Dextroamphetamine Modulates the Response of the Human Amygdala (original) (raw)
Main
Recent work in both animal and human subjects has implicated limbic-based circuitry, centered on the amygdala, in the generation and regulation of normal fearful responses as well as pathological anxiety (Damasio 1994; LeDoux 1997). Likewise, amphetamine and other dopaminergic psychostimulants have been shown to produce pronounced effects on emotional behavior, including the generation of fear and anxiety (Angrist and Gershon 1970; Ellinwood et al. 1973; Hall et al. 1988). Studies in animals suggest that such anxiogenic effects may reflect dopamine (DA) augmentation of amygdala activity (Willick and Kokkinidis 1995; Harmer et al. 1997; Harmer and Phillips 1999a,b; Rosenkranz and Grace 1999, 2001, 2002). These convergent findings lead to the intriguing possibility that the emotion enhancing properties of amphetamine may be related to effects on the amygdala. We used dextroamphetamine (DEX), a potent monoaminergic agonist and anxiogenic, in a double-blind placebo controlled functional magnetic resonance imaging (fMRI) study to explore this possibility in human subjects.
METHODS
Twelve healthy subjects (five males and seven females, mean age = 33 years) gave written informed consent according to the guidelines of the National Institute of Mental Health Institutional Review Board. Each subject participated in two fMRI sessions, separated by three to ten days, with all conditions kept constant across sessions. Approximately 120 min before each session, subjects received an oral dose of either placebo (PBO) or DEX (0.25 mg/kg body weight) in a double-blind manner. The order of drug administration was counterbalanced across subjects by the pharmacy. Blood was drawn at the beginning of each session and serum DEX levels were measured using gas chromatography.
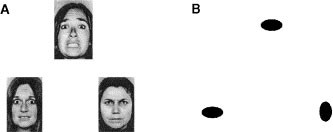
During each session, subjects completed a blocked fMRI paradigm consisting of two tasks. Subject performance (accuracy and reaction time) was monitored during all scans. In an emotion task, subjects were required to match the expression (either angry or afraid) of one of two faces to that of a simultaneously presented target face (Figure 1, panel A). These stimuli were employed because previous neuroimaging studies have revealed that facial expressions, especially those of negative affect, elicit a robust amygdala response (Davis and Whalen 2001). As a sensorimotor control task, subjects were required to match one of two geometric shapes with a simultaneously presented target shape (Figure 1, panel B). We employed simple geometric shapes and not neutral facial expressions as control stimuli because subjects may perceive such “neutral” faces ambiguously and thus elicit an amygdala response (Thomas et al. 2001). Thus, by contrasting matching of affective facial expressions with matching of simple geometric shapes we sought to maximize our ability to consistently and reliably engage the amygdala.
Figure 1
Experimental paradigm. (A) During the emotion task, subjects viewed a trio of faces and had to select one of two faces (bottom) that expressed the same emotion as the target face (top). The identity of all three faces was always different and an equal number of male and female faces was presented. (B) During the sensorimotor control, the subjects viewed a trio of geometric shapes and had to select one of two shapes (bottom) identical to the target shape (top).
Blood oxygenation-level dependent (BOLD) fMRI was conducted on a GE Signa 3T scanner (Milwaukee, WI) with a gradient echo EPI sequence, covering 24 axial slices (4 mm thick, 1 mm gap), beginning at the cerebral vertex and encompassing the entire cerebrum and the majority of the cerebellum (TR/TE = 2000/28 msec, FOV = 24 cm, matrix = 64 × 64). Whole-brain image analysis was completed using SPM99 (www.fil.ion.ucl.ac.uk/spm). Images for each subject were realigned to the first volume in the time series to correct for head motion, spatially normalized into a standard stereotactic space (Montreal Neurological Institute template) using a 12 parameter affine model and smoothed to minimize noise and residual differences in gyral anatomy with a Gaussian filter, set at 8 mm full-width at half-maximum. Voxel-wise signal intensities were ratio normalized to the whole-brain global mean. Predetermined condition effects at each voxel were calculated using a t-statistic, producing a statistical image for the contrast of the emotion task versus the sensorimotor control for each subject. These individual contrast images were then used in second-level random effects models, that account for both scan-to-scan and subject-to-subject variability, to determine task-specific regional responses at the group-level with one sample (main effects of task) and paired _t_-tests (DEX vs. PBO). A statistical threshold of p < .05, corrected for multiple comparisons based on the volumes of the regions of interest such as the amygdala, was used to identify significant responses for all comparisons (Worsley et al. 1996).
