Serum markers lactate dehydrogenase and S100B predict independently disease outcome in melanoma patients with distant metastasis (original) (raw)
Main
The prognosis of melanoma patients with distant metastasis is poor with a median survival of 9 months (Neuman et al, 2008). The American Joint Committee on Cancer (AJCC) 2009 staging system includes the site of involved organs and serum lactate dehydrogenase (LDH) to classify stage IV into the M categories: M1a (soft tissue metastasis), M1b (pulmonary involvement), and M1c (involvement of other visceral organs or elevated LDH) (Balch et al, 2009). Lactate dehydrogenase has been part of the AJCC staging system since the sixth edition released in 2001 (Balch et al, 2001), as it had been identified as an independent prognostic factor before start of systemic treatment for unresectable disease by different research groups (Sirott et al, 1993; Eton et al, 1998; Manola et al, 2000). Nevertheless, differences in survival according to M categories are small and current factors do neither allow the identification of patients with the worst prognosis nor those with the chance for long-term survival.
In addition to LDH, S100B is another prognostic serum factor for melanoma patients (Deichmann et al, 1999; Hauschild et al, 1999; Mohammed et al, 2001; Schmidt et al, 2005; Smit et al, 2005; Egberts et al, 2008; Tarhini et al, 2009). However, the role of S100B in interaction with LDH has not yet been well defined in patients with distant metastasis. All studies which addressed this question involving multivariate Cox regression analysis were performed in small patient cohorts ranging from 64 to 145 patients (Deichmann et al, 1999; Hauschild et al, 1999; Mohammed et al, 2001; Schmidt et al, 2005; Smit et al, 2005; Egberts et al, 2008). In all six studies, both serum markers were found to predict the patients’ prognosis significantly in bivariate analyses. However, during multivariate analyses, only either LDH or S100B remained a significant prognostic factor; interestingly, three times LDH and three times S100B. It seems possible that the findings of a significant impact for only one serum marker per study depended on the relatively small sample sizes. Therefore, an investigation of this question in a large collective of stage IV patients is required.
Several research groups have reported favourable survival for patients after complete metastasectomy of distant metastases (Ollila et al, 1996; Leo et al, 2000; Meyer et al, 2000; Wood et al, 2001; Essner et al, 2004; Neuman et al, 2007; Wasif et al, 2011) with 5-year survival rates up to 41% (Ollila et al, 1996). Nevertheless, the impact of this procedure is still unclear, because a randomised trial has never been published and the majority of analyses comprised selected patient subgroups only. The decision, whether a patient is selected for complete metastasectomy, is apart from technical feasibility, influenced by prognostic factors, the age of the patient, and co-morbidities. This leads to a selection bias of prognostically favourable patients for surgery (Ollila, 2006).
The present study investigated prognostic factors in a cohort of 855 melanoma patients with distant metastases and thorough follow-up. The main aim was to clarify the prognostic impact of the serum markers LDH and S100B, adjusting for all potential and established prognostic factors like the number of distant sites and the organ involvement. Furthermore, the impact of complete metastasectomy and its interaction with the other significant prognostic factors has been examined.
Patients and Methods
Patients
Patients with distant metastasis treated between 1996 and 2010, at the University Department of Dermatology in Tübingen, Germany, were identified in the Central Malignant Melanoma Registry (CMMR) database, which prospectively records patients from more than 60 dermatological centres in Germany. Of 1140 stage IV patients with follow-up, those with unknown primary melanoma at the time of initial diagnosis (_n_=204), ocular (_n_=45), or mucosal localisation (_n_=36) were excluded, resulting in a final sample size of 855 after individual file review. All patients had given their written informed consent to have their data recorded by the CMMR. The aims and methods of data collection by the CMMR have previously been reported in detail (Lasithiotakis et al, 2007). Staging algorithms according to national surveillance guidelines were used (Garbe et al, 2007). These algorithms consider the individual risk of relapse for each patient based on prognostic factors considered in the AJCC classification. Stage II patients are followed-up via clinical inspections, lymph node ultrasound, and the evaluation of S100B. Stage III patients are additionally staged once a year by whole body by computerised tomography scan.
