Senescence in premalignant tumours (original) (raw)
- Brief Communication
- Published: 04 August 2005
Tumour biology
- Jesús Gil2,
- Alejo Efeyan1,
- Carmen Guerra1,
- Alberto J. Schuhmacher1,
- Marta Barradas1,
- Alberto Benguría3,
- Angel Zaballos3,
- Juana M. Flores4,
- Mariano Barbacid1,
- David Beach5 &
- …
- Manuel Serrano1
Nature volume 436, page 642 (2005)Cite this article
- 19k Accesses
- 1347 Citations
- 14 Altmetric
- Metrics details
Abstract
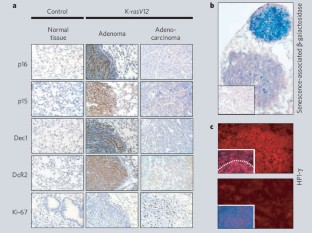
Oncogene-induced senescence is a cellular response that may be crucial for protection against cancer development1,2, but its investigation has so far been restricted to cultured cells that have been manipulated to overexpress an oncogene. Here we analyse tumours initiated by an endogenous oncogene, ras, and show that senescent cells exist in premalignant tumours but not in malignant ones. Senescence is therefore a defining feature of premalignant tumours that could prove valuable in the diagnosis and prognosis of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout
Additional access options:
Figure 1: Premalignant lung adenomas induced by oncogenic K-ras are positive for markers of senescence, whereas malignant adenocarcinomas are negative.
Similar content being viewed by others
References
- Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Cell 88, 593–602 (1997).
Article CAS Google Scholar - Lowe, S. W., Cepero, E. & Evan, G. Nature 432, 307–315 (2004).
Article ADS CAS Google Scholar - Guerra, C. et al. Cancer Cell 4, 111–120 (2003).
Article MathSciNet CAS Google Scholar - Dimri, G. P. et al. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Article ADS CAS Google Scholar - Narita, M. et al. Cell 113, 703–716 (2003).
Article CAS Google Scholar - Palmero, I., Pantoja, C. & Serrano, M. Nature 395, 125–126 (1998).
Article ADS CAS Google Scholar - Tuveson, D. A. et al. Cancer Cell 5, 375–387 (2004).
Article CAS Google Scholar
Author information
Authors and Affiliations
- Spanish National Cancer Centre (CNIO), Madrid, 28029, Spain
Manuel Collado, Alejo Efeyan, Carmen Guerra, Alberto J. Schuhmacher, Marta Barradas, Mariano Barbacid & Manuel Serrano - MRC Clinical Sciences Centre, Imperial College, Hammersmith, London, W12 0NN, UK
Jesús Gil - Spanish National Centre of Biotechnology (CNB-CSIC), Madrid, 28049, Spain
Alberto Benguría & Angel Zaballos - Department of Animal Surgery and Medicine, Complutense University, Madrid, 28040, Spain
Juana M. Flores - Centre for Cutaneous Research, Institute of Cell and Molecular Science, London, E1 2AT, UK
David Beach
Authors
- Manuel Collado
- Jesús Gil
- Alejo Efeyan
- Carmen Guerra
- Alberto J. Schuhmacher
- Marta Barradas
- Alberto Benguría
- Angel Zaballos
- Juana M. Flores
- Mariano Barbacid
- David Beach
- Manuel Serrano
Corresponding author
Correspondence toManuel Serrano.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Collado, M., Gil, J., Efeyan, A. et al. Senescence in premalignant tumours.Nature 436, 642 (2005). https://doi.org/10.1038/436642a
- Issue date: 04 August 2005
- DOI: https://doi.org/10.1038/436642a
This article is cited by
Editorial Summary
Cell senescence and cancer
Cellular senescence, a growth-arrest program that limits the lifespan of mammalian cells and prevents unlimited cell proliferation, is attracting considerable interest because of its links to tumour suppression. Using a mouse model in which the oncogene Ras is activated in the haematopoietic compartment of bone marrow, Braig et al. show that cellular senescence can block lymphoma development. Genetic inactivation of the histone methyltransferase Suv39h1 that controls senescence by ‘epigenetic’ modification of DNA-associated proteins, or a pharmacological approach that mimics loss of this enzyme, allow the formation of malignant lymphomas in response to oncogenic Ras. This work has important implications for both tumour development and tumour therapy. Michaloglou et al. report that oncogene-induced senescence may be a physiologically important process in humans, keeping moles in a benign state for many years: unchecked they develop into malignant melanomas. Chen et al. also find that cellular senescence blocks tumorigenesis in vivo: they show that acting together, the p53 tumour suppressor and the cellular senescence system can prevent prostate cancer induction in mice by the PTEN mutation. Collado et al. show that cellular senescence is a defining feature of Ras-initiated premalignant tumours; this could prove valuable in the diagnosis and prognosis of cancer.
See the web focus.