient’s characteristics, cognition, function, medical conditions, and social situation, which is used to identify problems and appropriately manage them through individualized care plans [90]. Though there is variation in the execution of the CGA, drug review is an integral component, with the minimal requirement of medication count and assessment, preferably at each visit and at least annually [76]. If time limitation is a concern, the minimal requirement is to focus on those drugs with the highest risk or highest benefit [28]. Multidisciplinary team approaches using the CGA in frail hospitalized older adults have been shown to significantly reduce serious ADEs and IMUP, when compared with usual care [91]. Therefore, the CGA in itself may serve as an important tool for reducing IMUP [88, 89].
2.3.2 Medication Appropriateness Index
The Medication Appropriateness Index is a judgment-based process measure of prescribing appropriateness that assesses 10 elements of prescribing: indication, effectiveness, dose, correct directions, practical directions, drug–drug interactions, drug–disease interactions, duplication, duration, and cost. The Medication Appropriateness Index is the only implicit tool with validated inter-rater reliability [92]. In heterogeneous smaller studies, the Index reduced ADEs compared with usual care [93], but the tool has not been extensively used in larger settings.
2.3.3 Prescribing Optimization Method
The Prescribing Optimization Method is based on six questions: Is undertreatment present and the addition of medication indicated? Does the patient adhere to his/her medication schedule? Which drugs can be withdrawn or which drugs are inappropriate for the patient? Which adverse effects are present? Which clinically relevant interactions are to be expected? Should the dose, dose frequency, or form of drug be adjusted? [94].
2.3.4 SMART (Specific, Measurable, Acceptable, Realistic, and Time-framed) Tool
The SMART (Specific, Measurable, Acceptable, Realistic and Time-framed) Tool is a method for reviewing complex geriatric drug regimens. It consists of 10 questions that draw attention to the appropriateness and safety of the drug plan. Therapeutic objectives are developed to improve quality of life, which the authors of the tool believe lead to a better understanding of geriatric clinical pharmacology, the special needs of older patients, and appropriate use of healthcare resources [95].
2.3.5 Patient-Focused Drug Surveillance
Patient-focused drug surveillance was developed in Swedish nursing homes [96]. The intervention involved a physician-led, patient-focused approach that optimizes medication therapy and reduces polypharmacy by taking the patient’s health conditions into account. This approach advocates for a discussion of the benefits and risks of drug therapy with frail older people, accompanied by close monitoring and re-evaluation.
2.3.6 CRIME (CRIteria to Assess Appropriate Medication Use Among Elderly Complex Patients)
CRIME (CRIteria to assess appropriate Medication use among Elderly complex patients) offers 19 explicit recommendations on pharmacological treatments of only 5 common conditions (diabetes mellitus, hypertension, congestive heart failure, atrial fibrillation, and coronary heart disease). In line with vulnerability characteristics, CRIME takes into consideration complex aspects of aging (i.e. limited life expectancy, functional/cognitive impairments, and geriatric syndromes) that may negatively affect a drug’s benefit/risk ratio and therefore reduce its efficacy [97].
2.3.7 PATH (Palliative and Therapeutic Harmonization) Program
The PATH (Palliative and Therapeutic Harmonization) program was developed in Canada [98]. One goal of this program is to achieve frailty-specific treatment guidelines to replace conventional clinical practice guidelines using an evidence review of common chronic conditions, such as hypertension, diabetes, and statin use for the prevention of cardiovascular disease [[99](#ref-CR99 "Dalhousie University. Faculty of Medicine continuing medical education. 8 Jan 2014. Available from: http://cme.medicine.dal.ca/ADS.htm
. Accessed 2 May 2018."),[100](#ref-CR100 "Mallery LH, et al. Evidence-informed guidelines for treating frail older adults with type 2 diabetes: from the Diabetes Care Program of Nova Scotia (DCPNS) and the Palliative and Therapeutic Harmonization (PATH) program. J Am Med Dir Assoc. 2013;14(11):801–8."),[101](/article/10.1007/s40266-018-0554-2#ref-CR101 "Mallery LH, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med. 2014;81(7):427–37.")\]. The guidelines consider the clinical relevance of commonly accepted outcomes when there is frailty and suggest that even the outcome of reduced mortality may not be relevant with frailty owing to multiple competing risks for mortality. Based on an evidence review, the panel of experts conclude that rigid blood pressure, serum glucose, and cholesterol targets may be harmful in patients with severe frailty.2.3.8 10-Step Discontinuation Guide
The 10-Step Discontinuation Guide was formulated by Scott et al. and synthesizes suggestions and guidance raised in other frameworks [28, 86, 102, 103]. The guide recommends a case-specific framework for each patient, with confirmed face validity showing that doctors who used the guide suggested more drugs for discontinuation for a hypothetical case [104].
