Smaller Volume of Prefrontal Lobe in Polysubstance Abusers: A Magnetic Resonance Imaging Study (original) (raw)
Main
The accumulated evidence suggests that abnormalities in the prefrontal cortex are associated with substance abuse. For example, recent studies using positron emission tomography (PET) and single photon emission computerized tomography (SPECT) have demonstrated abnormalities of glucose metabolism in the prefrontal cortex of subjects with histories of illicit substance abuse. Stapleton et al. (1995) reported higher normalized rates of glucose metabolism in the orbitofrontal cortex of polysubstance abusers than in controls. Volkow et al. (1991) found that cocaine abusers, within 1 week of cocaine withdrawal, had higher levels of glucose metabolism in the orbitofrontal cortex, whereas those who were abstinent from drugs for 1 to 6 weeks showed lower glucose metabolism in the frontal cortex than controls (Volkow et al. 1992). Furthermore, several studies using PET and SPECT have reported deficits in cerebral blood flow in the prefrontal cortex of subjects who abused cocaine (Volkow et al. 1988; Tumeh et al. 1990; Weber et al. 1993).
Behavioral and personality problems observed in substance abusers include failure to inhibit inappropriate responses, aggressiveness, difficulty in sustaining attention, and poor judgment of future consequences. Similar deficits have also been reported in patients with injuries of the frontal or prefrontal lobe (Stuss and Benson 1984; Eslinger et al. 1992; Bechara et al. 1994). In addition, some of these behavioral abnormalities are similar to those observed in children with attention deficit hyperactivity disorder (ADHD) and conduct disorder (Robins and Price 1991), two conditions that are risk factors for substance abuse, as documented in several prospective studies (Bukstein et al. 1989; Mannuzza et al. 1991; Robins and Price 1991). These childhood behavioral problems have been related to deficits in the prefrontal cortex (Lueger and Gill 1990; Benson 1991).
We therefore hypothesized that individuals with histories of polysubstance abuse have structural deficits in the prefrontal cortex. As a first step toward evaluating this hypothesis, we have measured the volume of the prefrontal lobe (gray and white matter) using high-resolution magnetic resonance imaging (MRI) in polysubstance abusers and controls with no history of substance abuse. Because the temporal cortex has extensive neural connections with the prefrontal cortex, and because lesions in the temporal cortex can produce dysfunction of the prefrontal cortex, as observed in schizophrenia (Wible et al. 1995), we also measured volumes of the temporal lobe. Herein, we report that research volunteers with histories of polysubstance abuse have smaller volumes of the prefrontal lobe in comparison to those of controls.
METHODS
Subjects
To reduce potential confounding factors, such as sex, age, and handedness, only right-handed, physically healthy, male subjects between the ages of 21 to 44 years were included in this study. A total of 40 paid volunteers, including 26 polydrug abusers and 14 control subjects, met the general criteria for the study.
All subjects showed no illness in a complete physical examination and on standard diagnostic tests, including complete blood cell count, serum electrolyte assay, liver function tests, fasting blood glucose concentration, prothrombin and partial prothrombin times, thyroid function tests, urinalysis, electrocardiogram, and tests for exposure to tuberculosis, viral hepatitis, and human immunodeficiency virus-I. No subject had a history of head trauma with loss of consciousness or seizure. However, one of the substance abusers showed encephalomalacic change in the right frontotemporal lobe on the MRI scan, and was therefore excluded from data analysis, leaving 25 subjects in the substance abuse group. Demographics of the subjects are reported in Table 1. Information on the mother's educational level was also collected as a variable reflecting the socioeconomic background of the subject because level of education in substance abusers could be compromised by factors, such as substance abuse and/or personality problems associated with it.