Data obtained for each patient included gender, age at stage IV diagnosis (<60 _vs_ ⩾60 years), stage at initial diagnosis (I/II _vs_ III _vs_ IV), the time interval between initial diagnosis and stage IV diagnosis (<36 _vs_ ⩾36 months), the date of the last follow-up, and the date and cause of death, if applicable. The following characteristics of the primary tumour were analysed: anatomical localisation (axial _vs_ extremities), Breslow’s tumour thickness (⩽2 _vs_ >2 mm), Clark’s level of invasion (I–III vs IV vs V), ulceration, histopathological signs of regression, and subtype (superficial spreading melanoma vs nodular melanoma vs lentigo maligna melanoma vs acral lentiginous melanoma). At the time of stage IV diagnosis, the following variables were evaluated: site of visceral involvement (soft tissue metastasis vs pulmonary involvement vs other visceral sites), the number of involved distant sites (distant soft tissue, lung, CNS, liver, intestine, bone, other visceral sites), serum LDH (normal vs >upper limit of normal (ULN)), and S100B (normal vs >ULN). S100B was detected using the Sangtec S100 ELISA (Diasorin Inc., Stillwater, OK, USA; ULN=0.15 _μ_g l−1) until December 2003 and thereafter by the Elecsys S100 electrochemiluminescence immunoassay (Roche Diagnostics AG, Rotkreuz, Switzerland, ULN=0.10 _μ_g l−1) according to the instructions of the manufacturers.
The first treatment after diagnosis of stage IV melanoma was categorised as either complete metastasectomy vs all other treatment modalities, for example, systemic treatment, irradiation, or best supportive care. In our analysis, complete metastasectomy was defined as a surgical procedure following the intention of complete resection (even if the surgical outcome finally was an incomplete resection) of all clinically or radiographic suspect lesions. In case of brain metastases, stereotactic irradiation was classified as a surgical excision. At our institution, a patient is selected for surgical treatment if there is a high probability of complete resection of all detectable tumour manifestations according to national guidelines (Garbe et al, 2008). The decision is usually made after tumour board discussion accounting for the number of involved distant sites (ideally <3 for metastasectomy), technical feasibility, and treatment-related risks.
Statistical analysis
The closest clinically relevant cutoff was used for categorisation of characteristics instead of the median value for age (cutoff at 60 instead of median 62 years), Breslow’s tumour thickness of the primary melanoma (2.0 instead of median 2.5 mm), and time interval (3 years instead of 2.5 years). The categorisation of the body site of the primary melanoma was performed as described before by studies based on the AJCC database (Soong et al, 2010). As only 3 of the 24 patients had primary melanomas with Clark levels I or II, these were analysed as one group together with those with Clark level III. Categorised variables were dummy coded to adhere to the linearity assumption of multivariable regression analysis. Follow-up time was defined from the date of diagnosis of stage IV disease to the date of last follow-up or death. Estimates of cumulative survival probabilities according to Kaplan–Meier were described together with 95% confidence intervals and compared using two-sided log-rank test statistics. Median survival times (MST) are presented. For the analysis of overall survival, patients who were alive at the last follow-up were censored, although patients who had died were considered an ‘event’.
Multivariable Cox proportional hazard models were used to determine independent prognostic factors. All characteristics described above were considered in multivariable analysis. Missing values were assessed independently as a separate group for each variable except for LDH and S100B. Patients with missing LDH or S100B were excluded from multivariable testing. Forward and backward stepwise procedures of the multivariable modelling process were conducted. Results of the Cox models were described by means of hazard ratios (HRs) together with 95% confidence intervals; _P_-values were based on the Wald test.
Confounding was assessed by checking the effect of each remaining non-significant variable, which was not in a model, on factors in the model. If changes in the estimate of factors in the model of 5% or more occurred, the variable was considered a confounder. Throughout the analysis, _P_-values less than 0.05 were considered as statistically significant. All statistical analyses were carried out using the SPSS Version 19 (IBM SPSS, Chicago, IL, USA).
