Crystal chemistry of birefringent spessartine | Powder Diffraction | Cambridge Core (original) (raw)
Abstract
The crystal structure of one isotropic [(1) Brazil] and three birefringent spessartine samples [(2) California, (3) Tanzania, and (4) Colorado] were refined using the Rietveld method, cubic space group 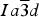 Iaoverline3dIa\overline 3 dIaoverline3d , and monochromatic synchrotron high-resolution powder X-ray diffraction (HRPXRD) data. The results of electron-microprobe analysis (EMPA) indicate homogeneous compositions, in terms of end-members, as follows: (1) Sps54Alm43, (2) Sps90Alm8, (3) Sps64Prp27Grs3, and (4) Sps73Alm19. Their crystal structures were modeled well as indicated by the Rietveld refinement statistics where the reduced χ 2 and overall R (F 2) values for each sample are: (1) 1.395 and 0.0329, (2) 1.082 and 0.0354, (3) 1.025 and 0.0347, and (4) 1.016 and 0.0413. Two cubic phases occur in samples 2–4, and a single cubic phase occurs in sample-1. The dominant cubic phase-1 with locality, weight fraction (%), unit-cell parameter (Å), distances (Å), and site occupancy factors (sofs) are as follows: (1) Brazil: 100%, a = 11.581 54 (1), average <Mn–O> = 2.3156, Al–O = 1.8949 (3), Si–O = 1.6376 (3) Å, Mn(sof) = 0.961(1), Al(sof) = 0.945(1), and Si(sof) = 0.936(1); (2) California: 96.67(7)%, a = 11.613 32(1), average <Mn–O> = 2.3249, Al–O = 1.8956 (4), Si–O = 1.6416 (4) Å, Mn(sof) = 0.951(1), Al(sof) = 0.946(1), and Si(sof) = 0.927(1); (3) Tanzania: 69.46(6)%, a = 11.598 45(1), average <Mn–O> = 2.3199, Al–O = 1.8964 (5), Si–O = 1.6398 (5) Å, Mn(sof) = 0.808(1), Al(sof) = 0.942(1), and Si(sof) = 0.922(1); and (4) Colorado: 98.58(6)%, a = 11.606 89(1), average <Mn–O> = 2.3204, Al-O = 1.8948 (6), Si–O = 1.6450 (6) Å, Mn(sof) = 0.949(1), Al(sof) = 0.967(2), and Si(sof) = 0.913(2). The two-phase intergrowth causes strain that arises from mismatch of the structural parameters and gives rise to strain-induced birefringence.
Iaoverline3dIa\overline 3 dIaoverline3d , and monochromatic synchrotron high-resolution powder X-ray diffraction (HRPXRD) data. The results of electron-microprobe analysis (EMPA) indicate homogeneous compositions, in terms of end-members, as follows: (1) Sps54Alm43, (2) Sps90Alm8, (3) Sps64Prp27Grs3, and (4) Sps73Alm19. Their crystal structures were modeled well as indicated by the Rietveld refinement statistics where the reduced χ 2 and overall R (F 2) values for each sample are: (1) 1.395 and 0.0329, (2) 1.082 and 0.0354, (3) 1.025 and 0.0347, and (4) 1.016 and 0.0413. Two cubic phases occur in samples 2–4, and a single cubic phase occurs in sample-1. The dominant cubic phase-1 with locality, weight fraction (%), unit-cell parameter (Å), distances (Å), and site occupancy factors (sofs) are as follows: (1) Brazil: 100%, a = 11.581 54 (1), average <Mn–O> = 2.3156, Al–O = 1.8949 (3), Si–O = 1.6376 (3) Å, Mn(sof) = 0.961(1), Al(sof) = 0.945(1), and Si(sof) = 0.936(1); (2) California: 96.67(7)%, a = 11.613 32(1), average <Mn–O> = 2.3249, Al–O = 1.8956 (4), Si–O = 1.6416 (4) Å, Mn(sof) = 0.951(1), Al(sof) = 0.946(1), and Si(sof) = 0.927(1); (3) Tanzania: 69.46(6)%, a = 11.598 45(1), average <Mn–O> = 2.3199, Al–O = 1.8964 (5), Si–O = 1.6398 (5) Å, Mn(sof) = 0.808(1), Al(sof) = 0.942(1), and Si(sof) = 0.922(1); and (4) Colorado: 98.58(6)%, a = 11.606 89(1), average <Mn–O> = 2.3204, Al-O = 1.8948 (6), Si–O = 1.6450 (6) Å, Mn(sof) = 0.949(1), Al(sof) = 0.967(2), and Si(sof) = 0.913(2). The two-phase intergrowth causes strain that arises from mismatch of the structural parameters and gives rise to strain-induced birefringence.