Table 1 Demographics of the Subjects
The National Institute of Mental Health Diagnostic Interview Schedule (DIS) (Robins et al. 1981) was used to screen for axis I psychiatric disorders. Diagnoses were based upon criteria of the Diagnostic and Statistical Manual of Mental Disorders, DSM-III-R (American Psychiatric Association 1987). Two subjects (one control and one substance abuser) were not administered the DIS, but profiles of their psychiatric symptoms were obtained on the SCL-90 Scale (Derogatis 1983). The score of the control subject was within the normal range on the SCL-90 Scale, whereas the substance abuser presented a symptom profile between that of normal subjects and psychiatric outpatients. None of the control subjects met any of the criteria for axis I diagnoses, except for one individual who met criteria for nicotine dependence (cigarette smoking). No subjects in the substance abuse group met criteria for any axis I diagnoses other than substance abuse or dependence, except for one who met criteria for post-traumatic stress disorder. The DIS also screens for the axis II diagnosis of antisocial personality disorder, which was observed in four substance abusers but not in controls.
Self reports of drug history (Table 2) were obtained from all subjects, using the Addictive Drug Survey (Smith 1991), a two-page questionnaire developed at the NIDA Addiction Research Center. It should be noted that history of drug use and medical problems reported by polysubstance abusers might contain inaccuracies, because subjects drawn from a drug abusing population tend to underreport their drug use and/or deny its consequences (Spitz and Rosecan 1987; Mensch and Kandel 1988). In addition, potential research volunteers may inaccurately report their drug use in order to match perceived criteria for entry into a research protocol for which they would be paid. Therefore, a urine screen for recent use of amphetamines, barbiturates, benzodiazepines, cocaine metabolites, methadone, opioids, phencyclidine, and cannabinoids was performed for each subject prior to their MRI scan. All but three of the substance abusers screened positive for use of at least one illicit drug (most often cocaine). The three subjects who had a negative drug screen on admission all had a positive drug screen at this center when they participated in other research protocols. No control subject had a positive drug screen report.
Table 2 Self-Reported Use of Substances of Abuse
Subjects in the substance abuse group were housed on a closed research ward for at least 15 days to prevent self-administration of drugs while awaiting their MRI scan. None of the subjects exhibited any significant withdrawal signs during the course of the study. This was not surprising, because individuals who would experience severe withdrawal generally do not volunteer for residential research studies. Furthermore, 23 of the 25 subjects in the abuse group reported cocaine as their primary drug of choice, and withdrawal signs induced by abstinence from cocaine are usually mild, even in patients who report heavy use of cocaine (Margolin et al. 1996).
Magnetic Resonance Imaging
Studies were performed using a General Electric Signa scanner (1.5 Tesla). After two scout scans to estimate brain size and define a transaxial plane across the anterior and posterior commissures (AC-PC), a double-echo T2/proton density-weighted scan, parallel to the AC-PC plane, was obtained to detect gross brain abnormalities, and a spoiled gradient-recall acquisition at steady state (Spoiled Grass, SPGR) scan (echo time = 13 ms, repetition time = 38 ms, matrix = 256 × 192, field of view = 24 cm, flip angle = 45 degrees, slice thickness = 1.5 mm) was obtained for image analysis. The SPGR scan contained 124 coronal slices covering the entire brain. The scans were evaluated by a clinical neuroradiologist blind to subject identity.
Image Analysis
The SPGR scans were coded by number in a random order by an individual who was not involved in image analysis. The 124 MRI slices were converted into a volume image file, which was readable by the ANALYZE© program (Robb 1991). All scans were reformatted to a constant orientation in which the transaxial planes were parallel to the AC-PC line and perpendicular to the interhemispheric commissure, and the transaxial, saggital, and coronal planes were orthogonal. The scans were resliced to an isotropic volume with voxel size of 0.9375 × 0.9375 × 0.9375 mm3. This procedure enhanced the capability of using three-dimensional information for segmentation.
Volumetric assessments were performed using a semi-automated procedure developed in our laboratory (Figure 1 ) (Liu et al. 1995). A specific autotrace algorithm was used to classify tissue on the basis of the range of intensity. The rater adjusted the intensity range to include a given type of tissue (e.g., brain versus ventricle) on the basis of his perception of the anatomical structure. As the intensity range was changed by the rater, traces that outlined the area within the intensity range were adjusted on the displayed image so that the rater could visualize results of the segmentation. Errors in the semi-automated tracing were corrected by manually drawing new traces or “limits” on the image. Then, the segmented area(s) were saved as object map(s) for later volume rendering and calculation.