Results
Description of sample
Patients’ characteristics are shown in Table 1. A total of 855 patients (56.4% male) with primary cutaneous melanoma were included in the survival analysis at the time of stage IV diagnosis. The median age was 62 years (inter quartile range (IQR) 50–71 years). The median follow-up for patients who died was 9 months (IQR: 5–16) and 25 months (IQR: 10.5–64.5) for patients who were alive at the last date of observation. The MST according to Kaplan–Meier was 11 months. Cumulative survival rates were 46.5 (1 year), 22.9 (2 years), 10.3 (5 years), and 7.9% (10 years). Based on the site of distant metastases, the majority of patients (54.9%) was aligned to the M1c category (M1a=18.5%; M1b=26.7%). An elevated serum S100B or LDH was observed in 55% or 28% of patients, respectively. Metastasectomy, as a first treatment for distant metastases, was performed in 220 patients. In 77% of patients with metastasectomy, distant metastasis occurred at only one distant site; 16% of patients had two and 7% more than two involved sites. Soft tissue metastases, pulmonal lesions, and brain metastases were removed in 49%, 31%, and 24%, respectively. Metastasectomy of liver lesions was performed in 4% and in intestinal metastasis in 6%.
Table 1 Descriptive statistics and 2-year survival rates according to Kaplan–Meier for 855 melanoma patients with distant metastasis
Survival analysis according to Kaplan–Meier
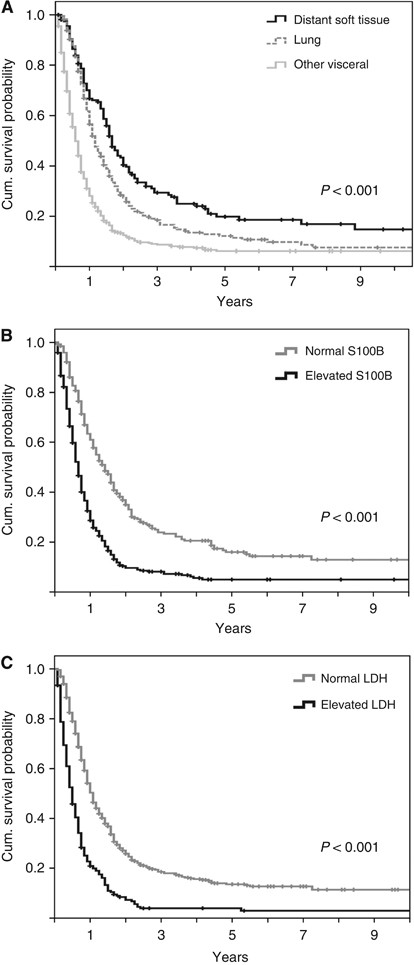
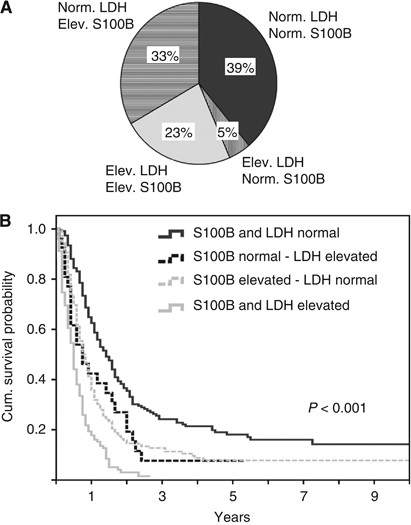
Both factors included in the AJCC staging system (site of distant metastases and LDH) were correlated with survival. If distant lesions were limited to soft tissue, MST was 20 months, although it was shorter for patients with pulmonary involvement (14 months, _P_=0.002 in log-rank test) or other visceral lesions (7 months; P<0.001; Figure 1A). Serum S100B had a strong impact on survival (_P_<0.001; Figure 1B). Patients with normal LDH had a MST of 13 months, and those with elevated LDH had a MST of 6 months (_P_<0.001; Figure 1C). The degree of elevation of both serum markers was also prognostically relevant (Supplementary Figure 1). A higher than two-fold elevation of S100B or LDH was associated with a remarkably worse prognosis compared with an elevation lower than two-fold (MST 3 _vs_ 7 months for LDH and 7 _vs_ 10 months for S100B, both _P_<0.001). A longer survival since stage IV diagnosis was observed, if the site of the primary melanoma was located on the extremities compared with axial lesions (_P_=0.005), if the interval between initial diagnosis and stage IV diagnosis was >36 months (_P_=0.001) and if only few distant sites were involved (P<0.001). Patients who underwent complete metastasectomy of their initial distant metastases had a significantly longer MST compared with patients receiving other treatments (17 vs 9 months; P<0.001). No differences in prognosis were evident for age, gender, stage at initial diagnosis, or histopathological characteristics of the primary melanoma (Table 1). Patients with combined elevation of S100B and LDH had an unfavourable prognosis. The 2-year survival rate was 3.1% in contrast to patients with normal values in one or both serum analyses (Figure 2). Median survival times of patients with brain metastases was 6 months. Significant survival differences for this subgroup of patients were observed according to the initial treatment, S100B, LDH and the number of involved distant sites (Supplementary Table 1).