I. INTRODUCTION
Birefringence is unexpected in ideal high-symmetry cubic minerals, such as common silicate garnet, [8]X 3[6]Y 2[4]Z 3[4]O12. Such birefringence was reported over a century ago (Brewster, Reference Brewster1853; Mallard, Reference Mallard1876; Brauns, Reference Brauns1891), but the origin remains debatable, and is addressed in this study with regard to spessartine, ideally {Mn2+ 3}[Al2](Si3)O12. Some spessartine, grossular, {Ca3}[Al2](Si3)O12; andradite, {Ca3}[Fe3+ 2](Si3)O12; uvarovite, {Ca3}[Cr3+ 2](Si3)O12, etc. may show birefringence under cross-polarized light, which may indicate that they are not optically cubic (Deer et al., Reference Deer, Howie and Zussman1992). Several reasons were given as the cause of the birefringence, but the main one appears to be cation order in the X or Y site and symmetry reduction from cubic to lower symmetry in which their structure refinements were carried out (Takéuchi et al., Reference Takéuchi, Haga, Umizu and Sato1982; Akizuki, Reference Akizuki1984; Allen and Buseck, Reference Allen and Buseck1988; Kingma and Downs, Reference Kingma and Downs1989; Griffen et al., Reference Griffen, Hatch, Phillips and Kulaksiz1992; Akizuki et al., Reference Akizuki, Takéuchi, Terada and Kudoh1998; Shtukenberg et al., Reference Shtukenberg, Punin, Frank-Kamenetskaya, K. and Sokolov2001, Reference Shtukenberg, Popov and Punin2005; Wildner and Andrut, Reference Wildner and Andrut2001; Frank-Kamenetskaya et al., Reference Frank-Kamenetskaya, Rozhdestvenskaya, Shtukenberg, Bannova and Skalkina2007; Boiocchi et al., Reference Boiocchi, Bellatreccia, Della Ventura and Oberti2012). Synthetic MgSiO3 majorite (and analogues CaGeO3, CdGeO3, and MnSiO3) were reported to be birefringent with tetragonal space group _I_41/a, which has important implications for phase transitions in the transition zone in the mantle (Prewitt and Sleight, Reference Prewitt and Sleight1969; Fujino et al., Reference Fujino, Momoi, Sawamoto and Kumazawa1986; Angel et al., Reference Angel, Finger, Hazen, Kanzaki, Weidner, Liebermann and Veblen1989; Parise et al., Reference Parise, Wang, Gwanmesia, Zhang, Sinelnikov, Chmielowski, Weidner and Liebermann1996; Nakatsuka et al., Reference Nakatsuka, Yoshiasa, Yamanaka and Ito1999a, Reference Nakatsuka, Yoshiasa, Yamanaka, Ohtaka, Katsura and Ito1999b, Reference Nakatsuka, Chaya and Yoshiasa2005). The various reasons for the birefringence in garnet were recently discussed by Antao and Klincker (Reference Antao and Klincker2013a, Reference Antao and Klincker2013b) and Antao (Reference Antao2013a, Reference Antao2013b, Reference Antao2013c). They showed that birefringent silicate garnet contained an intergrowth of a few cubic phases, which were not observed in the previous studies.
Novak and Gibbs (Reference Novak and Gibbs1971) reported the cubic structure of a spessartine [Mn2.58Fe0.34Ca0.08Al1.99Fe0.01Si3O12, Sps86Alm11Grs3, a = 11.612(1); Si–O = 1.637(1) Å] from Minas Gerais, Brazil. Smyth et al. (Reference Smyth, Madel, McCormick, Munoz and Rossman1990) examined the cubic structure of a (F,OH)-rich spessartine, [Mn2.67Fe0.33Ca0.05Al1.97Si2.70O10.7F1.0(OH)0.36, Sps87Alm11Grs2, a = 11.628(1); Si–O = 1.656(1) Å] from Clear Creek County, Colorado, with a striking birefringence of δ_n_ ≈ 0.008, similar to hydrogarnet. The structure of spessartine was refined in space group Iaoverline3dIa\overline{3} dIaoverline3d by several other researchers (e.g., Gramaccioli et al., Reference Gramaccioli, Pilati and Demartin2002; Rodehorst et al., Reference Rodehorst, Geiger and Armbruster2002). For synthetic end-member spessartine, Geiger and Armbruster (Reference Geiger and Armbruster1997) obtained a = 11.619(1) and Si–O = 1.640(1) Å, which are similar to those obtained by Novak and Gibbs (Reference Novak and Gibbs1971). For a spessartine sample from S. Piero, Campo, Elba Isle, Gramaccioli et al. (Reference Gramaccioli, Pilati and Demartin2002) obtained a = 11.630(1) and Si–O = 1.646 Å. Recently, Boiocchi et al. (Reference Boiocchi, Bellatreccia, Della Ventura and Oberti2012) examined the structure of a birefringent (F,OH)-rich spessartine [(Mn2.87Fe0.09Ca0.04)(Al1.94Fe0.06)Si2.52O10.08F0.81(OH)1.11, a tet = 11.6347 (3) Å, c tet = 11.6449 (3) Å; a cub = 11.6380 (3) Å] in the tetragonal space group _I_41/acd. Using the polarizing microscope, they observed that the broken fragments showed prominent zoned extinction. Their tetragonal unit-cell metrics are, in fact, nearly cubic (c/a = 1.006). Their a cub cells are larger than that reported by Smyth et al. (Reference Smyth, Madel, McCormick, Munoz and Rossman1990) because their samples contain more (F,OH)4 groups. They indicated that the cubic-structure refinement of Smyth et al. (Reference Smyth, Madel, McCormick, Munoz and Rossman1990) should be reconsidered and that the tetragonal symmetry and birefringence are related to ordering of the (F,OH)4 groups. All the above samples were reported as chemically homogeneous and multiple cubic phases in birefringent spessartine were not observed in their single-crystal studies.
