The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals (original) (raw)
References
- Henkemeyer, M. et al. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell 86, 35–46 (1996).
Article CAS Google Scholar - Birgbauer, E., Cowan, C. A., Sretavan, D. W. & Henkemeyer, M. Kinase independent function of EphB receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development 127, 1231–1241 (2000).
Article CAS Google Scholar - Mellitzer, G., Xu, Q. & Wilkinson, D. G. Eph receptors and ephrins restrict cell intermingling and communication. Nature 400, 77–81 (1999).
Article ADS CAS Google Scholar - Xu, Q., Mellitzer, G., Robinson, V. & Wilkinson, D. G. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature 399, 267–271 (1999).
Article ADS CAS Google Scholar - Holland, S. J. et al. Bidirectional signaling through the Eph family receptor Nuk and its transmembrane ligands. Nature 383, 722–725 (1996).
Article ADS CAS Google Scholar - Bruckner, K., Pasquale, E. B. & Klein, R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science 275, 1640–1643 (1997).
Article CAS Google Scholar - George, S. E. et al. The VAB-1 Eph receptor tyrosine kinase functions in neural and epithelial morphogenesis in C. elegans. Cell 92, 633–643 (1998).
Article CAS Google Scholar - Davy, A. et al. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 13, 3125–3135 (1999).
Article CAS Google Scholar - Margolis, B. et al. High-efficiency expression/cloning of epidermal growth factor-receptor-binding proteins with Src homology 2 domains. Proc. Natl Acad. Sci. USA 89, 8894–8898 (1992).
Article ADS CAS Google Scholar - Chen, M. et al. Identification of Nck family genes, chromosomal localization, expression, and signaling specificity. J. Biol. Chem. 273, 25171–25178 (1998).
Article CAS Google Scholar - Tu, Y., Li, F. & Wu, C. Nck-2, a novel Src homology2/3-containing adaptor protein that interacts with the LIM-only protein PINCH and components of growth factor receptor kinase-signaling pathways. Mol. Biol. Cell 9, 3367–3382 (1998).
Article CAS Google Scholar - Garrity, P. A. et al. Drosophila photoreceptor axon guidance and targeting requires the dreadlocks SH2/SH3 adapter protein. Cell 85, 639–650 (1996).
Article CAS Google Scholar - Lu, W., Katz, S., Gupta, R. & Mayer, B. J. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr. Biol. 7, 85–94 (1997).
Article CAS Google Scholar - Chen, M., She, H., Kim, A., Woodley, D. T. & Li, W. Nck-beta adapter regulates actin polymerization in NIH 3T3 fibroblasts in response to platelet-derived growth factor bb. Mol. Cell. Biol. 20, 7867–7880 (2000).
Article CAS Google Scholar - Ren, R., Mayer, B. J., Cicchetti, P. & Baltimore, D. Identification of a ten-amino acid proline-rich SH3 binding site. Science 259, 1157–1161 (1993).
Article ADS CAS Google Scholar - Wunderlich, L., Farago, A. & Buday, L. Characterization of interactions of Nck with Sos and dynamin. Cell Signal 11, 25–29 (1999).
Article CAS Google Scholar - Hobert, O., Jallal, B., Schlessinger, J. & Ullrich, A. Novel signaling pathway suggested by SH3 domain-mediated p95vav/heterogeneous ribonucleoprotein K interaction. J. Biol. Chem. 269, 20225–20228 (1994).
Article CAS Google Scholar - Bustelo, X. R., Suen, K. L., Michael, W. M., Dreyfuss, G. & Barbacid, M. Association of the vav proto-oncogene product with poly(rC)-specific RNA-binding proteins. Mol. Cell. Biol. 15, 1324–1332 (1995).
Article CAS Google Scholar - Ribon, V., Herrera, R., Kay, B. K. & Saltiel, A. R. A role for CAP, a novel, multifunctional Src Homology 3 domain-containing protein in formation of actin stress fibers and focal adhesions. J. Biol. Chem. 273, 4073–4080 (1998).
Article CAS Google Scholar - Mandai, K. et al. Ponsin/SH3P12: an l-afadin- and vinculin-binding protein localized at cell–cell and cell–matrix adherens junctions. J. Cell Biol. 144, 1001–1017 (1999).
Article CAS Google Scholar - Kurakin, A., Hoffman, N. G. & Kay, B. K. Molecular recognition properties of the C-terminal SH3 domain of the Cbl associated protein, CAP. J. Pept. Res. 52, 331–337 (1998).
Article CAS Google Scholar - Zeng, L. et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulated embryonic axis formation. Cell 90, 181–192 (1997).
Article CAS Google Scholar - Ikeda, S. et al. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-dependent phosphorylation of β-catenin. EMBO J. 17, 1371–1384 (1998).