Figure 1
Bivariate analysis of 885 patients. Kaplan–Meier survival curves according to (A) the site of metastasis, (B) serum S100B, and (C) serum LDH. Censored events are indicated by vertical lines.
Figure 2
Combined analysis of LDH and S100B in 586 patients. (A) Proportions of subgroups with different pattern of LDH and S100B values. Elev.=elevated; Norm.=normal. (B) Kaplan–Meier survival curves for patients according to different combinations of S100B and LDH.
Multivariable Cox proportional hazard analysis
Patients with missing values for LDH or S100B were excluded resulting in a sample size of 586 patients for the multivariate Cox regression analyses. All variables except the initial treatment in stage IV disease were included in the first analysis (Table 2). In the final model, elevated LDH and S100B, an interval between initial diagnosis and stage IV diagnosis ⩽36 months, lung, or other visceral sites of distant metastasis and a higher number of involved distant sites than two, were significant factors that independently increased the risk to die. Visceral metastases other than lung (HR 1.8), elevated S100B (HR 1.7), three or more involved metastatic sites (HR 1.7) and elevated LDH (HR 1.6) were the factors with the highest negative impact on survival.
Table 2 Results of the Cox proportional hazard analysis excluding the initial treatment of distant metastasis
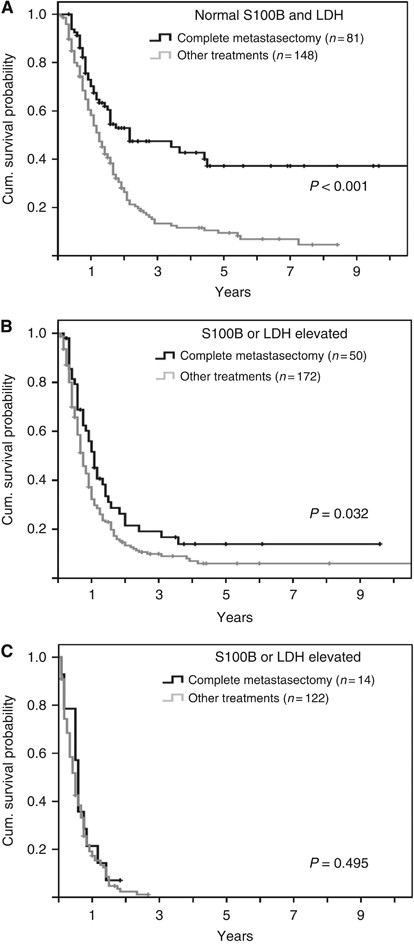
In a second analysis, the first treatment after occurrence of distant metastasis was additionally included (Table 3) to the established Cox model (Table 2). A treatment other than complete metastasectomy was found to represent an independent prognostic factor (HR 1.3; _P_=0.023). All other factors remained basically unchanged except the number of involved distant sites. The HR decreased to 1.5 if the patient had three or more involved sites. In patients with normal values of LDH and S100B, the difference in survival according to the initial treatment of distant metastasis was highest. The 5-year survival rate was 37.2% if complete metastasectomy was performed, although it was 9.5% for patient receiving other treatments (P<0.001; Figure 3A). This association between complete metastasectomy and favourable survival was still evident if only one serum marker, either S100B or LDH, was elevated (_P_=0.023; Figure 3B), but no prognostic impact was observed in patients with elevation of both markers (Figure 3C). The independent prognostic impact of LDH, S100B, the time interval between initial diagnosis and stage IV diagnosis, the site and the number of involved sites in model 1 and additionally of the first treatment in model 2 was also evident if age and tumour thickness were included as continuous variables in the Cox regression analysis.