In garnet, [8]X 3[6]Y 2[4]Z 3[4]O12, the eight-coordinated dodecahedral X site contains Mg, Ca, Mn2+, or Fe2+ cations, the six-coordinated octahedral Y site contains Al, Cr3+, Fe3+, Ti4+, or Zr4+ cations, and the four-coordinated tetrahedral Z site contains Si atoms (Novak and Gibbs, Reference Novak and Gibbs1971). There are Si-atom deficiencies in some garnet samples that are occupied by Fe3+, Al, or (F, O4H4) (e.g., Armbruster et al., Reference Armbruster, Birrer, Libowitzky and Beran1998). The crystal structure of garnet consists of alternating _Z_O4 tetrahedra and _Y_O6 octahedra with X cations filling cavities to form _X_O8 dodecahedra. The eight O atoms in the _X_O8 polyhedra occur at the corners of a distorted cube (Figure 1). Each O is coordinated by two X, one Y, and one Z cations in a tetrahedral configuration.
Figure 1. (Color online) Projection of the cubic garnet structure down c showing the ZO4 tetrahedra (grey), YO6 octahedra (yellow), and XO8 dodecahedra (blue) that occur as a distorted cubic shape. The dense packing of the polyhedral are obvious from the four unit cells displayed, which shows the prominent edge-sharing and zigzag arrangement of alternating octahedra and dodecahedra.
Some spessartine (this study), grossular (Antao, Reference Antao2013c), andradite-grossular (Antao, Reference Antao2013b), andradite (Antao and Klincker, Reference Antao and Klincker2013a, Reference Antao and Klincker2013b), and Ti-rich andradite (Antao, Reference Antao2013a) have high-resolution powder X-ray diffraction (HRPXRD) patterns that show splitting of reflections. All reflections were indexed using slightly different cubic unit-cell parameters (two or three different cubic phases) and the crystal structure of the multiple cubic phases was refined using the Rietveld method. These recent studies have shown that the birefringent garnet samples contain an intergrowth of two or more cubic phases, whereas isotropic garnet samples are single phase with cubic symmetry.
This study examines the crystal structure of four spessartine samples: three are birefringent and one is isotropic. Electron microprobe results indicate homogeneous compositions for each sample. The isotropic spessartine from Brazil is a single cubic phase. The other three birefringent samples contain an intergrowth of two different cubic phases that cause strain because of mismatch of the cubic structural parameters, and result in strain-induced birefringence. Preliminary reports were presented (Antao et al., Reference Antao, Klincker and Round2013a, Reference Antao, Klincker and Round2013b).
II. EXPERIMENTAL
A. Sample characterization and electron-microprobe analysis (EMPA)
The spessartine samples are from: (1) Navegador Mine, Conselheiro Pena, Minas Gerais, Brazil (burgundy/brown-red in color); (2) Little Three Mine, Ramona, San Diego County, California, USA (light orange/peach in color); (3) Loliondo, Tanzania (orange color); and (4) Ruby Mt., near Nathrop Chaffee County, Colarodo, USA (amber color). Except for sample 1 from Brazil, the other three samples are birefringent. They appear optically homogeneous in plane- and cross-polarized light. Some of the birefringent samples have a mottled, tweed texture, or zoned extinction. An example of the birefringent sample from Colorado is shown (Figure 2). Some birefringent garnet samples contain complex features such as twinning, “bow-tie”, lamellar, and concentric zoning (Akizuki, Reference Akizuki1984, Reference Akizuki1989; Allen and Buseck, Reference Allen and Buseck1988; Jamtveit, Reference Jamtveit1991; Brown and Mason, Reference Brown and Mason1994; Akizuki et al., Reference Akizuki, Takéuchi, Terada and Kudoh1998; Badar et al., Reference Badar, Akizuki and Hussain2010; Antao, Reference Antao2013b; Antao and Klincker, Reference Antao and Klincker2013a, Reference Antao and Klincker2013b; Badar et al., Reference Badar, Niaz, Hussain and Akizuki2013).