Figure 1
Method to segment and render volumes of brain regions. (A) Segmentation of prefrontal lobe (total volume: left, white matter: right). (B) Segmentation of temporal lobe and brain tissues without CSF (note that the CSF was excluded from the segmentation). (C) Volume rendering of prefrontal white matter. (D, E, F) Volume rendering of brain tissues with volumes of interest colored differently (prefrontal lobe: cyan and green, temporal lobe: magenta). Volumes of the rendered structures were calculated by Volume Measure algorithm of ANALYZE ©.
Definition of Brain Regions and Interrater Reliability
Brain without cerebral spinal fluid (CSF): The brain volume was defined as the volume of supratentorial brain tissues with the CSF excluded. Because the tissue intensity for the cerebellum and brain stem was similar to the cerebrum, after an outline was made to include the brain tissue, a trace or “limit” was drawn by the rater through the superior level of the cerebral peduncles to the base of the third ventricle to separate the brain stem and cerebellum from the rest of the brain. The CSF, which has a different intensity from brain tissue, was excluded (Figure 1). The interrater reliability, as tested by Pearson correlation between two independent raters (XL and JAM) was: r = 0.999 (five scans).
Brain with CSF: The purpose of measuring the brain with CSF was to obtain an estimate of the CSF volume (by subtracting the volume of the brain without CSF from this measure). This volume included the CSF within the ventricles and sulci, but not in the subaracnoid space. A different autotrace algorithm, which outlined only the contour of objects while disregarding intensity differences inside the contour, was used. When this algorithm was applied, an outline of the brain contour could be made to include brain tissues and CSF within the ventricles and sulci. The Pearson correlation on this measure between the two raters was: r = 0.998 (five scans).
Prefrontal lobe: The total prefrontal lobes (left and right) were defined as brain tissue extending from the frontal pole to the coronal plane just anterior to the genu of the corpus callosum. The white matter of the prefrontal lobe was segmented from the first slice showing white matter anteriorly to the last slice of prefrontal lobe posteriorly. Some motion artifacts in scans from four of the substance abusers did not cause difficulty in segmenting the entire prefrontal lobe, but made white matter segmentation extremely difficult. Therefore, volumes of the prefrontal gray and white matter were measured in only 21 of the 25 substance abusers. The Pearson correlations on measures of the total prefrontal lobe and prefrontal white matter between the two raters were: r = 0.982 and r = 0.933, respectively (five scans, volumes of left and right sides plotted together). The gray matter of the prefrontal lobe was estimated by subtracting the white matter from the volume of the total prefrontal lobe.
Temporal lobe: The temporal lobes (left and right) were defined as brain tissue extending from the first anterior slice showing the temporal lobe to the first coronal plane on which the posterior commissure was visible across the two hemispheres. The temporal lobes were separated from the rest of the brain through the base of the temporal stem. White matter segmentation was not performed for the temporal lobe because extensive manual editing would be required to separate the white matter from the amygdala and hippocampal formation using the available semi-automated procedure. The interrater reliability on measures of the temporal lobe between the two raters was: r = 0.979 (five scans, volumes of left and right sides were plotted together).
Statistical Analysis
Student's _t_-test was used to compare the differences in each measure between the two groups. Repeated measures analysis of variance, using group (substance abusers versus controls) as a between-subject factor, laterality (left and right hemispheres) and region [prefrontal lobe (left, right), temporal lobe (left, right)] as within-subject factors, was performed to test the interaction of these factors, the asymmetries of volumes and region by group interaction of the prefrontal and temporal lobes. Relative (normalized) volumes were obtained by dividing the absolute volume by total brain volume (without CSF) and then multiplying by 100. Statistics were performed separately on absolute and normalized measures.