Table 3 Results of the Cox proportional hazard analysis additionally accounting for the initial treatment of distant metastasis
Figure 3
Impact of complete metastasectomy according to LDH and S100B. Kaplan–Meier survival curves for patients with (A) normal LDH and normal S100B (B) one elevated serum marker and (C) elevated LDH and elevated S100B. Censored events are indicated by vertical lines.
Discussion
The present analysis showed for the first time that the serum markers LDH and S100B are both independent prognostic factors in melanoma patients with distant metastasis, additionally to the extent, organ pattern, and the time course of the metastasis. The HRs for death were 1.6 for patients with elevated LDH and 1.7 for patients with elevated S100B. Accordingly, the MST differed largely between patients with both serum markers in the normal range with 18 months vs one serum marker elevated (either LDH or S100B) with 9 months or both factors elevated with 6 months. It should be stressed that the independent impact of both serum markers was confirmed in multivariate analysis adjusted for all potential and established prognostic factors. The finding of the independent impact of both serum markers on prognosis was surprising, because one would expect a parallel increase of both factors. However, in 33% of patients only S100B was elevated, whereas 23% of patients showed elevation of both serum markers at the time of first diagnosis of distant metastasis. In contrast, only 5% had an isolated increase of LDH. Lactate dehydrogenase is expressed ubiquitously in different healthy tissues. An elevated serum concentration of the intracellular enzyme is mainly based on cell lysis, for example, after myocardial infarction or haemolysis. Moreover, an increased serum LDH occurs in different tumour entities and indicates a high turn-over of tumour cells as well as necrosis in fast-growing tumours. It is associated with high tumour burden and seems to be particularly elevated in liver metastases for which no clear explanation can be given (Finck et al, 1983; Heimdal et al, 1989; Sirott et al, 1993).
S100B shows many differences to LDH. It is tissue specific and mainly expressed in glial cells of the brain, melanocytes, and other cell types, which are originally derived from the neural crest. Additionally, it is detectable in chondrocytes and dendritic cells. Most melanomas strongly express S100B. A functional role of this protein in melanoma is conceivable due to its capacity to interact with p53 and to activate STK38/NDR1, a protein kinase involved in cell survival and proliferation (Donato, 2001; Hergovich et al, 2006). It has been shown in cell culture experiments that it can be actively secreted upon metabolic stress (Gerlach et al, 2006) and it is likewise elevated in patients with neural diseases showing metabolic abnormalities like schizophrenia or depression (Schroeter and Steiner, 2009). Therefore, the biology of S100B is clearly different from LDH and this may explain the independent prognostic impact shown by both markers. A complete lack of S100B expression can be observed by immunohistochemistry in a small proportion of melanoma patients (Argenyi et al, 1994). This downregulation of S100B might explain the normal serum S100B in spite of elevated LDH in 5% of our patients.