RESULTS
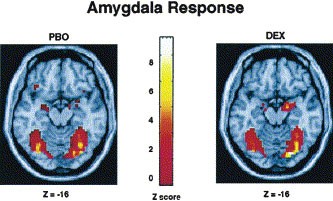
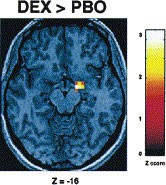
Table 1 provides a summary of brain regions that exhibited a significant BOLD fMRI response for all comparisons. Consistent with previous findings (Hariri et al. 2000), there was a strong bilateral amygdala response during the perceptual processing of facial expressions in comparison to the sensorimotor control for both PBO and DEX sessions (Figure 2). Direct comparison of the two sessions revealed a DEX induced significant increase in the response of the right amygdala (Figure 3). Additional responses during both sessions were identified in the bilateral posterior fusiform gyri, inferior parietal lobules and frontal eye fields, a distributed neural network involved in perceptual face processing (Haxby et al. 2002). However, DEX did not potentiate the responses of these other regions.
Table 1 Significant BOLD fMRI Responses for All Comparisons. Coordinates Represent Voxels in Each Region with the Most Significant Magnitude and Spatial Extent
Figure 2
Group statistical parametric maps illustrating significant bilateral BOLD responses during both PBO and DEX sessions in the amygdala when comparing the emotion task with the sensorimotor control (p < .05, corrected). Robust bilateral responses in the posterior fusiform gyri are also represented. See Table 1 for complete Talairach coordinates and voxel-level statistics.
Figure 3
Effects of DEX on BOLD responses. Group statistical parametric map illustrating a potentiated response (DEX > PBO) in the right amygdala (p < .05, corrected). See Table 1 for complete Talairach coordinates and voxel-level statistics.
There was no difference in performance for either task between PBO and DEX sessions (accuracy: F1,44 = 0.83, p = .37, and reaction time: F1,40 = 0.38, p = .54). Although subjects did report increased vigor and energy, DEX was not associated with heightened levels of anxiety or fear as indexed by either the Profile of Mood Scales or Spielberger anxiety scales. The absence of anxiogenic effects in the current study are not surprising given the relatively low physiological doses of DEX administered and the controlled environment. While measurable levels of DEX were detected in all subjects, ranging from 46 to 71 ng/ml (mean 57.3 ng/ml), previous work has illustrated that symptoms of intense anxiety and panic require persistent administration of amphetamine exceeding 150 mg/day (Ellinwood et al. 1973).
DISCUSSION
The potentiation of the amygdala response by DEX suggests that this structure may be involved in mediating the anxiogenic effects of amphetamine and perhaps other catecholaminergic stimulants. The regional specificity of this potentiation further implicates the amygdala as a critical brain structure for the extraction of affective information from environmental stimuli. The laterality of the potentiated amygdala response may reflect specialization of right hemisphere structures for the perceptual processing of emotional information, especially that of a visual nature. However, such hemispheric laterality may also reflect methodological phenomenon such as insufficient BOLD sensitivity and signal-to-noise ratio, especially in regions vulnerable to susceptibility artifacts such as the amygdala (LaBar et al. 2001). The consistency and nature of this observed laterality needs to be further explored in future studies.
Given the previously described task- and region-specific effects of DEX on brain regions subserving complex behavior (Mattay et al. 1996), the absence of DEX-potentiated responses in other brain regions, such as the posterior fusiform gyri, suggests that these regions, unlike the amygdala, may not be critical for the processing of the emotional content of stimuli specifically. Rather, they are likely involved in general perceptual processes such as recognizing faces from non-face objects (Haxby et al. 2002; Kanwisher et al. 1997). In addition, the absence of a DEX-potentiated response in these other regions suggests that the observed specific potentiation of the amygdala does not simply reflect global effects of DEX on either the neurovascular response or attentional phenomenon.