Pearson correlations between age and volume parameters in each group, and between the brain structural measures and self-reported drug use were also performed. Due to the large number of variables involved, some significant correlations may reflect random variation and, therefore, the correlational analysis should be viewed as exploratory and interpreted with caution. Because the potential effects of drugs of abuse on the brain of substance abusers could interact, partial correlations were also performed on these measures.
RESULTS
Demographic Data
Control subjects attained a higher level of education than did substance abusers (p < .05) (Table 1), but this difference was not found between their mothers’ levels of education. The difference in the educational level between the two groups, but not in their mothers’ education, suggested that substance abuse or factors that contribute to substance abuse may have prevented the participants from reaching their highest educational potentials.
MRI Measures
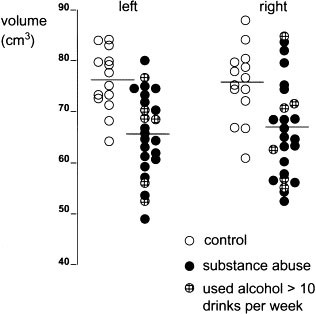
There were no significant differences in total brain volume and CSF volume between the two groups. No group by laterality effects were found for any measure. However, significant group main effects were found for the absolute volumes of the prefrontal and temporal lobes and for normalized prefrontal volumes. Group by region effects were also found for the absolute and normalized measures of the prefrontal and temporal lobes. Student's _t_-tests further demonstrated that the volumes of both the left and the right prefrontal lobes (absolute and normalized values) were significantly smaller in substance abusers than in controls (Table 3 and Figure 2 ). When the volumes of the prefrontal lobe were segmented by gray and white matter, significant differences were found in the gray matter (in both the left and right hemispheres) between the two groups, with subjects in the substance abuse group having smaller volumes. The absolute volume of white matter in the left prefrontal lobe was also significantly smaller in the substance abuse group; however, when the data were normalized the difference was no longer statistically significant. The absolute volumes of both the left temporal and right temporal lobes were also smaller in the substance abuse group than in the control group. The differences in left and right temporal lobe volumes became nonsignificant when the data were normalized.
Table 3 Morphometric Comparisons of Controls and Substance Abusers
Figure 2
Absolute volumes of prefrontal lobe in controls and substance abusers. Each dot represents data for a single research volunteer. Horizontal lines indicate means for each group. Substance abusers with average use of alcohol > 10 drinks per week were plotted as shaded circles.
There were no significant correlations between age and volumes of any measured structures, except for positive correlations between age and the volume of the left temporal lobe (r = 0.46, p < .05) and the CSF (r = 0.40, p < .05) in the substance abuse group. Because only two of the 20 correlations (10 for each group) revealed statistical significance, these correlations may reflect random variation. However, the positive correlations indicate that volume of the left temporal lobe or the CSF certainly were not reduced with increasing age in the substance abuse group. Age was also correlated with duration of substance use for alcohol (r = 0.52, p < .01), marijuana (r = 0.56, p < .01) and heroin (r = 0.57, p < .05).