Article CAS Google Scholar - Hamada, F. et al. Negative regulation of Wingless signaling by D-axin, a Drosophila homolog of axin. Science 283, 1739–1742 (1999).
Article ADS CAS Google Scholar - Fradkin, L. G., Noordermeer, J. N. & Nusse, R. The Drosophila Wnt protein DWnt-3 is a secreted glycoprotein localized on the axon tracts of the embryonic CNS. Dev. Biol. 168, 202–213 (1995).
Article CAS Google Scholar - Asakura, T. et al. Similar and differential behavior between the nectin-afadin-ponsin and cadherin-catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells 4, 573–581 (1999).
Article CAS Google Scholar - Loureiro, J. & Peifer, M. Roles of Armadillo, a Drosophila catenin, during central nervous system development. Curr. Biol. 8, 622–632 (1998).
Article CAS Google Scholar - Dai, Z. & Pendergast, A. M. Abi-2, a novel SH3-containing protein interacts with the c-Abl tyrosine kinase and modulates c-Abl transforming activity. Genes Dev. 9, 2569–2582 (1995).
Article CAS Google Scholar - Shi, Y., Alin, K. & Goff, S. P. Abl-interactor-1, a novel SH3 protein binding to the carboxy-terminal portion of the Abl protein, suppresses v-abl transforming activity. Genes Dev. 9, 2583–2597 (1995).
Article CAS Google Scholar - Keegan, K. & Cooper, J. A. Use of the two hybrid system to detect the association of the protein-tyrosine phosphatase, SHPTP2, with another SH2-containing protein, Grb7. Oncogene 12, 1537–1544 (1996).
CAS PubMed Google Scholar - Schlaepfer, D. D., Hauck, C. R. & Sieg, D. J. Signaling through focal adhesion kinase. Prog. Biophys. Mol. Biol. 71, 435–478 (1999).
Article CAS Google Scholar
Acknowledgements
We thank T. Saxton and T. Pawson for numerous discussions; B. Howell, S. Hollenberg and J. Cooper for two-hybrid vectors and brain library; F. Gertler for CAP peptide spots; K. Womak, X. Cao and S. Kennedy for help with two-hybrid screens; and J. Shay for technical assistance. These studies were made possible by a grant from the Welch Foundation (to M.H.).
Author information
Authors and Affiliations
- Center for Developmental Biology and Kent Waldrep Foundation Center for Basic Research on Nerve Growth and Regeneration, University of Texas Southwestern Medical Center, Dallas, 75390-9133, Texas, USA
Chad A. Cowan & Mark Henkemeyer
Authors
- Chad A. Cowan
- Mark Henkemeyer
Corresponding author
Correspondence toMark Henkemeyer.
Supplementary information
Reagents
Anti-ephrin-B1 (C-18), anti-HA (F-7/monoclonal), anti-HA (Y-11/polyclonal), anti-Myc (9E10/monoclonal), anti-Myc (A-14/polyclonal), and anti-OctA/FLAG (D-8G/polyclonal) antibodies were from Santa Cruz Biotechnology. Anti-Flag (M2/monoclonal) antibodies were from Sigma. Anti-Xpress antibodies were from Invitrogen. Anti-phosphotyrosine (monoclonal, 4G10), anti-Paxillin (monoclonal, 5H11), anti-Nckb (Grb4, polyclonal), and anti-CAP (polyclonal) antibodies were from Upstate Biotechnology. Polyclonal anti-phosphotyrosine-specific FAK antibodies were from Biosource International. Anti-pan FAK monoclonal antibodies were from Transduction Labs. Protein-A and Protein—G Sepharose was from Pharmacia. All secondary fluorescent (Cy2, Cy3, and Cy5) and HRP-conjugated antibodies were from Jackson Immunoresearch. HRP-conjugated antibodies were visualized with SuperSignal Dura Extend chemiluminescence substrate from Pierce. Immunoblots were stripped using ImmunoPure IgG elution buffer from Pierce. Complete protease inhibitor (Roche) and protease inhibitor cocktail (Sigma) were included in all lysis buffers.
Yeast two-hybrid
Detailed methods can be found in Keegan and Cooper, Oncogene 12, 1537-44 (1996).
Cell stimulations
Cells were serum-starved for 8 to 12h in DMEM, 10mM HEPES (pH7.4), and 2mg/ml BSA. A 2:1 molar ratio of soluble EphB2-Fc (R&D Systems) to anti-human IgG (Jackson Immunoresearch) was pre-clustered in PBS (from CellGro, without calcium or magnesium) for 2h at 4°C and then used at 5 to 10mg/ml in serum free media containing 2mg/ml of BSA. Cells were stimulated at 37°C for the times indicated.