2.3.9 Good Palliative Geriatric Practice Algorithm
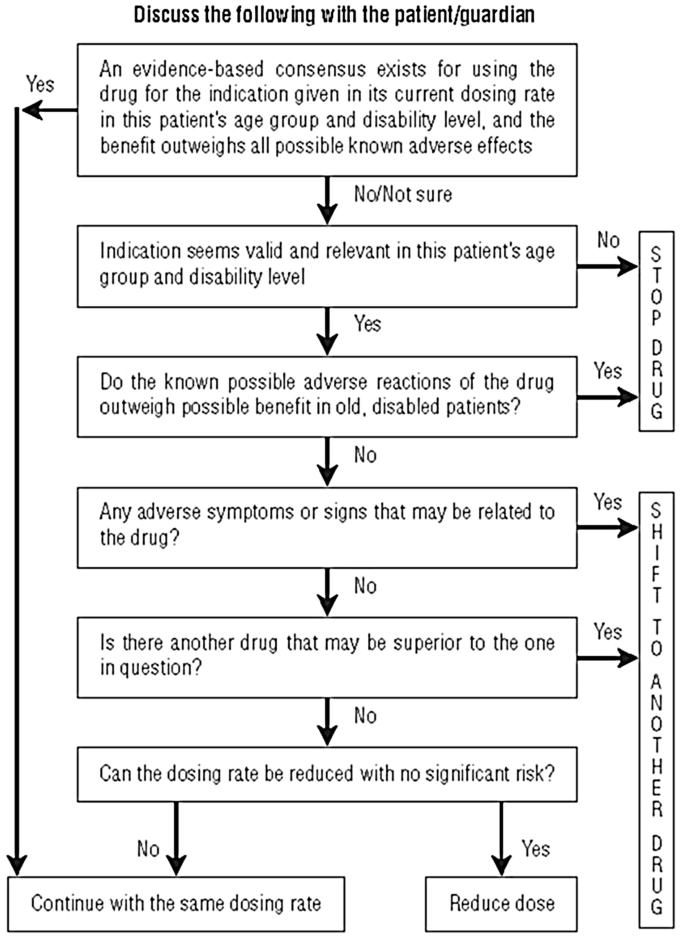
The Good Palliative Geriatric Practice Algorithm is a proactive process that simultaneously discontinues as many ‘non-life saving’ medications as possible where there is an absence of evidence in older people. Attention is paid to a patient’s circumstances and preference for care, and to providing follow-up monitoring (see Fig. 1) [50]. In nursing homes, the discontinuation of 2.8 drugs per patient led to a significant 24% reduction in mortality and 18% in referral to acute care facilities [105]. In community-dwelling elderly individuals, discontinuation of 4.4 drugs per patient led to an improvement of global health and well-being in 88% of the patients; only 2% of the discontinued drugs had to be re-administered and no significant adverse events were recorded as a result of deprescribing [50]. This approach is translatable into any setting; but requires knowledge of the limitations of standard guidelines. A longer follow-up (> 3 years) cohort study showed significantly better clinical outcomes in older people undergoing multiple deprescribing, compared with those who did not, suggesting benefits are sustained [51].
Fig. 1
Good Palliative Geriatric Practice Algorithm
3 Moving Forward
"Where is the Wisdom We Have Lost in Knowledge?"
This phrase, from TS Elliott’s Choruses from The Rock, reminds us that the competent use of multiple medicines requires the application of wisdom, based on a critical understanding of the limitations and generalizability of research and of patient circumstances and preferences. The original definition of evidence-based medicine (EBM) stated: “_Evidence_-_based medicine is the integration of best research evidence with clinical expertise and patient values_” [106]. The EBM diagram shows how these concepts are integrated using clinical judgment. More recently evidence-based medicine has been reconfigured as single disease guideline adherence, whereby evidence largely encourages clinicians to start medications, but the integration of clinical judgment and patient-centered care are lost. In this situation variation may be seen as poor practice.
The cornerstone of this position statement is that without evidence of definite and relevant benefit, when prescribing for many older patients ‘less is more’. The approach differs from standard guidance as the focus here is on what, when, and how to stop, rather than on when to start medications/interventions. The scope can be extended beyond frailty to include many subpopulations with multimorbidity, disability, or limited life expectancy. It extends beyond the list-based approach of any explicit tool.
Our principal goal is to promote a return to the original concept of EBM and restore the physician’s role to one of a professional providing care, rather than a disease or algorithm technician. This role requires integration of patient preference and context, clinical judgment, and scientific data (where it exists), using a drug prescribing approach that can be customized to each older adult in a fashion aligned with patient-centered medicine ideals. A return to the more nuanced EBM framework, as described, will produce variation in care; but consistency in care is not synonymous with best care.
Most elderly patients have not had a formal review of their long-term medications and there is evidence that most patients, if asked, would like to reduce their medications and there is some evidence that patient-directed approaches to reducing single medicines can be effective [[107](#ref-CR107 "Canadian Institute for Health Information. Seniors and the health care system: what Is the impact of multiple chronic conditions? 2011. Available from: https://secure.cihi.ca/free_products/air-chronic_disease_aib_en.pdf
. Accessed 2 May 2018."),[108](#ref-CR108 "Reeve E, et al. People’s attitudes, beliefs, and experiences regarding polypharmacy and willingness to deprescribe. J Am Geriatr Soc. 2013;61(9):1508–14."),[109](/article/10.1007/s40266-018-0554-2#ref-CR109 "Tannenbaum C, et al. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890–8.")\]. The potential benefits of medication review to healthcare systems and patients are substantial and include reduced drug costs, ADEs, morbidity, and hospitalizations. Reducing IMUP should be a shared goal of the highest urgency that requires an integrated international effort.There is a growing call for a medical system that better serves patients with multimorbidity [36, 58, 110,111,, 111]. Barriers to addressing inappropriate medication use derive from the limitations of research; an inadequate framework for the clinical care of patients with polypharmacy, performance measures and incentives that focus on starting but not stopping medications; commercial influences; health system design; inadequate education about medication risks; and insufficient funding, all of which impact prescribers, pharmacists, and patients. Addressing these barriers will require changes to medical education, research practice, diagnostic frameworks, measures of quality, and policy [36, 58, 112,113,114].
3.1 10 Recommendations of the International Group for Reducing Inappropriate Medication Use & Polypharmacy
We make 10 recommendations for action and 12 recommendations for research.
Individual and System Approaches to Inappropriate Medication Use and Polypharmacy
- Review the medications of all older adults with an eye to deprescribing, particularly those who are vulnerable to the adverse effects of medication.
Patients with polypharmacy deserve special attention. Consideration of medication reduction does not mean that medication reduction is appropriate for everyone. Likewise, after stopping a medication, the need to reintroduce that medication should not be seen as a failure.
- Before initiating a potentially ‘appropriate’ medication, consider the validity of the evidence based on patient characteristics and preferences.
Clinicians should bear in mind that disease-specific guidelines (and perceptions of ‘under-prescribing’) are driven by data from trials that almost always exclude (or do not separately evaluate) older adults, particularly those with vulnerability characteristics. When faced with polypharmacy, patients can be overwhelmed with the burden of treatment, which can compromise adherence. Thus, medications may need to be prioritized, recognizing that using all potentially ‘helpful’ medications may not be appropriate. Deprescribing based on the paradigm described may lead to labels of ‘under-prescribing’, but needs to be reframed as ideal prescribing in this context.