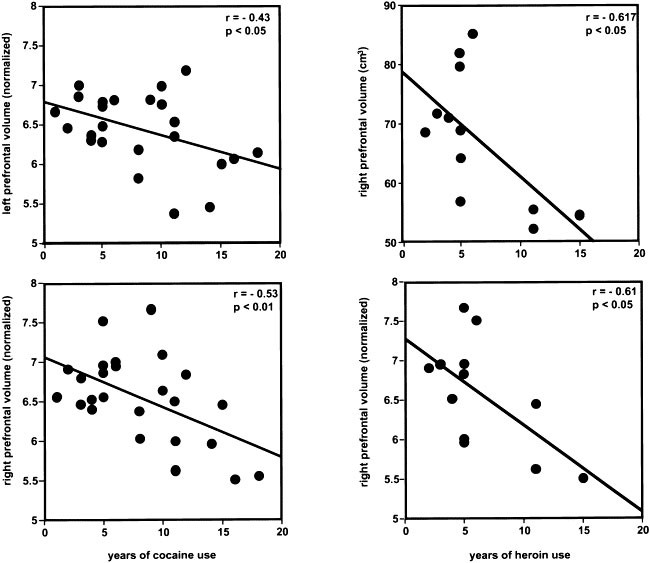
A number of significant correlations between the brain structural measures and the self-reported drug use data were found. Pearson correlation tests showed that there were negative relationships between years of cocaine use and normalized left and right prefrontal volumes (r = −0.43, p < .05 and r = −0.53, p < .01, respectively), years of heroin use and absolute and normalized right prefrontal volumes (r = −0.62, p < .05, r = −0.61, p < .05, respectively), and years of heroin use and absolute left prefrontal white matter and normalized left and right prefrontal white matters (r = −0.69, p < .05, r = −0.70, p < .05 and r = −0.68, p < .05, respectively). Positive correlations between average alcohol use and absolute and normalized volumes of the right temporal lobe (r = 0.54 and r = 0.58, respectively, p < .01) were also revealed. Scatterplots of the data showed that most of the correlations were driven by a few data points, except for the correlations between years of cocaine use or years of heroin use and the normalized volumes of the prefrontal lobes (Figure 3 ). Because of limited self-reported drug use data on heroin use, partial correlations were performed only on the self-reported data for alcohol, cocaine, and marijuana use. When correlation of brain structural measures and each item on the self-reported data were performed, the influence of other items and age were partialled out. For example, when correlation between the structural measures and average use of alcohol were calculated, the influence of years of alcohol, cocaine, and marijuana use, average use of cocaine and marijuana, and age were partialled out. The results revealed a significant positive partial correlation between average use of alcohol and normalized right temporal volume (r = 0.70, p < .01), average use of cocaine and normalized right prefrontal gray matter (r = 0.71, p < .05), and marginal negative correlation between years of cocaine use and normalized right and left prefrontal volumes (r = 0.49, p < .10 and r = 0.54, p < .06, respectively).
Figure 3
(Left side) Correlation between years of cocaine use and normalized prefrontal volumes (n = 25); (Right side) Correlation between years of heroin use and absolute and normalized right prefrontal volumes (n = 12).
DISCUSSION
The most striking finding in the present study was that the volumes of the prefrontal lobe in subjects with histories of polysubstance abuse were significantly smaller than in individuals without such a history. This observation is consistent with our hypothesis that subjects with substance abuse disorder have structural deficits in the prefrontal cortex. Because the substance abusers in this study were abstinent for more than 2 weeks before their MRI scans, the prefrontal structural deficits are likely to reflect either a preexisting condition or a long-term consequence of chronic substance abuse, but not the acute effects of alcohol or illicit drug abuse.
Our data provide supportive anatomical evidence for functional alterations in the prefrontal cortex in subjects with illicit substance abuse, as suggested by PET and SPECT studies (Volkow et al. 1988, 1991, 1992; Tumeh et al. 1990; Weber et al. 1993; Stapleton et al. 1995). Stapleton et al. (1995) found that whereas polysubstance abusers showed overall lower absolute glucose metabolic rate than controls, the relative glucose metabolic rate in the frontal and temporal areas, especially the orbitofrontal gyrus, was higher in the substance abusers than in controls. By pointing out that the orbitofrontal cortex has been implicated in disorders of emotional control, including aggressive and impulsive behaviors, they alluded to the possibility that an abnormality in this brain region contributed to compulsive drug-seeking behavior. In contrast, Volkow et al. (1992) found frontal hypometabolism in cocaine abusers and suggested that this phenomenon may reflect a long-term consequence of cocaine use. However, they also noted the possibility that the defects may have been present prior to the cocaine abuse and may have predisposed the subjects to substance abuse. In addition, they indicated that their results could represent the combined effects from administration of several different drugs, because their cocaine abusers, although not dependent on other substances, such as alcohol and marijuana, did use them. Similar arguments can be put forward to explain the present observations.