Figure 2. (Color online) Optical microscopy thin-section images for spessartine from Colorado. (a) plane-polarized with the corresponding cross-polarized light image (b). The scale bars represent 100 _µ_m (bottom).
The four samples were analyzed with a JEOL JXA-8200 WD-ED EMPA. The JEOL operating program on a Solaris platform was used for ZAF correction and data reduction. The wavelength-dispersive (WD) operating conditions were 15 kV, 20 nA, 5 µm beam diameter, and using various standards [e.g., almandine-pyrope (Mg_K_α), grossular (Ca_K_α), almandine (Fe_K_α, Al_K_α, Si_K_α), rutile (Ti_K_α), spessartine (Mn_K_α), and chromite (Cr_K_α)]. Based on the EMPA results from eight spots from different areas of each crystal (≈2 mm diameter in size), the samples are chemically homogeneous (Table I). Back-scattered images from EMPA were featureless. The EMPA data were analyzed using the method and spreadsheet from Locock (Reference Locock2008).
Table I. EMPA results for four spessartine samples.
B. Synchrotron HRPXRD
The four samples were studied by HRPXRD experiments that were performed at beamline 11-BM, Advanced Photon Source (APS), Argonne National Laboratory (ANL). A crystal fragment from each sample (≈2 mm in diameter) was crushed to a fine powder using an agate mortar and pestle. The crushed samples were loaded into Kapton capillaries (0.8 mm internal diameter) and rotated during the experiment at a rate of 90 rotations per second. The data were collected at 23 °C to a maximum 2_θ_ of about 50° with a step size of 0.001° and a step time of 0.1 s per step. The HRPXRD traces were collected with 12 silicon (111) crystal analyzers that increase detector efficiency, reduce the angular range to be scanned, and allow rapid acquisition of data. A silicon (NIST 640c) and alumina (NIST 676a) standard (ratio of ⅓ Si: ⅔ Al2O3 by weight) was used to calibrate the instrument and refine the monochromatic wavelength used in the experiment (Table II). Additional details of the experimental set-up are given elsewhere (Antao et al., Reference Antao, Hassan, Wang, Lee and Toby2008; Lee et al., Reference Lee, Shu, Ramanathan, Preissner, Wang, Beno, Von Dreele, Ribaud, Kurtz, Antao, Jiao and Toby2008; Wang et al., Reference Wang, Toby, Lee, Ribaud, Antao, Kurtz, Ramanathan, Von Dreele and Beno2008).
Table II. HRPXRD data and Rietveld refinement statistics for four spessartine samples.
C. Rietveld structure refinements
The HRPXRD data were analyzed using the Rietveld method (Rietveld, Reference Rietveld1969), as implemented in the GSAS program (Larson et al., Reference Larson and Von Dreele2000), and using the EXPGUI interface (Toby, Reference Toby2001). Scattering curves for neutral atoms were used. The starting atom coordinates, cell parameter, and space group Iaoverline3dIa\overline 3 dIaoverline3d , were taken from Antao (Reference Antao2013b). The background was modeled using a Chebyschev polynomial. The reflection-peak profiles were fitted using type-3 profile in the GSAS program. Full-matrix least-squares refinements were carried out by varying the parameters in the following sequence: a scale factor, cell parameter, atom coordinates, and isotropic displacement parameters. Except for the single-phase sample from Brazil, examination of the HRPXRD traces shows the presence of two cubic phases with different unit-cell parameters (Figures 3 and 4). The two phases were refined together with the site occupancy factors (sofs) in terms of the dominant atom in each of the X, Y, and Z sites. Toward the end of the refinement, all the parameters were allowed to vary simultaneously, and the refinement proceeded to convergence. The birefringent samples contain two cubic phases, but they are less obvious in the California sample-2 (Figure 4). The isotropic sample-1 from Brazil is a single cubic phase (Figure 4(a)). No impurity peaks were observed in the samples and all the diffraction peaks were indexed using one or two cubic unit-cell parameters.
Figure 3. A full HRPXRD trace for the isotropic spessartine from Brazil. The difference curve (I obs − I calc) is shown at the bottom. The short vertical lines indicate allowed reflection positions. The intensities for the trace and difference curve that are above 20° and 30° 2_θ_ are scaled by factors of ×5 and ×20, respectively.
Figure 4. Comparison of the same reflections in spessartine samples from (a) Brazil, (b) California, and (c) Tanzania. (d) The low-angle 2_θ_ region for the Colorado sample shows a second phase on the right shoulder of reflections from phase-1. Except for the single-phase sample from Brazil with narrow peak widths (a), the other three data sets were fitted using two different cubic phases (b–d) that are clearly observed in the Tanzania sample (c) and in the low angle 2_θ_ region of the Colorado sample (d). The two cubic phases in the California sample (b) are detected from the peak asymmetry and is a bit difficult to observe.