- Consider each medication for potential withdrawal, extending beyond standardized lists.
Many unnecessary and inappropriate medications for an individual are not included in lists of ‘potentially inappropriate medications’ [50].
- Employ mixed implicit and explicit approaches to polypharmacy.
Explicit criteria contribute to the detection of IMUP and are useful support systems, but inadequate on a stand-alone basis. Drugs-to-avoid criteria are insufficiently accurate, focus only on the more common causes of ADEs, and may provide false reassurance: prescribing 10–15 ‘non-list’ medications to patients is still likely to do more harm than good [50, 59, 115,116,117].
- Address the underrepresentation of older patients in clinical trials.
Older adults are largely excluded from randomized controlled trials; and those that are included are non-representative of an older population with multimorbidity [118,119,120,121,122]. Randomized controlled trial findings may, thus, overestimate the benefit-risk balance in favor of increased prescribing in the group least able to tolerate it. This approach is antithetical to good healthcare and is likely to increase health inequity [111, 123,124,125]. Beyond recruitment strategies, the impact of underrepresentation/misrepresentation of older patients in trials might be at least partially addressed by incorporating stratified randomization and analysis, where possible. However, trial participation is disproportionately burdensome to patients with physical and cognitive limitations, who may also be unable to consent. Thus, approaches to enhance the enrollment of older adults must be complemented by explicit recognition that trial findings often overstate benefit over risk in older adults.
We also need a better understanding of the dose–effect curve and benefit/risk ratio of drugs used by older adults and especially for the subpopulation of older adults with frailty and multimorbidity. While observational studies contribute to our understanding of how treatment effects may be different in older age groups, on balance multiple concurrent medication use in older adults is a global experiment with little data collection. We need data on the overall health effects of this type of prescribing.
- Acknowledge and address commercial influences on polypharmacy: trial results should not be implemented in older adults unless access to all available patient-level data is provided. Appropriate outcome measures should be required before licensing indications that include older populations.
The degree to which commercial interests can potentially distort scientific data is well documented [126,[127](#ref-CR127 "Golomb BA. Conflict of Interest in Medicine. 5 Oct 2008. Available from: http://thesciencenetwork.org/programs/beyond-belief-candles-in-the-dark/beatrice-golomb
. Accessed 2 May 2018."),[128](#ref-CR128 "Gotzsche P. Deadly medicines and organised crime. London: Radcliffe Medical Press; 2013."),[129](#ref-CR129 "Healy D. Let them eat Prozac: the unhealthy relationship between the pharmaceutical industry and depression. New York: New York University Press; 2006."),[130](#ref-CR130 "Healy D. Pharmageddon. California: University of California Press; 2012."),[131](/article/10.1007/s40266-018-0554-2#ref-CR131 "Healy D, Cattel D. Interface between authorship, industry and science in the domain of therapeutics. Br J Psychiatry. 2003;183(1):22–7.")\]. Trials can be structured to provide commercially favorable results and there is limited access to patient-level trial and adverse-event data, which are grounds for precautionary prescribing \[[132](/article/10.1007/s40266-018-0554-2#ref-CR132 "Doshi P, et al. Restoring invisible and abandoned trials: a call for people to publish the findings. BMJ. 2013;346:f2865.")\]. Use of intermediate outcomes, publication bias, and overhyping of new or immature research results by media and pharmaceutical companies result in a research narrative that overestimates efficacy, underestimates harms, and fuels IMUP \[[133](#ref-CR133 "Chan A, et al. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. J Am Med Assoc. 2004;291(20):2457–65."),[134](#ref-CR134 "Bero L, et al. Factors associated with findings of published trials of drug–drug comparisons: why some statins appear more efficacious than others. PLoS Med. 2007;4(6):e184."),[135](/article/10.1007/s40266-018-0554-2#ref-CR135 "Ciani O, et al. Comparison of treatment effect sizes associated with surrogate and final patient relevant outcomes in randomised controlled trials: meta-epidemiological study. BMJ. 2013;346:f457.")\]. Evidence bias is commonly compounded by biased interpretation, where key opinion leaders have industry conflicts of interest \[[136](/article/10.1007/s40266-018-0554-2#ref-CR136 "Choudhry NK, Stelfox HT, Detsky AS. Relationships between authors of clinical practice guidelines and the pharmaceutical industry. J Am Med Assoc. 2002;287(5):612–7.")\].- Medical education needs a stronger focus on IMUP and its potential negative impact. Education about generalist approaches to multimorbidity should teach prioritization skills and aim to improve the clinician's understanding of the strengths and weaknesses of evidence and how best to apply standard models of care to vulnerable older adults with multimorbidity.
Currently these topics are inadequately emphasized in the curriculum to doctors, nurses, and pharmacists [137] with insufficient education about the harm of polypharmacy, specific drug-class ADEs, the importance of medication reviews, and how specialty prescribing may increase IMUP and lead to prescribing cascades [138, 139].
- Medical training should review methods to stop treatments and provide equal attention to drug side effects and benefits.
When systematically discontinuing medications, physicians face multiple barriers, including fear of lawsuits, uncertainty about the best approach to combat IMUP, and lack of evidence-based studies on why, when, and how to stop medications [58, 140, 141]. Most patients would be agreeable to reducing medication numbers, if recommended. However, when elderly patients/families want to reduce the drug load, they are pressured not to discontinue medications [141, 142]. Providing more balanced communication on risk and benefit, such as reviewing numbers needed to treat along with the number not benefiting, may help. Likewise, the generalizability of accepted outcomes such as all-cause mortality for elderly individuals could be discussed.
- When patients have multimorbidity, the single disease model (and its incentivization) should be spurned.