Biochemistry
GST Pulldowns. Approximately 5mg of each GST fusion protein bound to Glutathione agarose beads (Pharmacia) were blocked for 2 hours at 4°C with 2mg/ml of BSA in PBS, and then incubated overnight at 4°C with 1mg/ml whole cell protein lysates prepared in PLC lysis buffer (50mM HEPES, pH 7.5, 150mM NaCl, 10% Glycerol, 0.1% Triton-X, 1.5mM MgCl2, 1mM EGTA, 10mM NaP2O7, 100mM NaF, and 1mM Na3VO4). Protein complexes were washed 3 times in PLC lysis buffer, 2 times in HNTG (20mM HEPES, pH 7.5, 150mM NaCl, 0.1% Triton-X, 10% Glycerol, and 1mM Na3VO4), once in HN (20mM HEPES, pH 7.5, 150mM NaCl), and boiled in Lammeli sample buffer. Bound proteins were resolved on 10% SDS.PAGE gels, transferred to nitrocellulose, and immunoblotted with the indicated antibodies.
EphB2-Fc Pulldowns and Immunoprecipitations.Briefly, 10µg of EphB2-Fc was coupled to protein-G sepharose beads (Pharmacia) or 5µg of anti-ephrin-B1 (C-18) was coupled to protein-A sepharose beads (Pharmacia) and incubated overnight at 4ûC with the indicated lysates (1mg/ml) prepared in PLC lysis buffer containing 1% NP-40. Bound proteins were then washed 3 times in HNTG for the EphB2-Fc pulldowns or 3 times in PLC-Lysis buffer followed by 2 washes in HNTG and 1 wash in HN for ephrin-B1 immunoprecipitations. Samples were then boiled 10 minutes in Lammeli sample buffer, resolved on 10% SDS PAGE gels, transferred to nitrocellulose and immunoblotted with the indicated antibodies.
Membrane Fractionation. A stable ephrin-B1 expressing NG108 cell line was plated on 15cm Nunclon dishes, serum starved for 12h, and then either left unstimulated or subjected to a timecourse of stimulation with 10mg/ml of pre-clustered EphB2-Fc. The cells were washed three times with cold PBS, collected by scraping into HNG buffer (20mM HEPES, 150mM NaCl, 10% Glycerol) supplemented with protease inhibitors, and then dounced 10 times in a 2ml Kontes homogenizer on ice. Following a 15 min low-speed centrifugation at 15K x G at 4°C to pellet nuclei, endoplasmic reticulum, and Golgi membranes, the supernatant was centrifuged at high-speed for 1h at 100K x G at 4°C to pellet the plasma membrane, which was gently washed twice with HN. Pelleted membranes were boiled in Lammeli sample buffer and associated proteins were resolved on 10% SDS.PAGE gels, transferred to nitrocellulose, and immunoblotted with anti-Paxillin and anti-ephrin-B1 (C-18) antibodies.
FAK Immunoblotting.A stable ephrin-B1 expressing NG-108 cell line was plated on 15cm Nunclon dishes, serum starved for 12 hours, and then either left unstimulated or subjected to a timecourse of stimulation with 10µg/mL of pre-clustered EphB2-Fc reagent. The cells were washed 3 times with cold PBS, lysed in PLC-lysis buffer supplemented with protease inhibitors, collected in microcentrifuge tubes and clarified by centrifugation at 14,000 rpm for 10 minutes at 4ûC. Lysates were boiled in Lammeli sample buffer and resolved on 7.5% SDS PAGE gels, transferred to nitrocellulose and immunoblotted with six different FAK phosphotyrosine-specific antibodies and a pan-FAK monoclonal antibody.
Filter Binding Assay.16-mer peptides encompassing the PQQP motif in CAP/Ponsin as well as 16-mer peptides in which one or both proline residues in the motif have been replaced by alanine residues (PQQA, AQQP, and AQQA) were spotted onto a cellulose paper matrix by an Abimed peptide synthesizer. Bacterially produced GST-Grb4 SH3 domain fusion proteins were generated by cloning into PGEX residues 1-258 of human Grb4 for PGEX-Grb4 SH3.1-3.3 and residues 104-174 for pGEX-Grb4 SH3.2. The peptide blot was blocked in PBB (10mM Tris, pH 7.6, 150mM NaCl, 0.1% Triton-X, 5% Powdered Milk and 1% BSA) overnight at 4ûC. 20µg of each GST fusion protein was pre-incubated with antibodies directed against GST and a secondary antibody coupled to HRP for 2 hours at 4ûC, and then incubated with the peptide blot overnight at 4ûC at a final concentration of 2µg/mL in PBB. The blot was washed 3 times in TBST (10mM Tris pH7.5, 150mM NaCl, and 0.1% Triton-X 100) and visualized with a chemiluminescent substrate.