It is an interesting finding of the present study that complete metastasectomy was associated with a favourable prognosis. The subgroup of patients with no elevation of the two serum markers LDH and S100B in combination had the longest survival after complete metastasectomy. In general, it has to be considered that the association between metastasectomy and survival may be due to a selection bias of prognostically favourable patients towards surgery. As expected, a higher percentage of our patients with metastasectomy had soft-tissue metastases only and normal serum markers. The mean number of involved distant sites was lower with 1.3 compared with 2.1 in patients with other treatments (Supplementary Table 2). Therefore, we accounted for these factors in the multivariate analyses. Although the independent impact of complete metastasectomy was confirmed in the Cox regression analysis, we acknowledge that the true effect of this procedure can only be assessed in a prospective randomised controlled trial. Thus far, surgery of distant metastasis has frequently been found to be associated with favourable survival of selected patients subgroups (Ollila et al, 1996; Wood et al, 2001; Neuman et al, 2007), but only two research groups analysed the role of complete metastasectomy vs other treatments using Cox regression analyses in unselected patients with distant metastasis (Meyer et al, 2000; Wasif et al, 2011). Both groups found an independent positive impact on prognosis of this procedure but in both studies LDH was not available. The present study is the first that accounted for LDH as well as the site of distant metastasis in Cox regression analysis and therefore included both relevant factors of the current AJCC staging system (Balch et al, 2009). However, there could be other yet unknown prognostic factors that we have not adjusted for and which only a randomised study can control.
A general problem of prognostic studies in patients with distant metastasis is that only a part of these studies analysed patients at the time of the diagnosis of distant metastases (Neuman et al, 2008; Wasif et al, 2011), whereas others investigated prognostic factors before the start of systemic treatment (Sirott et al, 1993; Eton et al, 1998; Manola et al, 2000; Unger et al, 2001). The latter studies did not include patients who were initially treated by surgical modalities or by best supportive care. Furthermore, many systemic treatment schedules exclude patients with brain metastases. Therefore, these studies analysed already selected patient subgroups. In the present study of prognostic factors in patients with distant metastases such selection bias was avoided and all patients have been included at the time point of their initial diagnosis of distant metastasis.
In conclusion, the serum markers LDH and S100B were found to be independent prognostic factors in melanoma patients with distant metastasis, and both factors were associated with similar HRs. Other features of the metastasis like its extent, the organ pattern, and the time course of its development remained independent prognostic factors. Furthermore, complete metastasectomy had an independent favourable prognostic impact in particular for the patient subgroup with both LDH and S100B in their normal range.
Change history
17 July 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
- Argenyi ZB, Cain C, Bromley C, Nguyen AV, Abraham AA, Kerschmann R, LeBoit PE (1994) S-100 protein-negative malignant melanoma: fact or fiction? A light-microscopic and immunohistochemical study. Am J Dermatopathol 16 (3): 233–240
Article CAS PubMed Google Scholar - Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF (2001) Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 19 (16): 3635–3648
Article CAS PubMed Google Scholar - Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Morton DL, Ross MI, Sober AJ, Sondak VK (2009) Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27 (36): 6199–6206