About half of people over 65 years of age have at least three coexisting chronic conditions and one in five has five or more [143, 144]. The single disease approach with adherence to clinical guidelines for each illness make polypharmacy and inappropriate medication use inevitable. Boyd et al. [123] demonstrates this clearly: a patient experiencing five chronic conditions will receive 19 doses of 12 different medications, taken at five times during the day, carrying the risk of ten attendant interactions and adverse events. Quality measures that assess care on a single disease basis might rate this compliance with guidelines as "good care" but what appears measurably better is meaningfully worse for the patient. When performance pay or professional re-certifications are linked to guideline adherence, physicians may feel coerced into persuading patients to follow treatments that do not serve their best interests.
A single disease-by-disease approach obscures the multimorbid patient’s individual pattern of symptoms and overrides their preferences for care. Data also suggest that treatments that focus on preventing single diseases at the end of the survival curve may simply change the cause of death and morbidity without making life longer or better; in essence, altering the manner of dying rather than the quality of living [145]. Efforts to reframe guidance in terms of multimorbidity also note that support for preventive drugs for single diseases weakens as life expectancy decreases and that the additional benefit provided by individual drugs may reduce (or reverse) when combined [146].
- Decisions in older complex patients should routinely consider expected survival and quality of life, giving the highest priority to patient/family preferences.
One physician should co-ordinate decisions within a shared framework (preferably in a generalist setting suited to consideration of multimorbidity and polypharmacy ideally with a pharmacist partner). Care for patients with multimorbidity should balance the burden of treatment, potential to benefit, potential harms, and personal priorities. The term ‘personalized medicine’ could be re-harnessed to describe these considerations.
Many important research and clinical needs arise from these 10 recommendations; we have proposed 12 polypharmacy research priorities in Table 2.
Table 2 12 polypharmacy research priorities
4 Conclusions
Reducing IMUP should be a high priority target. The morbidity and mortality attributed to the adverse effects of IMUP underscore this imperative. Studies suggest benefit from judicious deprescribing that may be equivalent to benefits achieved from medical treatments. Our goal is to promote a return to the original concept of EBM that integrates patient preference, context, clinical judgment, and scientific data (where it exists). There is a pressing need to reconceptualize the framework of medical care to better serve patients with multimorbidity, which will require shifts in medical education, quality measures, and policy. To reverse this epidemic of iatrogenic morbidity and mortality, an integrated global effort from health professionals, policy makers, and consumers is required.
References
- Kaufman DW, et al. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002;287(3):337–44.
Article PubMed Google Scholar - Lombardi TP, Kennicutt JD. Promotion of a safe medication environment: focus on the elderly and residents of long-term care facilities. Medsc Pharm. 2001;2(1):23–8.
Google Scholar - Thomsen LA, et al. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007;41(9):1411–26.
Article PubMed Google Scholar - Topinkova E. Aging, disability and frailty. Ann Nutr Metab. 2008;52(Suppl. 1):6–11.
CAS PubMed Google Scholar - Gill TM, Robison JT, Tinetti ME. Predictors of recovery in activities of daily living among disabled older persons living in the community. J Gen Intern Med. 1997;12(12):757–62.
Article CAS PubMed PubMed Central Google Scholar - Alagiakrishnan K, Wiens C. An approach to drug induced delirium in the elderly. Postgrad Med J. 2004;80(945):388–93.
Article CAS PubMed PubMed Central Google Scholar - Larson EB, et al. Adverse drug reactions associated with global cognitive impairment in elderly persons. Ann Intern Med. 1987;107(2):169–73.
Article CAS PubMed Google Scholar - Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15(1):15–28.
Article CAS PubMed Google Scholar - Huffman GB. Evaluating and treating unintentional weight loss in the elderly. Am Fam Physician. 2002;65(4):640–50.
PubMed Google Scholar - Jyrkka J, et al. Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population. Pharmacoepidemiol Drug Saf. 2011;20(5):514–22.
Article PubMed Google Scholar - Gnjidic D, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65(9):989–95.
Article PubMed Google Scholar - Lai SW, et al. Polypharmacy correlates with increased risk for hip fracture in the elderly: a population-based study. Medicine (Baltimore). 2010;89(5):295–9.
Article PubMed Google Scholar - Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc. 1999;47(1):40–50.
Article CAS PubMed Google Scholar - Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: I. Psychotropic drugs. J Am Geriatr Soc. 1999;47(1):30–9.
Article CAS PubMed Google Scholar - Thapa PB, et al. Antidepressants and the risk of falls among nursing home residents. N Engl J Med. 1998;339(13):875–82.
Article CAS PubMed Google Scholar - Talasz H, Lechleitner M. Polypharmacy and incontinence. Z Gerontol Geriatr. 2012;45(6):464–7.
Article CAS PubMed Google Scholar - Akazawa M, et al. Potentially inappropriate medication use in elderly Japanese patients. Am J Geriatr Pharmacother. 2010;8(2):146–60.
Article PubMed Google Scholar - Lopez-Sendon JL, Mena MA, de Yebenes JG. Drug-induced parkinsonism in the elderly: incidence, management and prevention. Drugs Aging. 2012;29(2):105–18.
Article CAS PubMed Google Scholar - Onder G, et al. Adverse drug reactions as cause of hospital admissions: results from the Italian Group of Pharmacoepidemiology in the Elderly (GIFA). J Am Geriatr Soc. 2002;50(12):1962–8.
Article PubMed Google Scholar - Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash). 2001;41(2):192–9.
Article CAS Google Scholar - Bates DW, et al. The costs of adverse drug events in hospitalized patients: Adverse Drug Events Prevention Study Group. JAMA. 1997;277(4):307–11.
Article CAS PubMed Google Scholar - Fick DM, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716–24.
Article PubMed Google Scholar - Johnson JA, Bootman JL. Drug-related morbidity and mortality: a cost-of-illness model. Arch Intern Med. 1995;155(18):1949–56.
Article CAS PubMed Google Scholar - Perry DP. When medicine hurts instead of helps. Consult Pharm. 1999;14:1326–30.
Google Scholar - Gurwitz JH, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med. 2000;109(2):87–94.
Article CAS PubMed Google Scholar - Han BH, et al. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: the ALLHAT-LLT randomized clinical trial. JAMA Intern Med. 2017;177(7):955–65.