Immunofluorescence
CHP100 cells or transfected BHK cells were plated on glass coverslips (Baxter) and either left unstimulated or stimulated with pre-clustered EphB2-Fc for desired timepoints, washed with cold PBS 3 times, fixed for 15 min with 3.7% paraformaldehyde in PBS at 4°C, and then either incubated at -20°C in methanol for 5 min (membrane proteins) or in acetone for 2 min (cytoskeletal proteins). After two washes in cold PBS, nonspecific binding sites were blocked by incubation in PBS with either 5% heat inactivated goat serum (Vector labs) in PBS or 5% heat inactivated donkey serum (Sigma) for 1h at RT. Primary antibodies were diluted in the blocking buffer and added to coverslips for 1h at RT. Secondary antibodies and Oregon Green Phalloidin (Molecular Probes) were also diluted in blocking buffer and incubated for 45 min at RT. Primary and secondary antibody incubations were followed by three washes in PBS and cells were mounted using Aqua Polymount (Polysciences). Images were acquired with a confocal microscope (Leica DM DRB or Zeiss LSM 510) and processed with Adobe Photoshop. In some instances, single channel images are displayed in gray scale for better visualization.
Experimental N-values
The data shown in Fig. 1 are representative of 9 (a), 3 (b), and 3 (c) independent experiments.
The data shown in Fig. 2 are representative images obtained from 5 (a), 11 (b), and 2 (c) independent experiments.
The data shown in Fig. 3 are representative images obtained from 8 (a), 5 (b), 6 (c), 2 (d), and 4 (e) independent experiments.
The data shown in Fig. 4 are representative images obtained from 9 (Grb4) and 2 (Nck) (a), 3 (b), 5 (c), 3 (d), 2 (e), and 2 (f) independent experiments.

Figure 2
(GIF 15.3 KB)
a, HA-tagged Nck was co-expressed with ephrin-B1 in BHK cells and stimulated for 45 minutes with pre-clustered EphB2-Fc. Although ephrin-B1became aggregated to spots on the plasma membrane (red), Nck did not colocalize (green).
b, HA-tagged Grb4 was co-expressed in BHK cells with a truncated version of ephrin-B1 lacking the majority of its cytoplasmic domain (DC). Followingstimulation with EphB2-Fc, the truncated ephrin-B1 did become clustered (red), but this did not lead to colocalization of Grb4 molecules (green).
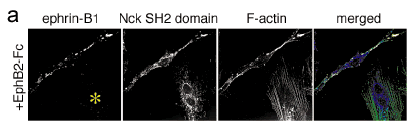
Figure 3
(GIF 12.1 KB)
a, Unlike the Grb4 SH2 domain, expression of the isolated Nck SH2 domain did not block the cell rounding, shrinking, detachment, and collapse of F-actin stress fibers in response to ephrin-B1 reverse signaling. Note that the adjacent cell in this panel which expressed the Nck SH2 domain but notephrin-B1 (yellow asterisk) showed no response to reverse signaling as indicated by a normal flat morphology and many stress fibers that spanned thelength of the cell.

Figure 4
(GIF 20.4 KB)
a, A 16-mer peptide encompassing the PQQP motif in CAP/Ponsin was spotted on a filter membrane and reacted with a soluble GST fusion proteincomposed of the three Grb4 SH3 domains. Binding of the SH3 domains was greatly diminished if one or both of the proline residues in the peptide waschanged to alanine. No binding was observed when the filter was reprobed with the isolated second Grb4 SH3 domain, indicating that the SH3.1 and/orSH3.3 domains are important for binding to CAP. As a control, a set of peptides corresponding to a different Grb4 SH3 domain binding protein(G4BP#98) was recognized by both fusion proteins indicating it is bound by the SH3.2 domain of Grb4.
b, Co-expression of ephrin-B1 and the isolated Grb4 SH2 domain in BHK cells blocked the recruitment of endogenous CAP to ephrin-B1 followingreverse signaling.
c, Expression of the isolated Nck SH2 domain was not able to block the recruitment of endogenous CAP to spots of activated ephrin-B1. Note that thecell shown exhibits the typical rounding and shrinkage in response to reverse signaling which is not observed when signaling is blocked using thetruncated ephrin-B1 or the isolated Grb4 SH2 domain.
Rights and permissions
About this article
Cite this article
Cowan, C., Henkemeyer, M. The SH2/SH3 adaptor Grb4 transduces B-ephrin reverse signals.Nature 413, 174–179 (2001). https://doi.org/10.1038/35093123
- Received: 22 May 2001
- Accepted: 17 July 2001
- Issue Date: 13 September 2001
- DOI: https://doi.org/10.1038/35093123