The unit-cell parameters and the Rietveld refinement statistics for the four samples are listed in Table II. Atom coordinates, isotropic displacement parameters, and sofs are given in Table III. Bond distances are given in Table IV.
Table III. Atom coordinatesa, isotropic displacement parameters, U (Å2), and sofs for four spessartine samples.
Table IV. Selected distances (Å) for four spessartine samples.
III. RESULTS AND DISCUSSION
The EMPA results for the four homogeneous samples are (Table I):
- (1) {Mn2+ 1.61Fe2+ 1.28Ca0.03}Σ2.92[Al1.96Fe3+ 0.12]Σ2.08(Si2.92Al0.08)Σ3O12, Sps54Alm43 (Brazil);
- (2) {Mn2+ 2.70Fe2+ 0.24Ca0.01}Σ2.94[Al1.98Fe3+ 0.08]Σ2.06(Si2.94Al0.06)Σ3O12, Sps90Alm8 (California);
- (3) {Mn2+ 2.03Mg0.81Ca0.10}Σ2.94[Al1.93Fe3+ 0.08Mn3+ 0.06]Σ2.06(Si2.94Al0.06)Σ3O12, Sps64Prp27 (Tanzania); and
- (4) {Mn2+ 2.19Fe2+ 0.64Mg0.05Ca0.03}Σ2.90[Al1.88Fe3+ 0.20Ti4+ 0.02]Σ2.10(Si2.88Al0.12)Σ3O12, Sps73Alm19 (Colorado).
The dominant cation in the X site is Mn, Y site is Al, and Z site is Si. The X site in sample-2 contains the most Mn2+ cations (2.7 apfu). The Al atoms in the Y site are ≥1.9 apfu. The Si atoms in the Z site are 2.9 apfu in sample-4 from Colorado, which may indicate a minor OH content, but in the EMPA calculation, the Si deficiency is assumed to be filled with Al (Table I). Previous EMPA studies on other spessartine samples show the same general features (see the Introduction section).
The same diffraction peaks for three samples are compared (Figures 4(a)–4(c)), and that for sample-4 is for a different 2_θ_ range (Figure 4(d)). Sample-1 is a single cubic phase and has sharp narrow peaks that show no splitting (Figure 4(a)). The peaks in the three birefringent samples are split into two, indicating two different cubic phases (Figures 4(b)–4(d)). The two phases in sample-2 are not clear, but they can be observed from the large peak width and asymmetry in the peak shape (Figure 4(b)). However, splitting of the reflections is clearly seen in Figures 4(c) and 4(d).
The sofs calculated from the EMPA chemical analysis are shown for comparison to the sofs obtained by the Rietveld refinements, and their values are similar (Table III). The deficient refinement sof values for the Si site indicate that there may be minor (O4H4) replacing SiO4, as the Si–O distance is nearly constant. The two separate phases in the birefringent samples could not be detected using EMPA, indicating that they occur as a fine-scale intergrowth. However, the HRPXRD technique clearly shows two different cubic phases (Figure 4). Many refinements of the garnet structure have used the single-crystal method, and those studies may miss the minor phases in birefringent multi-phase samples. The single-crystal method is not the appropriate technique to examine such multi-phase samples.
The unit-cell parameters and bond distances for the four samples compare well to each other and to other published work (Figure 5). In general, the calculated distances based on radii sum are also reasonable, so the EMPA results are acceptable (Table IV; Figure 5). Smyth et al. (Reference Smyth, Madel, McCormick, Munoz and Rossman1990; a = 11.628 Å) obtained a large cubic unit-cell parameter because their sample contains some (F,OH)4 groups that give rise to an Si–O = 1.656(1) compared to 1.637(1) Å for the anhydrous sample studied by Novak and Gibbs (Reference Novak and Gibbs1971). Our Brazil and Tanzania samples have Si–O = 1.6376 (3) and 1.6398 (5) Å, respectively (Table IV). However, our California and Colorado samples have Si–O = 1.6416 (4) and 1.6450 (6) Å, respectively. These larger values indicate minor O4H4 substitution for SiO4, as is indicated by the chemical analyses, especially for the sample from Colorado (Table I).
Figure 5. (Color online) Structural variations across the part of the pyralspite series. The mean <_D_–O> distance varies linearly with the a parameter across the full series. Linear trend lines are based on literature data and only some relevant data points are displayed [see Antao (Reference Antao2013b) for details]. Data of this study are between those from Smyth et al. (Reference Smyth, Madel, McCormick, Munoz and Rossman1990; a = 11.628 Å) and almandine (Alm; a = 11.531 Å).