Article PubMed PubMed Central Google Scholar - Jyrkka J, et al. Patterns of drug use and factors associated with polypharmacy and excessive polypharmacy in elderly persons: results of the Kuopio 75+ study: a cross-sectional analysis. Drugs Aging. 2009;26(6):493–503.
Article PubMed Google Scholar - Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “There’s got to be a happy medium”. JAMA. 2010;304(14):1592–601.
Article CAS PubMed PubMed Central Google Scholar - Fu AZ, et al. Potentially inappropriate medication use and healthcare expenditures in the US community-dwelling elderly. Med Care. 2007;45(5):472–6.
Article PubMed Google Scholar - Hug BL, et al. The costs of adverse drug events in community hospitals. Jt Comm J Qual Patient Saf. 2012;38(3):120–6.
PubMed Google Scholar - Rochon PA, TJ, Gill SS, Gurwitz JH. In: Halter JB, Tinetti ME, Studenski S, High KP, Asthana S, editors. Hazzard’s Geriatric Medicine and Gerontology. New York (NY): McGraw-Hill; 2009. p. 289–302.
- Bootman JL, Harrison DL, Cox E. The health care cost of drug-related morbidity and mortality in nursing facilities. Arch Intern Med. 1997;157(18):2089–96.
Article CAS PubMed Google Scholar - Hoonhout LH, et al. Direct medical costs of adverse events in Dutch hospitals. BMC Health Serv Res. 2009;9:27.
Article PubMed PubMed Central Google Scholar - Rottenkolber D, Hasford J, Stausberg J. Costs of adverse drug events in German hospitals: a microcosting study. Value Health. 2012;15(6):868–75.
Article PubMed Google Scholar - Santibanez-Beltran S, et al. Economic cost of polypharmacy in the elderly in primary health care. Rev Med Inst Mex Seguro Soc. 2013;51(2):192–9 (in Spanish).
PubMed Google Scholar - Mangin D, Heath I, Jamoulle M. Beyond diagnosis: rising to the multimorbidity challenge. BMJ. 2012;44:e3526.
Article Google Scholar - de Ruijter W, et al. Use of Framingham risk score and new biomarkers to predict cardiovascular mortality in older people: population based observational cohort study. BMJ. 2009;338:a3083.
Article PubMed PubMed Central Google Scholar - Golomb BA, Bui AK. Fasting glucose positively predicts word memory performance in older men. Circulation. 2014;130(Suppl. 2):A13365.
Google Scholar - BA, G. The starving cell: metabolic syndrome as an adaptive process. Available from: Nature Precedings; 2011.
- The Action to Control Cardiovascular Risk in Diabetes. Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59.
Article Google Scholar - Tinetti ME, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174(4):588–95.
Article PubMed PubMed Central Google Scholar - Lipska KJ, et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA Intern Med. 2014;174(7):1116–24.
Article PubMed PubMed Central Google Scholar - Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279(15):1200–5.
Article CAS PubMed Google Scholar - Makary MA, Daniel M. Medical error: the third leading cause of death in the US. BMJ. 2016;353:i2139.
Article PubMed Google Scholar - Garfinkel D. Geriatric boom catastrophe: a major medical, economic and social nightmare of the 21st century. Proceedings of the 16th Congress of the International Association of Gerontology 1997; 364.
- Boyle P. Global burden of cancer. Lancet. 1997;349(Suppl. 2):23–6.
Article Google Scholar - Ershler WB. Geriatrics and medical oncology: finding the common ground. J Gerontol A Biol Sci Med Sci. 1997;52(6):M327–8.
CAS PubMed Google Scholar - Garfinkel D. The tsunami in 21st century healthcare: the age-related vicious circle of co-morbidity. J Nutr Health Aging. 2013;17(Suppl. 1):SS24, 227–C1, S96–7.
- Bahat G, et al. Comorbidities, polypharmacy, functionality and nutritional status in Turkish community-dwelling female elderly. Aging Clin Exp Res. 2014;26(3):255–9.
Article PubMed Google Scholar - Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170(18):1648–54.
Article PubMed Google Scholar - Garfinkel D. Poly-de-prescribing to treat polypharmacy: efficacy and safety. Ther Adv Drug Saf. 2018;9(1):25–43.
Article PubMed Google Scholar - Potter K, et al. Deprescribing in frail older people: a randomised controlled trial. PLoS One. 2016;11(3):e0149984.
Article PubMed PubMed Central CAS Google Scholar - Page AT, et al. Deprescribing in older people. Maturitas. 2016;91:115–34.
Article PubMed Google Scholar - Gnjidic D, et al. Deprescribing trials: methods to reduce polypharmacy and the impact on prescribing and clinical outcomes. Clin Geriatr Med. 2012;28(2):237–53.
Article PubMed Google Scholar - Lynch T. Management of drug–drug interactions: considerations for special populations: focus on opioid use in the elderly and long term care. Am J Manag Care. 2011;17(Suppl. 11):S293–8.
PubMed Google Scholar - Wolfstadt JI, et al. The effect of computerized physician order entry with clinical decision support on the rates of adverse drug events: a systematic review. J Gen Intern Med. 2008;23(4):451–8.
Article PubMed PubMed Central Google Scholar - Ancker JS, et al. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):36.
Article PubMed PubMed Central Google Scholar - Garfinkel D, Ilhan B, Bahat G. Routine deprescribing of chronic medications to combat polypharmacy. Ther Adv Drug Saf. 2015;6(6):212–33.
Article CAS PubMed PubMed Central Google Scholar - Chang CB, Chan DC. Comparison of published explicit criteria for potentially inappropriate medications in older adults. Drugs Aging. 2010;27(12):947–57.
Article PubMed Google Scholar - Beers MH, et al. Explicit criteria for determining inappropriate medication use in nursing home residents: UCLA Division of Geriatric Medicine. Arch Intern Med. 1991;151(9):1825–32.
Article CAS PubMed Google Scholar - American Geriatrics Society. 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227–46.