While DEX is generally regarded as a nonspecific monoaminergic agonist, there is considerable evidence suggesting that its primary mode of action is via DA neurotransmission. For example, DA D1 receptor and DA transporter knockout mice are insensitive to the psychostimulant effects of both cocaine and amphetamine (Zhang and Xu 2001), and behavioral sensitization to amphetamine is primarily mediated through mesocorticolimbic dopamine projections (Pierce and Kalivas 1997). Furthermore, additional studies have illustrated the importance of DA in the response of the amygdala to stress (Inglis and Moghaddam 1999) as well as in conditioned fear behavior (Guarraci et al. 1999; Nader and LeDoux 1999). However, the effects of DEX may also be mediated by serotonergic and noradrenergic systems, both of which have been implicated in emotional reactivity and behavior (Goldstein et al. 1996). Future work using specific monoaminergic agonists is needed to clarify the contributions of each system to amygdala activity.
Our results provide the first in vivo demonstration in human subjects of a powerful potentiation of the amygdala by DEX and provide insight to the underlying neural substrate of some of the emotion enhancing effects of amphetamine as well as neuropharmacological modulation of amygdala activity. The potentiated amygdala response we report may reflect DA gating of amygdala inputs and subsequent amygdala neural firing. Recently, Rosenkranz and Grace have shown that DA potentiates the response of the amygdala by attenuating the effect of inhibitory input from the prefrontal cortex and augmenting the effect of excitatory input from sensory cortices (Rosenkranz and Grace 2001). Consistent with their findings, our fMRI data may reflect such DA mediated augmentation of excitatory sensory input to the amygdala and concurrent attenuation of inhibitory prefrontal input. It is important to note that prefrontal inhibition of the amygdala has been described in human studies (Hariri et al. 2000) and may be another critical element of emotional regulation. Furthermore, our finding that amphetamine did not change responses in other brain regions supports the interpretation that its effects on the amygdala reflect local information processing at the level of the amygdala itself.
References
- Angrist BM, Gershon S . (1970): The phenomenology of experimentally induced amphetamine psychosis–preliminary observations. Biol Psychiatry 2: 95–107
CAS PubMed Google Scholar - Damasio AR . (1994): Descartes' Error. New York, Grosset/Putnam
Google Scholar - Davis M, Whalen PJ . (2001): The amygdala: vigilance and emotion. Mol Psychiatry 6: 13–34
Article CAS Google Scholar - Ellinwood EH Jr, Sudilovsky A, Nelson LM . (1973): Evolving behavior in the clinical and experimental amphetamine (model) psychosis. Am J Psychiatry 130: 1088–1093
Article Google Scholar - Goldstein LE, Rasmusson AM, Bunney BS, Roth RH . (1996): Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci 16: 4787–4798
Article CAS Google Scholar - Guarraci FA, Frohardt RJ, Kapp BS . (1999): Amygdaloid D1 dopamine receptor involvement in Pavlovian fear conditioning. Brain Res 827: 28–40
Article CAS Google Scholar - Hall RC, Popkin MK, Beresford TP, Hall AK . (1988): Amphetamine psychosis: clinical presentations and differential diagnosis. Psychiatr Med 6: 73–79
CAS PubMed Google Scholar - Hariri AR, Bookheimer SY, Mazziotta JC . (2000): Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport 11: 43–48
Article CAS Google Scholar - Harmer CJ, Hitchcott PK, Morutto SL, Phillips GD . (1997): Repeated d-amphetamine enhances stimulated mesoamygdaloid dopamine transmission. Psychopharmacology (Berl) 132: 247–254
Article CAS Google Scholar - Harmer CJ, Phillips GD . (1999a): Enhanced conditioned inhibition following repeated pretreatment with d-amphetamine. Psychopharmacology (Berl) 142: 120–131