In ideal spessartine, the X and Y sites are completely occupied with Ca and Al atoms, respectively, so the question of cation order does not arise. In our birefringent spessartine samples, all the diffraction peaks in the HRPXRD trace belongs to two cubic phases. There is no impurity or un-indexed peaks. All the refinements were done in the cubic space group, so spessartine contains no cation order.
Based on thin-film observations, the ideas presented by Kitamura and Komatsu (Reference Kitamura and Komatsu1978) can explain the birefringence in the samples used in this study and those birefringent garnet samples used in the previous studies that show concentric, oscillatory, or lamellar zoning, or tweed-like features (e.g., Akizuki, Reference Akizuki1984; Antao and Klincker, Reference Antao and Klincker2013a; Badar et al., Reference Badar, Niaz, Hussain and Akizuki2013). If a garnet sample contains a few cubic phases, a fine-scale mixture will show birefringence and EMPA will show a homogeneous sample. If the phases occur as large-scale lamellae, EMPA will show compositional variations. In both cases, HRPXRD will show different cubic unit-cell parameters. So a unique solution to the birefringence problem in spessartine is provided where two-phase intergrowths result in mismatch of the cubic structural parameters that gives rise to strain-induced birefringence. Similar multi-phase intergrowths occur in other birefringent samples such as andradite (Antao, Reference Antao2013a, Reference Antao2013b; Antao and Klincker, Reference Antao and Klincker2013a), grossular (Antao, Reference Antao2013c), spessartine (this study), almandine, morimotoite, schorlomite, uvarovite, etc. (unpublished results). Isotropic garnet occurs as a single cubic phase. The two-phase intergrowth in spessartine is similar to hetero-epitaxial or epitaxial intergrowths because of the similarity of the structural parameters.
The formation of two-phase intergrowths may be related to changes in oxygen fugacity (f O2), activity of SiO2 (a SiO2), etc., as the crystals grow at low temperature that prevents diffusion or homogenization. Alternatively, the multiphase assemblage may be the stable form. Intergrowths in minerals are not uncommon and were also observed in the helvine-group minerals (Antao and Hassan, Reference Antao and Hassan2010), apatite (Baikie et al., Reference Baikie, Schreyer, Wong, S.S., Klooster, Ferraris, McIntyre and White2012), and many silicate garnets.
ACKNOWLEDGEMENTS
R. Marr is thanked for help with the EMPA data collection. The HRPXRD data were collected at the X-ray Operations and Research beamline 11-BM, Advanced Photon Source (APS), Argonne National Laboratory (ANL). Use of the APS was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. This work was supported with a NSERC Discovery Grant and an Alberta Ingenuity Award to SMA.
References
Akizuki, M. (1984). “Origin of optical variations in grossular-andradite garnet,” American Mineralogist 66, 403–409.Google Scholar
Akizuki, M. (1989). “Growth structure and crystal symmetry of grossular garnets from the Jeffrey mine, Asbestos, Quebec, Canada,” Am. Mineral. 74, 859–864.Google Scholar
Akizuki, M., Takéuchi, Y., Terada, T., and Kudoh, Y. (1998). “Sectoral texture of a cubo-dodecahedral garnet in grandite,” Neues Jahrbuch für Mineralogie, Monatshefte 12, 565–576.Google Scholar
Allen, F. M. and Buseck, P. R. (1988). “XRD, FTIR, and TEM studies of optically anisotropic grossular garnets,” Am. Mineral. 73, 568–584.Google Scholar
Angel, R., Finger, L. W., Hazen, R. M., Kanzaki, M., Weidner, D. J., Liebermann, R. C., and Veblen, D. R. (1989). “Structure and twinning of single-crystal MgSiO3 garnet synthesized at 17 GPa and 1800 °C,” Am. Mineral. 74, 509–512.Google Scholar
Antao, S. M. (2013a). “The mystery of birefringent garnet: is the symmetry lower than cubic?,” Powder Diffr. 28, 265–272.Google Scholar
Antao, S. M. (2013b). “Three cubic phases intergrown in a birefringent andradite-grossular garnet and their implications,” Phys. Chem. Miner. 40, 705–716.Google Scholar