Article Google Scholar - Gallagher P, Barry P, O’Mahony D. Inappropriate prescribing in the elderly. J Clin Pharm Ther. 2007;32(2):113–21.
Article CAS PubMed Google Scholar - Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Ageing. 2008;37(6):673–9.
Article PubMed Google Scholar - Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89(6):845–54.
Article CAS PubMed Google Scholar - Hamilton H, et al. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011;171(11):1013–9.
Article PubMed Google Scholar - O’Mahony D, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213–8.
Article PubMed Google Scholar - Holt S, Schmiedl S, Thürmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Ärztebl Int. 2010;107(31–32):543–51.
PubMed PubMed Central Google Scholar - Laroche ML, Charmes JP, Merle L. Potentially inappropriate medications in the elderly: a French consensus panel list. Eur J Clin Pharmacol. 2007;63(8):725–31.
Article PubMed Google Scholar - Rognstad S, et al. The Norwegian General Practice (NORGEP) criteria for assessing potentially inappropriate prescriptions to elderly patients: a modified Delphi study. Scand J Prim Health Care. 2009;27(3):153–9.
Article PubMed PubMed Central Google Scholar - Naugler CT, et al. Development and validation of an improving prescribing in the elderly tool. Can J Clin Pharmacol. 2000;7(2):103–7.
CAS PubMed Google Scholar - McLeod P, et al. Defining inappropriate practices in prescribing for elderly people: a national consensus panel. CMAJ. 1997;156:385–91.
CAS PubMed PubMed Central Google Scholar - Rancourt C, et al. Potentially inappropriate prescriptions for older patients in long-term care. BMC Geriatr. 2004;4:9.
Article PubMed PubMed Central Google Scholar - Basger BJ, Chen TF, Moles RJ. Inappropriate medication use and prescribing indicators in elderly Australians: development of a prescribing indicators tool. Drugs Aging. 2008;25(9):777–93.
Article PubMed Google Scholar - Basger BJ, Chen TF, Moles RJ. Validation of prescribing appropriateness criteria for older Australians using the RAND/UCLA appropriateness method. BMJ Open. 2012;2(5):e001431.
Article PubMed PubMed Central Google Scholar - Winit-Watjana W, Sakulrat P, Kespichayawattana J. Criteria for high-risk medication use in Thai older patients. Arch Gerontol Geriatr. 2008;47(1):35–51.
Article PubMed Google Scholar - Shrank WH, Polinski JM, Avorn J. Quality indicators for medication use in vulnerable elders. J Am Geriatr Soc. 2007;55(Suppl. 2):S373–82.
Article PubMed Google Scholar - Shekelle P, Maclean C, Morton S, Wenger N. ACOVE quality indicators. Ann Intern Med. 2001;135:653–67.
Article CAS PubMed Google Scholar - Hanlon JT, et al. Use of inappropriate prescription drugs by older people. J Am Geriatr Soc. 2002;50(1):26–34.
Article PubMed Google Scholar - Lapane KL, et al. Effect of a pharmacist-led multicomponent intervention focusing on the medication monitoring phase to prevent potential adverse drug events in nursing homes. J Am Geriatr Soc. 2011;59(7):1238–45.
Article PubMed PubMed Central Google Scholar - Salahudeen MS, Hilmer SN, Nishtala PS. Comparison of anticholinergic risk scales and associations with adverse health outcomes in older people. J Am Geriatr Soc. 2015;63(1):85–90.
Article PubMed Google Scholar - Tosato M, et al. Potentially inappropriate drug use among hospitalised older adults: results from the CRIME study. Age Ageing. 2014;43(6):767–73.
Article PubMed Google Scholar - Wehling M. Multimorbidity and polypharmacy: how to reduce the harmful drug load and yet add needed drugs in the elderly? Proposal of a new drug classification: fit for the aged. J Am Geriatr Soc. 2009;57(3):560–1.
Article PubMed Google Scholar - Wehling M, et al. VALFORTA: a randomised trial to validate the FORTA (Fit fOR The Aged) classification. Age Ageing. 2016;45(2):262–7.
Article PubMed Google Scholar - Kuhn-Thiel AM, Weiss C, Wehling M. Consensus validation of the FORTA (Fit fOR The Aged) list: a clinical tool for increasing the appropriateness of pharmacotherapy in the elderly. Drugs Aging. 2014;31(2):131–40.
Article CAS PubMed Google Scholar - BPAC. A practical guide to stopping medicines in older people. Best Pract J. 2010;27;10–23.
Google Scholar - Scott IA, et al. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med. 2012;125(6):529–37.e4.
Article PubMed Google Scholar - Farrell B, et al. What are priorities for deprescribing for elderly patients? Capturing the voice of practitioners: a modified delphi process. PLoS One. 2015;10(4):e0122246.
Article PubMed PubMed Central CAS Google Scholar - Onder G, et al. Strategies to reduce the risk of iatrogenic illness in complex older adults. Age Ageing. 2013;42(3):284–91.
Article PubMed Google Scholar - Petrovic M, van der Cammen T, Onder G. Adverse drug reactions in older people: detection and prevention. Drugs Aging. 2012;29(6):453–62.
Article PubMed Google Scholar - Ellis G, et al. Comprehensive geriatric assessment for older adults admitted to hospital: meta-analysis of randomised controlled trials. BMJ. 2011;343:d6553.
Article PubMed PubMed Central Google Scholar - Schmader KE, et al. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. Am J Med. 2004;116(6):394–401.
Article PubMed Google Scholar - Hanlon JT, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45(10):1045–51.
Article CAS PubMed Google Scholar - Schmader KE, et al. Inappropriate prescribing and health outcomes in elderly veteran outpatients. Ann Pharmacother. 1997;31(5):529–33.
Article CAS PubMed Google Scholar - Drenth-van Maanen AC, et al. Prescribing optimization method for improving prescribing in elderly patients receiving polypharmacy: results of application to case histories by general practitioners. Drugs Aging. 2009;26(8):687–701.