A number of biological and environmental factors may predispose an individual toward substance abuse. Genetic traits that interact with the environment have been reported to contribute to antisocial behavior, conduct disorder, aggressiveness as well as substance abuse (Cadoret et al. 1995). Personality and behavioral changes after frontal lobe injuries have been recognized since the middle of the 19th century, when the famous case of Phineas Gage was reported (Harlowe 1868). A number of studies (Stuss and Benson 1984; Eslinger et al. 1992; Bechara et al. 1994) have subsequently demonstrated that injuries in the prefrontal cortex may cause profound personality and behavioral problems with features similar to those observed in individuals who abuse illicit drugs. It can thus be argued that a causative or associative relationship might exist between structural deficits in the prefrontal cortex and substance abuse. Although the present study does not reveal the precise cause for our observations, a number of related scenarios can be envisioned. For example, the reduced volumes of gray matter in the prefrontal lobe of substance abusers may reflect developmental hypoplasia. This hypothetical hypoplasia would lead to improper neural connections between the prefrontal cortex and other cortical and/or subcortical areas. Such a defect might underlie the comorbidity of conduct disorder, ADHD, and antisocial personality disorder with substance abuse. In this regard, delayed myelination of prefrontal cortex has been proposed to be responsible for the frontal symptomatology of ADHD (Benson 1991).
On the other hand, the findings of the present study may reflect chronic effects of substance use on the prefrontal cortex. Studies of cognitive function in alcoholics and/or illicit substance abusers have shown that neurobehavioral deficits were most frequently related to prefrontal functioning (Miller 1985; O'Malley et al. 1992; Eckardt et al. 1995). Although one cannot exclude the possibility that these deficits might have existed prior to addiction, most investigators had suggested that chronic drug abuse was the main factor. It is notable that studies of youths at high risk for alcohol abuse have provided evidence of cognitive vulnerability in these populations. Specifically, Harden and Pihl (1995) reported that boys at high risk for alcoholism performed more poorly on tests reflecting frontal cognitive function than controls, whereas Wiers et al. (1994) proposed that children of primary alcoholic parents may suffer from inherited dysfunction of the prefrontal cortex with an enhanced risk of alcoholism.
Structural brain imaging studies of alcoholic subjects have also demonstrated volume reductions in the frontal lobe, with deficits in gray matter predominant in younger subjects (i.e., under 45 years of age), whereas both gray and white matter volumes are reduced in older subjects (Pfefferbaum et al. 1997). Neuropathological studies suggest that gray matter reductions could be caused by shrinkage of neuronal cell bodies or loss of axonal and/or dendritic processes (Harper and Kril 1990). These changes may be reversible, whereas cell death would not be. Similar mechanisms may underlie for the gray matter reductions observed in the present study.
Smaller absolute volumes of the left and right temporal lobes were also observed in the substance abuse group compared with controls. This difference became marginal when the data were normalized. Although further studies are warranted, deficits in the temporal lobes of substance abusers in this study could be a consequence of the prefrontal deficits due to the extensive interconnections between the temporal lobe and the prefrontal cortex.
Although the negative correlations between years of cocaine use or of heroin use and volumes of the prefrontal lobes provide evidence of reduced prefrontal lobes as function of cocaine or heroin abuse, we interpret these data with caution. Many factors could influence the results. First of all, the self-reported data might suffer some degree of inaccuracy, as noticed in other studies (Spitz and Rosecan 1987; Mensch and Kandel 1988; Hser et al. 1992). Second, some significant correlations may be due to random effects because of large number of variables involved. Third, combined effects of the drugs of abuse on brain structure could be more complex than a simple linear interaction. Other factors may include missing data and uneven use of other drugs of abuse among subjects. These factors could contribute to the puzzling results, such as the positive correlation between average use of cocaine and the normalized volume of the right prefrontal gray matter, and between average use of alcohol and right temporal volume. Further studies are needed to clarify these issues.
In summary, whether findings in the present study indicate hypoplasia and/or atrophy of the prefrontal lobe of substance abusers remains to be determined. In either case, structural deficits in the prefrontal lobe could play an essential role in the neuropathological basis of functional impairments in substance abusers demonstrated by functional brain imaging and cognitive studies. Therefore, the present findings emphasize the importance of our approach, which seeks to establish what role the prefrontal cortex plays in substance abuse disorder.