Article PubMed PubMed Central Google Scholar - Deichmann M, Benner A, Bock M, Jackel A, Uhl K, Waldmann V, Naher H (1999) S100-Beta, melanoma-inhibiting activity, and lactate dehydrogenase discriminate progressive from nonprogressive American Joint Committee on Cancer stage IV melanoma. J Clin Oncol 17 (6): 1891–1896
Article CAS PubMed Google Scholar - Donato R (2001) S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 33 (7): 637–668
Article CAS PubMed Google Scholar - Egberts F, Pollex A, Egberts JH, Kaehler KC, Weichenthal M, Hauschild A (2008) Long-term survival analysis in metastatic melanoma: serum S100B is an independent prognostic marker and superior to LDH. Onkologie 31 (7): 380–384
Article CAS PubMed Google Scholar - Essner R, Lee JH, Wanek LA, Itakura H, Morton DL (2004) Contemporary surgical treatment of advanced-stage melanoma. Arch Surg 139 (9): 961–966
Article PubMed Google Scholar - Eton O, Legha SS, Moon TE, Buzaid AC, Papadopoulos NE, Plager C, Burgess AM, Bedikian AY, Ring S, Dong Q, Glassman AB, Balch CM, Benjamin RS (1998) Prognostic factors for survival of patients treated systemically for disseminated melanoma. J Clin Oncol 16 (3): 1103–1111
Article CAS PubMed Google Scholar - Finck SJ, Giuliano AE, Morton DL (1983) LDH and melanoma. Cancer 51 (5): 840–843
Article CAS PubMed Google Scholar - Garbe C, Hauschild A, Volkenandt M, Schadendorf D, Stolz W, Reinhold U, Kortmann RD, Kettelhack C, Frerich B, Keilholz U, Dummer R, Sebastian G, Tilgen W, Schuler G, Mackensen A, Kaufmann R (2007) Evidence and interdisciplinary consense-based German guidelines: diagnosis and surveillance of melanoma. Melanoma Res 17 (6): 393–399
Article PubMed Google Scholar - Garbe C, Hauschild A, Volkenandt M, Schadendorf D, Stolz W, Reinhold U, Kortmann RD, Kettelhack C, Frerich B, Keilholz U, Dummer R, Sebastian G, Tilgen W, Schuler G, Mackensen A, Kaufmann R (2008) Evidence and interdisciplinary consensus-based German guidelines: surgical treatment and radiotherapy of melanoma. Melanoma Res 18 (1): 61–67
Article PubMed Google Scholar - Gerlach R, Demel G, Konig HG, Gross U, Prehn JH, Raabe A, Seifert V, Kogel D (2006) Active secretion of S100B from astrocytes during metabolic stress. Neuroscience 141 (4): 1697–1701
Article CAS PubMed Google Scholar - Hauschild A, Michaelsen J, Brenner W, Rudolph P, Glaser R, Henze E, Christophers E (1999) Prognostic significance of serum S100B detection compared with routine blood parameters in advanced metastatic melanoma patients. Melanoma Res 9 (2): 155–161
Article CAS PubMed Google Scholar - Heimdal K, Hannisdal E, Gundersen S (1989) Regression analyses of prognostic factors in metastatic malignant melanoma. Eur J Cancer Clin Oncol 25 (8): 1219–1223
Article CAS PubMed Google Scholar - Hergovich A, Stegert MR, Schmitz D, Hemmings BA (2006) NDR kinases regulate essential cell processes from yeast to humans. Nat Rev Mol Cell Biol 7 (4): 253–264
Article CAS PubMed Google Scholar - Lasithiotakis KG, Leiter U, Eigentler T, Breuninger H, Metzler G, Meier F, Garbe C (2007) Improvement of overall survival of patients with cutaneous melanoma in Germany, 1976–2001: which factors contributed? Cancer 109 (6): 1174–1182
Article PubMed Google Scholar - Leo F, Cagini L, Rocmans P, Cappello M, Geel AN, Maggi G, Goldstraw P, Pastorino U (2000) Lung metastases from melanoma: when is surgical treatment warranted? Br J Cancer 83 (5): 569–572
Article CAS PubMed PubMed Central Google Scholar - Manola J, Atkins M, Ibrahim J, Kirkwood J (2000) Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol 18 (22): 3782–3793
Article CAS PubMed Google Scholar - Meyer T, Merkel S, Goehl J, Hohenberger W (2000) Surgical therapy for distant metastases of malignant melanoma. Cancer 89 (9): 1983–1991
Article CAS PubMed Google Scholar - Mohammed MQ, Abraha HD, Sherwood RA, MacRae K, Retsas S (2001) Serum S100beta protein as a marker of disease activity in patients with malignant melanoma. Med Oncol 18 (2): 109–120