Article PubMed Google Scholar - Vogt-Ferrier N. Reviewing a complicated geriatric drug regimen. Eur Geriatr Med. 2010;1(3):198–202.
Article Google Scholar - Olsson IN, Curman B, Engfeldt P. Patient focused drug surveillance of elderly patients in nursing homes. Pharmacoepidemiol Drug Saf. 2010;19(2):150–7.
Article PubMed Google Scholar - Onder G, et al. Recommendations to prescribe in complex older adults: results of the CRIteria to assess appropriate Medication use among Elderly complex patients (CRIME) project. Drugs Aging. 2014;31(1):33–45.
Article PubMed Google Scholar - Moorhouse P, Mallery LH. Palliative and therapeutic harmonization: a model for appropriate decision-making in frail older adults. J Am Geriatr Soc. 2012;60(12):2326–32.
Article PubMed Google Scholar - Dalhousie University. Faculty of Medicine continuing medical education. 8 Jan 2014. Available from: http://cme.medicine.dal.ca/ADS.htm. Accessed 2 May 2018.
- Mallery LH, et al. Evidence-informed guidelines for treating frail older adults with type 2 diabetes: from the Diabetes Care Program of Nova Scotia (DCPNS) and the Palliative and Therapeutic Harmonization (PATH) program. J Am Med Dir Assoc. 2013;14(11):801–8.
Article PubMed Google Scholar - Mallery LH, et al. Promoting higher blood pressure targets for frail older adults: a consensus guideline from Canada. Cleve Clin J Med. 2014;81(7):427–37.
Article PubMed Google Scholar - Scott IA, et al. Deciding when to stop: towards evidence-based deprescribing of drugs in older populations. Evid Based Med. 2013;18(4):121–4.
Article PubMed Google Scholar - Pollock M, Bazaldua OV, Dobbie AE. Appropriate prescribing of medications: an eight-step approach. Am Fam Physician. 2007;75(2):231–6.
PubMed Google Scholar - Scott IA, Gray LC, Martin JH, et al. Effects of a drug minimisation guide on prescribing intentions in elderly persons with polypharmacy. Drugs Aging. 2012;29:659–67.
PubMed Google Scholar - Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. Israel Med Assoc J. 2007;9(6):430–4.
Google Scholar - Sackett DL, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312(7023):71–2.
Article CAS PubMed PubMed Central Google Scholar - Canadian Institute for Health Information. Seniors and the health care system: what Is the impact of multiple chronic conditions? 2011. Available from: https://secure.cihi.ca/free_products/air-chronic_disease_aib_en.pdf. Accessed 2 May 2018.
- Reeve E, et al. People’s attitudes, beliefs, and experiences regarding polypharmacy and willingness to deprescribe. J Am Geriatr Soc. 2013;61(9):1508–14.
Article PubMed Google Scholar - Tannenbaum C, et al. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890–8.
Article PubMed CAS Google Scholar - Starfield B. Threads and yarns: weaving the tapestry of comorbidity. Ann Fam Med. 2006;4(2):101–3.
Article PubMed PubMed Central Google Scholar - Tinetti ME, Fried T. The end of the disease era. Am J Med. 2004;116(3):179–85.
Article PubMed Google Scholar - May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803.
Article PubMed Google Scholar - Mangin D, Heath I. Multimorbidity and quaternary prevention (P4). Revista Brasileira de Medicina de Família e Comunidade. 2015;10(35):5.
Article Google Scholar - Starfield B, Mangin D. An international perspective on the basis for payment for performance. Qual Prim Care. 2010;18:399–404.
PubMed Google Scholar - Chutka DS, Takahashi PY, Hoel RQW. Inappropriate medications for elderly patients. Mayo Clin Proc. 2004;79(1):122–39.
Article CAS PubMed Google Scholar - Morton A. Inappropriately defining “inappropriate medication for the elderly”. J Am Geriatr Soc. 2004;52(9):1580.
Article PubMed Google Scholar - Steinman MA, et al. Agreement between drugs-to-avoid criteria and expert assessments of problematic prescribing. Arch Intern Med. 2009;169(14):132632.
Article Google Scholar - de Souto Barreto P, Ferrandez AM, Saliba-Serre B. Are older adults who volunteer to participate in an exercise study fitter and healthier than nonvolunteers? The participation bias of the study population. J Phys Act Health. 2013;10(3):359–67.
Article Google Scholar - Golomb BA, et al. The older the better: are elderly study participants more non-representative? A cross-sectional analysis of clinical trial and observational study samples. BMJ Open. 2012;2(6):e000833.
Article PubMed PubMed Central Google Scholar - Kaiser C, et al. Selection bias of elderly patients with chronic angina referred for catheterization. Int J Cardiol. 2006;110(1):80–5.
Article CAS PubMed Google Scholar - Sugisawa H, Kishino H, Sugihara Y, Okabayashi H, Shibata H. Comparison of characteristics between respondents and nonrespondents in a national survey of Japanese elderly using six year follow-up study. Nihon Koshu Eisei Zasshi. 1999;46(7):551–62 (in Japanese).
CAS PubMed Google Scholar - Sugisawa H, Kishino H, Sugihara Y, Shibata H. Characteristics of dropouts and participants in a twelve-year longitudinal research of Japanese elderly. Nihon Koshu Eisei Zasshi. 2000;47(4):337–49 (in Japanese).
CAS PubMed Google Scholar - Boyd CM, et al. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–24.
Article CAS PubMed Google Scholar - Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351(27):2870–4.
Article CAS PubMed Google Scholar - Starfield B, Gérvas J, Mangin D. Clinical care and health disparities. Annu Rev Public Health. 2012;33:89–106.
Article CAS PubMed Google Scholar - Angell M. The truth about drug companies: how they deceive us and what to do about it. Random House; 2005.
- Golomb BA. Conflict of Interest in Medicine. 5 Oct 2008. Available from: http://thesciencenetwork.org/programs/beyond-belief-candles-in-the-dark/beatrice-golomb. Accessed 2 May 2018.
- Gotzsche P. Deadly medicines and organised crime. London: Radcliffe Medical Press; 2013.