Article CAS Google Scholar - Harmer CJ, Phillips GD . (1999b): Enhanced dopamine efflux in the amygdala by a predictive, but not a non-predictive, stimulus: facilitation by prior repeated D-amphetamine. Neuroscience 90: 119–130
Article CAS Google Scholar - Haxby JV, Hoffman EA, Gobbini MI . (2002): Human neural systems for face recognition and social communication. Biol Psychiatry 51: 59–67
Article Google Scholar - Inglis FM, Moghaddam B . (1999): Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem 72: 1088–1094
Article CAS Google Scholar - Kanwisher N, McDermott J, Chun MM . (1997): The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci 17: 4302–4311
Article CAS Google Scholar - LaBar KS, Gitelman DR, Mesulam MM, Parrish TB . (2001): Impact of signal-to-noise on functional MRI of the human amygdala. Neuroreport 12: 3461–3464
Article CAS Google Scholar - LeDoux J . (1997): The Emotional Brain. New York, Touchstone
Google Scholar - Mattay VS, Berman KF, Ostrem JL, Esposito G, Van Horn JD, Bigelow LB, Weinberger DR . (1996): Dextroamphetamine enhances “neural network-specific” physiological signals: a positron-emission tomography rCBF study. J Neurosci 16: 4816–4822
Article CAS Google Scholar - Nader K, LeDoux JE . (1999): Inhibition of the mesoamygdala dopaminergic pathway impairs the retrieval of conditioned fear associations. Behav Neurosci 113: 891–901
Article CAS Google Scholar - Pierce RC, Kalivas PW . (1997): A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25: 192–216
Article CAS Google Scholar - Rosenkranz JA, Grace AA . (1999): Modulation of basolateral amygdala neuronal firing and afferent drive by dopamine receptor activation in vivo. J Neurosci 19: 11027–11039
Article CAS Google Scholar - Rosenkranz JA, Grace AA . (2001): Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci 21: 4090–4103
Article CAS Google Scholar - Rosenkranz JA, Grace AA . (2002): Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J Neurosci 22: 324–337
Article CAS Google Scholar - Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ . (2001): Amygdala response to facial expressions in children and adults. Biol Psychiatry 49: 309–316
Article CAS Google Scholar - Willick ML, Kokkinidis L . (1995): Cocaine enhances the expression of fear-potentiated startle: evaluation of state-dependent extinction and the shock-sensitization of acoustic startle. Behav Neurosci 109: 929–938
Article CAS Google Scholar - Worsley KJ, Marrett P, Neelin AC, Friston KJ, Evans AC . (1996): A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73
Article CAS Google Scholar - Zhang J, Xu M . (2001): Toward a molecular understanding of psychostimulant actions using genetically engineered dopamine receptor knockout mice as model systems. J Addict Dis 20: 7–18
Article CAS Google Scholar
Acknowledgements
We would like to thank Saumitra Das and Sam Lee for technical assistance. This research was supported by the National Institute of Mental Health Intramural Research Program.
Author information
Authors and Affiliations
- Clinical Brain Disorders Branch, Intramural Research Program, National Institute of Mental Health, National Institutes of Health, Bethesda, MD, USA
Ahmad R Hariri Ph.D, Venkata S Mattay MD, Alessandro Tessitore MD, Francesco Fera MD, William G Smith BS & Daniel R Weinberger MD
Authors
- Ahmad R Hariri Ph.D
- Venkata S Mattay MD
- Alessandro Tessitore MD
- Francesco Fera MD
- William G Smith BS
- Daniel R Weinberger MD
Corresponding author
Correspondence toAhmad R Hariri Ph.D.
Rights and permissions
About this article
Cite this article
Hariri, A., Mattay, V., Tessitore, A. et al. Dextroamphetamine Modulates the Response of the Human Amygdala.Neuropsychopharmacol 27, 1036–1040 (2002). https://doi.org/10.1016/S0893-133X(02)00373-1
- Received: 25 January 2002
- Revised: 04 April 2002
- Accepted: 13 May 2002
- Published: 16 May 2002
- Issue Date: 01 December 2002
- DOI: https://doi.org/10.1016/S0893-133X(02)00373-1