References
- American Psychiatric Association. (1987): Diagnostic and Statistical Manual of Mental Disorders, 3rd ed. rev. Washington, DC, American Psychiatric Association
- Bechara A, Damasio AR, Damasio H, Anderson SW . (1994): Insensitivity to future consequences following damage to human prefrontal cortex. Cognition 50: 7–15
Article CAS Google Scholar - Benson DF . (1991): The role of frontal dysfunction in attention deficit hyperactivity disorder. J Child Neurol 6: S9–S12
Article Google Scholar - Bukstein OG, Brent DA, Kaminer Y . (1989): Comorbidity of substance abuse and other psychiatric disorders in adolescents. Am J Psychiatry 146: 1131–1141
Article CAS Google Scholar - Cadoret RJ, Yates WR, Troughton E, Woodworth G, Stewart MA . (1995): Genetic-environmental interaction in the genesis of aggressivity and conduct disorders. Arch Gen Psychiatry 52: 916–924
Article CAS Google Scholar - Derogatis LR . (1983): SCL-90-R Administration, scoring and procedures manual-II for the revised version and other instruments of the psychopathology rating scale series. Towson, MI, Clinical Psychometric Research
- Eckardt MJ, Stapleton JM, Rawlings RR, Davis EZ, Grodin DM . (1995): Neuropsychological functioning in detoxified alcoholics between 18 and 35 years of age. Am J Psychiatry 152: 53–59
Article CAS Google Scholar - Eslinger PJ, Grattan LM, Damasio H, Damasio AR . (1992): Developmental consequences of childhood frontal lobe damage. Arch Neurol 49: 764–769
Article CAS Google Scholar - Harden PW, Pihl RO . (1995): Cognitive function, cardiovascular reactivity, and behavior in boys at high risk for alcoholism. J Abnorm Psychol 104: 94–103
Article CAS Google Scholar - Harlowe JM . (1868): Recovery from severe injury to the head. Publ Mass Med Soc 2: 327–346
Google Scholar - Harper CG, Kril JJ . (1990): Neuropathology of alcoholism. Alcohol Alcohol 25: 207–216
Article CAS Google Scholar - Hser Y-I, Anglin MD, Chou C-P . (1992): Reliability of retrospective self-report by narcotics addicts. Psychol Assess 4: 207–213
Article Google Scholar - Liu X, Phillips RL, Resnick SM, Wong DF, Stapleton JM, Stauffer R, London ED . (1995): Magnetic resonance imaging reveals no ventriculomegaly in polydrug abusers. Acta Neurol Scand 92: 83–90
Article CAS Google Scholar - Lueger RJ, Gill KJ . (1990): Frontal-lobe cognitive dysfunction in conduct disorder adolescents. J Clin Psychol 46: 696–706
CAS PubMed Google Scholar - Mannuzza S, Klein RG, Bonagura N, Malloy P, Giampino TL, Addalli KA . (1991): Hyperactive boys almost grown up. Arch Gen Psychiatry 48: 77–83
Article CAS Google Scholar - Margolin A, Avants SK, Kosten TR . (1996): Abstinence symptomatology associated with cessation of chronic cocaine abuse among methadone-maintained patients. Am J Drug Alcohol Abuse 22: 377–388
Article CAS Google Scholar - Mensch BS, Kandel DB . (1988): Underreporting of substance use in a national longitudinal youth cohort: Individual and interviewer effects. Public Opin Q 52: 100–124
Article Google Scholar - Miller L . (1985): Neuropsychological assessment of substance abusers: Review and recommendations. J Subst Abuse Treat 2: 5–17
Article CAS Google Scholar - O'Malley S, Adamse M, Heaton RK, Gawin FH . (1992): Neuropsychological impairment in chronic cocaine abusers. Am J Drug Alcohol Abuse 18: 131–144
Article CAS Google Scholar - Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO . (1997): Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res 21: 521–529
Article CAS Google Scholar - Robb RA . (1991): ANALYZE Reference Manual (Version 5.0). Rochester, MN, Biomedical Imaging Resource Mayo Foundation