Google Scholar - Healy D. Let them eat Prozac: the unhealthy relationship between the pharmaceutical industry and depression. New York: New York University Press; 2006.
Google Scholar - Healy D. Pharmageddon. California: University of California Press; 2012.
Book Google Scholar - Healy D, Cattel D. Interface between authorship, industry and science in the domain of therapeutics. Br J Psychiatry. 2003;183(1):22–7.
Article PubMed Google Scholar - Doshi P, et al. Restoring invisible and abandoned trials: a call for people to publish the findings. BMJ. 2013;346:f2865.
Article PubMed PubMed Central Google Scholar - Chan A, et al. Empirical evidence for selective reporting of outcomes in randomized trials: comparison of protocols to published articles. J Am Med Assoc. 2004;291(20):2457–65.
Article CAS Google Scholar - Bero L, et al. Factors associated with findings of published trials of drug–drug comparisons: why some statins appear more efficacious than others. PLoS Med. 2007;4(6):e184.
Article PubMed PubMed Central CAS Google Scholar - Ciani O, et al. Comparison of treatment effect sizes associated with surrogate and final patient relevant outcomes in randomised controlled trials: meta-epidemiological study. BMJ. 2013;346:f457.
Article PubMed PubMed Central Google Scholar - Choudhry NK, Stelfox HT, Detsky AS. Relationships between authors of clinical practice guidelines and the pharmaceutical industry. J Am Med Assoc. 2002;287(5):612–7.
Article Google Scholar - Gordon J. The under-representation of elderly patients in a problem-based medical school curriculum. Med Teach. 2007;29(8):844.
Article PubMed Google Scholar - Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997;315(7115):1096–9.
Article CAS PubMed PubMed Central Google Scholar - Gill SS, et al. A prescribing cascade involving cholinesterase inhibitors and anticholinergic drugs. Arch Intern Med. 2005;165(7):808–13.
Article PubMed Google Scholar - Anderson K, et al. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open. 2014;4(12):e006544.
Article PubMed PubMed Central Google Scholar - Reeve E, et al. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging. 2013;30(10):793–807.
Article PubMed Google Scholar - Checkland K, et al. Biomedicine, holism and general medical practice: responses to the 2004 General Practitioner contract. Sociol Health Illn. 2008;30(5):788–803.
Article PubMed Google Scholar - Marengoni A, et al. Prevalence of chronic diseases and multimorbidity among the elderly population in Sweden. Am J Public Health. 2008;98(7):1198–200.
Article PubMed PubMed Central Google Scholar - Barnett K, et al. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43.
Article PubMed Google Scholar - Mangin D, Sweeney K, Heath I. Preventive health care in elderly people needs rethinking. BMJ. 2007;335(7614):285–7.
Article PubMed PubMed Central Google Scholar - Multimorbidity: clinical assessment and management, National Institute For Health and Care Excellence guideline [NG56]. 2016: United Kingdom nice.org.uk/guidance/ng56.
Acknowledgements
The authors acknowledge Hannah Lamb, McMaster University, for her invaluable and elegant work in helping edit the manuscript, and the reviewers whose thoughtful comments improved the final version of the manuscript. Note on the International Group for Reducing Inappropriate Medication Use & Polypharmacy (IGRIMUP): all authors, along with many others worldwide, work with the shared goal of reducing the negative health and economic effects of inappropriate medication use. The idea of creating an international group to support this work via information sharing, research, and communication was born in a symposium at the 2009 International Association of Gerontology and Geriatrics Congress in Paris. The association was established at the 2013 International Association of Gerontology and Geriatrics Congress in Seoul. While the authors represent the IGRIMUP early members, IGRIMUP now includes more than 100 members from 26 countries and the efforts of this wider group are acknowledged.
Author information
Authors and Affiliations
- Department of Family Medicine, Faculty of Health Sciences, McMaster University, 100 Main Street West, Hamilton, ON, Canada
Dee Mangin - Division of Geriatrics, Department of Internal Medicine, Istanbul Medical School, Istanbul University, Istanbul, Turkey
Gülistan Bahat - Department of Medicine, University of California San Diego, San Diego, CA, USA
Beatrice A. Golomb - Division of Geriatric Medicine, Department of Medicine, Dalhousie University, Halifax, NS, Canada
Laurie Herzig Mallery & Paige Moorhouse - Department of Geriatrics, Università Cattolica del Sacro Cuore, IRCCS Fondazione Policlinico Universitario A. Gemelli, Rome, Italy
Graziano Onder - Department of Internal Medicine, Section of Geriatrics, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium
Mirko Petrovic - Wolfson Medical Center, Holon, Israel
Doron Garfinkel - Homecare Hospice Israel Cancer Association, Holon, Israel
Doron Garfinkel - Department of General Practice, University of Otago, Christchurch, New Zealand
Dee Mangin
Authors
- Dee Mangin
You can also search for this author inPubMed Google Scholar - Gülistan Bahat
You can also search for this author inPubMed Google Scholar - Beatrice A. Golomb
You can also search for this author inPubMed Google Scholar - Laurie Herzig Mallery
You can also search for this author inPubMed Google Scholar - Paige Moorhouse
You can also search for this author inPubMed Google Scholar - Graziano Onder
You can also search for this author inPubMed Google Scholar - Mirko Petrovic
You can also search for this author inPubMed Google Scholar - Doron Garfinkel
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toDee Mangin.
Ethics declarations
Funding
No funding was received for the preparation of this article.
Conflict of Interest
All authors have completed the Author Declaration Form and have no conflicts directly relevant to the content of this article.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mangin, D., Bahat, G., Golomb, B.A. et al. International Group for Reducing Inappropriate Medication Use & Polypharmacy (IGRIMUP): Position Statement and 10 Recommendations for Action.Drugs Aging 35, 575–587 (2018). https://doi.org/10.1007/s40266-018-0554-2
- Published: 14 July 2018
- Issue Date: July 2018
- DOI: https://doi.org/10.1007/s40266-018-0554-2