Antao, S. M. (2013c). “Can birefringent near end-member grossular be non-cubic? New evidence from synchrotron diffraction,” Can. Mineral. In Press.Google Scholar
Antao, S. M. and Hassan, I. (2010). “A two-phase intergrowth of genthelvite from Mont Saint-Hilaire, Quebec,” Can. Mineral. 48, 1217–1223.Google Scholar
Antao, S. M. and Klincker, A. M. (2013a). “Origin of birefringence in andradite from Arizona, Madagascar, and Iran,” Phys. Chem. Miner. 40, 575–586.CrossRefGoogle Scholar
Antao, S. M. and Klincker, A. M. (2013b). “Crystal structure of a birefringent andradite-grossular from Crowsnest Pass, Alberta, Canada,” Powder Diffr. 29, 20–27.Google Scholar
Antao, S. M., Hassan, I., Wang, J., Lee, P. L., and Toby, B. H. (2008). “State-of-the-art high-resolution powder X-ray diffraction (HRPXRD) illustrated with Rietveld structure refinement of quartz, sodalite, tremolite, and meionite,” Can. Miner. 46, 1501–1509.Google Scholar
Antao, S. M., Klincker, A. M., and Round, S. A. (2013a). “Origin of birefringence in common silicate garnet: intergrowth of different cubic phases,” Am. Geophys. Union Conference, Cancun, Mexico, 14–17 May, 2013.Google Scholar
Antao, S. M., Klincker, A. M., and Round, S. A. (2013b). Some Garnets are Cubic and Birefringent, Why? Am. Crystallographic Association Conference, Hawaii, USA, 20–24 July, 2013.CrossRefGoogle Scholar
Armbruster, T., Birrer, J., Libowitzky, E., and Beran, A. (1998). “Crystal chemistry of Ti-bearing andradites,” Eur. J. Mineral. 10, 907–921.Google Scholar
Badar, M. A., Akizuki, M., and Hussain, S. (2010). “Optical anomaly in iridescent andradite from the Sierra Madre mountains, Sonora, Mexico,” Can. Mineral. 48, 1195–1203.Google Scholar
Badar, M. A., Niaz, S., Hussain, S., and Akizuki, M. (2013). “Lamellar texture and optical anomaly in andradite from the Kamaishi mine, Japan,” Eur. J. Mineral. 25, 53–60.Google Scholar
Baikie, T., Schreyer, M. K., Wong, C. L., S.S., P., Klooster, W. T., Ferraris, C., McIntyre, G. J., and White, T. J. (2012). “A multi-domain gem-grade Brazilian apatite,” Am. Mineral. 97, 1574–1581.Google Scholar
Boiocchi, M., Bellatreccia, F., Della Ventura, G. D., and Oberti, R. (2012). “On the symmetry and atomic ordering in (OH,F)-rich spessartine: towards a new hydrogarnet end-member,” Zeitschrift für Kristallographie 227, 385–395.CrossRefGoogle Scholar
Brauns, R. (1891). Die optischen Anomalien der Kristalle (Preisschr. Jablonowski Ges., Leipzig, Germany).Google Scholar
Brewster, D. (1853). “On the optical figures produced by the disintegrated surfaces of crystals,” Phil. Mag. Ser. 4(6), 16–30.Google Scholar
Brown, D. and Mason, R. A. (1994). “An occurrence of sectored birefringence in almandine from the Gangon terrane, Labrador,” Can. Miner. 32, 105–110.Google Scholar
Deer, W. A., Howie, R. A., and Zussman, J. (1992). An Introduction to the Rock-Forming Minerals (John Wiley, New York, NY), 2nd ed. Google Scholar
Frank-Kamenetskaya, O. V., Rozhdestvenskaya, L. V., Shtukenberg, A. G., Bannova, I. I., and Skalkina, Y. A. (2007). “Dissymmetrization of crystal structures of grossular-andradite garnets Ca3(Al, Fe)2(SiO4)3 ,” Struct. Chem. 18, 493–503.Google Scholar
Fujino, K., Momoi, H., Sawamoto, H., and Kumazawa, M. (1986). “Crystal structure and chemistry of MnSiO3 tetragonal garnet,” Am. Mineral. 71, 781–785.Google Scholar
Geiger, C. A. and Armbruster, T. (1997). “Mn3Al2Si3O12 spessartine and Ca3Al2Si3O12 grossular garnet: Structural dynamic and thermodynamic properties,” American Mineralogist. 82, 740–747.Google Scholar
Gramaccioli, C. M., Pilati, T., and Demartin, F. (2002). “Atomic displacement parameters for spessartine Mn3Al2Si3O12 and their lattice-dynamical interpretation,” Acta Crystallogr. B58, 965–969.Google Scholar
Griffen, D. T., Hatch, D. M., Phillips, W. R., and Kulaksiz, S. (1992). “Crystal chemistry and symmetry of a birefringent tetragonal pyralspite75-grandite25 garnet,” Am. Mineral. 77, 399–406.Google Scholar
Jamtveit, B. (1991). “Oscillatory zonation patterns in hydrothermal grossular-andradite garnet: nonlinear dynamics in regions of immiscibility.,” American Mineralogist 76, 1319–1327.Google Scholar