- Robins LN, Helzer JE, Croughan J, Ratcliff KS . (1981): National Institute of Mental Health Diagnostic Interview Schedule: Its history, characteristics, and validity. Arch Gen Psychiatry 38: 381–389
Article CAS Google Scholar - Robins LN, Price RK . (1991): Adult disorders predicted by childhood conduct problems: Results from the NIMH epidemiologic catchment area project. Psychiatry 54: 116–132
Article CAS Google Scholar - Smith SS . (1991): Addictive Drug Survey (ADS) Manual. Baltimore, MD, NIDA Addiction Research Center
- Spitz HI, Rosecan JS . (1987): Cocaine Abuse: New Directions in Treatment and Research. New York, Brunner/Mazel
- Stapleton JM, Morgan MJ, Phillips RL, Wong DF, Yung BCK, Shaya EK, Dannals RF, Liu X, Grayson RL, London ED . (1995): Cerebral glucose utilization in polysubstance abuse. Neuropsychopharmacology 13: 21–31
Article CAS Google Scholar - Stuss DT, Benson DF . (1984): Neuropsychological studies of the frontal lobes. Psychol Bull 95: 3–28
Article CAS Google Scholar - Tumeh SS, Nagel JS, English RJ, Moore M, Holman BL . (1990): Cerebral abnormalities in cocaine abusers: Demonstration by SPECT perfusion brain scintigraphy. Radiology 176: 821–824
Article CAS Google Scholar - Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K . (1988): Cerebral blood flow in chronic cocaine users: A study with positron emission tomography. Br J Psychiatry 152: 641–648
Article CAS Google Scholar - Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A . (1991): Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry 148: 621–626
Article CAS Google Scholar - Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L . (1992): Long-term frontal brain metabolic changes in cocaine abusers. Synapse 11: 184–190
Article CAS Google Scholar - Weber DA, Franceschi D, Ivanovic M, Atkins HL, Cabahug C, Wong CTC, Susskind H . (1993): SPECT and planar brain imaging in crack abuse: Iodine-123-iodoamphetamine uptake and localization. J Nucl Med 34: 899–907
CAS PubMed Google Scholar - Wible CG, Shenton ME, Hokama H, Kikinis R, Jolesz FA, Metcalf D, McCarley RW . (1995): Prefrontal cortex and schizophrenia. A quantitative magnetic resonance imaging study. Arch Gen Psychiatry 52: 279–288
Article CAS Google Scholar - Wiers RW, Sergeant JA, Gunning WB . (1994): Psychological mechanisms of enhanced risk of addiction in children of alcoholics: A dual pathway? Acta Paediatr Scand Suppl 404: 9–13
Article CAS Google Scholar
Acknowledgements
We thank Drs. R.N. Bryan, Department of Radiology, Johns Hopkins School of Medicine, and S.M. Resnick, Gerontology Research Center, National Institute on Aging, for generously advising and consulting with us on the design of this study, and Loretta Spurgeon and Greg Wood for assistance with data collection.
Author information
Authors and Affiliations
- Neuroscience Branch, Intramural Research Program, National Institute on Drug Abuse, 5500 Nathan Shock Drive, Baltimore, Maryland
Xiang Liu MD, John A Matochik Ph.D, Jean-Lud Cadet MD & Edythe D London Ph.D
Authors
- Xiang Liu MD
- John A Matochik Ph.D
- Jean-Lud Cadet MD
- Edythe D London Ph.D
Rights and permissions
About this article
Cite this article
Liu, X., Matochik, J., Cadet, JL. et al. Smaller Volume of Prefrontal Lobe in Polysubstance Abusers: A Magnetic Resonance Imaging Study.Neuropsychopharmacol 18, 243–252 (1998). https://doi.org/10.1016/S0893-133X(97)00143-7
- Received: 12 May 1997
- Revised: 11 August 1997
- Accepted: 12 August 1997
- Issue Date: 01 April 1998
- DOI: https://doi.org/10.1016/S0893-133X(97)00143-7