Kingma, K. J. and Downs, J. W. (1989). “Crystal-structure analysis of a birefringent andradite,” Am. Mineral. 74, 1307–1316.Google Scholar
Kitamura, K. and Komatsu, H. (1978). “Optical anisotropy associated with growth striation of yttrium garnet, Y3(Al,Fe)5O12 ,” Kristallographie und Technik 13, 811–816.Google Scholar
Larson, A. C. and Von Dreele, R. B. (2000). “Generalized Structure Analysis System (GSAS),” Report No LAUR 86–748, Los Alamos National Laboratory, Los Alamos, NM.Google Scholar
Lee, P. L., Shu, D., Ramanathan, M., Preissner, C., Wang, J., Beno, M. A., Von Dreele, R. B., Ribaud, L., Kurtz, C., Antao, S. M., Jiao, X., and Toby, B. H. (2008). “A twelve-analyzer detector system for high-resolution powder diffraction,” J. Synchrotron Radiat. 15, 427–432.Google Scholar
Locock, A. J. (2008). “An excel spreadsheet to recast analyses of garnet into end-member components, and a synopsis of the crystal chemistry of natural silicate garnets,” Comput. Geosci. 34, 1769–1780.Google Scholar
Mallard, E. (1876). “Anomalies optiques,” Ann. Mines Mem. VII Ser. 10, 60.Google Scholar
Nakatsuka, A., Yoshiasa, A., Yamanaka, T., and Ito, E. (1999a). “Structure refinement of a birefringent Cr-bearing majorite Mg3(Mg0.34Si0.34Al0.18Cr0.14)2Si3O12 ,” Am. Mineral. 84, 199–202.Google Scholar
Nakatsuka, A., Yoshiasa, A., Yamanaka, T., Ohtaka, O., Katsura, T., and Ito, E. (1999b). “Symmetry change of majorite solid-solution in the system Mg3Al2Si3O12-MgSiO3 ,” Am. Mineral. 84, 1135–1143.Google Scholar
Nakatsuka, A., Chaya, H., and Yoshiasa, A. (2005). “Crystal structure of single-crystal CaGeO3 tetragonal garnet synthesized at 3 GPa and 1000 °C,” Am. Mineral. 90, 755–757.Google Scholar
Novak, G. A. and Gibbs, G. V. (1971). “The crystal chemistry of the silicate garnets,” Am. Mineral. 56, 1769–1780.Google Scholar
Parise, J. B., Wang, Y., Gwanmesia, G. D., Zhang, J., Sinelnikov, Y., Chmielowski, J., Weidner, D. J., and Liebermann, R. C. (1996). “The symmetry of garnets on the pyrope (Mg3Al2Si3O12) – majorite (MgSiO3) join,” Geophys. Res. Lett. 23, 3799–3802.CrossRefGoogle Scholar
Prewitt, C. T. and Sleight, A. W. (1969). “Garnet-like structures of high-pressure cadmium germanate and calcium germanate,” Science 163, 386–387.Google Scholar
Rietveld, H. M. (1969). “A profile refinement method for nuclear and magnetic structures,” J. Appl. Crystallogr. 2, 65–71.Google Scholar
Rodehorst, U., Geiger, C. A., and Armbruster, T. (2002). “The crystal structures of grossular and spessartine between 100 and 600 K and the crystal chemistry of grossular-spessartine solid soutions,” Am. Mineral. 87, 542–549.Google Scholar
Shannon, R. D. (1976). “Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides,” Acta Crystallogr. A32, 751–767.Google Scholar
Shtukenberg, A. G., Punin, Y. O., Frank-Kamenetskaya, O. V., K., O. G., and Sokolov, P. B. (2001). “On the origin of anomalous birefringence in grandite garnets,” Mineral. Mag. 65, 445–459.Google Scholar
Shtukenberg, A. G., Popov, D. Y., and Punin, Y. O. (2005). “Growth ordering and anomalous birefringence in ugrandite garnets,” Mineral. Mag. 69, 537–550.Google Scholar
Smyth, J. R., Madel, R. E., McCormick, T. C., Munoz, J. L., and Rossman, G. R. (1990). “Crystal-structure refinement of a F-bearing spessartine garnet,” Am. Mineral. 75, 314–318.Google Scholar
Takéuchi, Y., Haga, N., Umizu, S., and Sato, G. (1982). “The derivative structure of silicate garnets in grandite,” Zeitschrift für Kristallographie 158, 53–99.Google Scholar
Toby, B. H. (2001). “EXPGUI, a graphical user interface for GSAS,” J. Appl. Crystallogr. 34, 210–213.Google Scholar
Wang, J., Toby, B. H., Lee, P. L., Ribaud, L., Antao, S. M., Kurtz, C., Ramanathan, M., Von Dreele, R. B., and Beno, M. A. (2008). “A dedicated powder diffraction beamline at the advanced photon source: commissioning and early operational results,” Rev. Sci. Instrum. 79, 085105.Google Scholar
Wildner, M. and Andrut, M. (2001). “The crystal chemistry of birefringent natural uvarovites. Part II. Single-crystal X-ray structures,” Am. Mineral. 86, 1231–1251.Google Scholar