Article CAS PubMed Google Scholar - Neuman HB, Patel A, Hanlon C, Wolchok JD, Houghton AN, Coit DG (2007) Stage-IV melanoma and pulmonary metastases: factors predictive of survival. Ann Surg Oncol 14 (10): 2847–2853
Article PubMed Google Scholar - Neuman HB, Patel A, Ishill N, Hanlon C, Brady MS, Halpern AC, Houghton A, Coit DG (2008) A single-institution validation of the AJCC staging system for stage IV melanoma. Ann Surg Oncol 15 (7): 2034–2041
Article PubMed Google Scholar - Ollila DW (2006) Complete metastasectomy in patients with stage IV metastatic melanoma. Lancet Oncol 7 (11): 919–924
Article PubMed Google Scholar - Ollila DW, Essner R, Wanek LA, Morton DL (1996) Surgical resection for melanoma metastatic to the gastrointestinal tract. Arch Surg 131 (9): 975–979
Article CAS PubMed Google Scholar - Schmidt H, Sorensen BS, Fode K, Nexo E, von der MH (2005) Tyrosinase messenger RNA in peripheral blood is related to poor survival in patients with metastatic melanoma following interleukin-2-based immunotherapy. Melanoma Res 15 (5): 409–416
Article CAS PubMed Google Scholar - Schroeter ML, Steiner J (2009) Elevated serum levels of the glial marker protein S100B are not specific for schizophrenia or mood disorders. Mol Psychiatry 14 (3): 235–237
Article CAS PubMed Google Scholar - Sirott MN, Bajorin DF, Wong GY, Tao Y, Chapman PB, Templeton MA, Houghton AN (1993) Prognostic factors in patients with metastatic malignant melanoma. A multivariate analysis. Cancer 72 (10): 3091–3098
Article CAS PubMed Google Scholar - Smit LH, Korse CM, Hart AA, Bonfrer JM, Haanen JB, Kerst JM, Nieweg OE, de Gast GC (2005) Normal values of serum S-100B predict prolonged survival for stage IV melanoma patients. Eur J Cancer 41 (3): 386–392
Article CAS PubMed Google Scholar - Soong SJ, Ding S, Coit D, Balch CM, Gershenwald JE, Thompson JF, Gimotty P (2010) Predicting survival outcome of localized melanoma: an electronic prediction tool based on the AJCC Melanoma Database. Ann Surg Oncol 17 (8): 2006–2014
Article PubMed PubMed Central Google Scholar - Tarhini AA, Stuckert J, Lee S, Sander C, Kirkwood JM (2009) Prognostic significance of serum S100B protein in high-risk surgically resected melanoma patients participating in Intergroup Trial ECOG 1694. J Clin Oncol 27 (1): 38–44
Article PubMed PubMed Central Google Scholar - Unger JM, Flaherty LE, Liu PY, Albain KS, Sondak VK (2001) Gender and other survival predictors in patients with metastatic melanoma on Southwest Oncology Group trials. Cancer 91 (6): 1148–1155
Article CAS PubMed Google Scholar - Wasif N, Bagaria SP, Ray P, Morton DL (2011) Does metastasectomy improve survival in patients with stage IV melanoma? a cancer registry analysis of outcomes. J Surg Oncol 104 (2): 111–115
Article PubMed PubMed Central Google Scholar - Wood TF, DiFronzo LA, Rose DM, Haigh PI, Stern SL, Wanek L, Essner R, Morton DL (2001) Does complete resection of melanoma metastatic to solid intra-abdominal organs improve survival? Ann Surg Oncol 8 (8): 658–662
Article CAS PubMed Google Scholar
Acknowledgements
This work was supported by Deutsche Forschungsgemeinschaft grant SFB685.
Author information
Author notes
- B Weide and M Elsässer: These authors contributed equally to this work.
Authors and Affiliations
- Department of Dermatology, Center for Dermatooncology, University Medical Center, Liebermeisterstrasse 25, Tübingen, 72076, Germany
B Weide, M Elsässer, A Pflugfelder, U Leiter, T K Eigentler, J Bauer, F Meier & C Garbe - Skin Cancer Research Group, School of Public Health, Tropical Medicine and Rehabilitation Sciences, James Cook University, Townsville, 4811, Queensland, Australia
P Büttner & C Garbe - Department of General and Transplant Surgery, University Medical Center, Hoppe-Seyler-Street 3, Tübingen, 72076, Germany
M Witte
Authors
- B Weide
- M Elsässer
- P Büttner
- A Pflugfelder
- U Leiter
- T K Eigentler
- J Bauer
- M Witte
- F Meier
- C Garbe
Corresponding author
Correspondence toB Weide.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Supplementary Information accompanies the paper on British Journal of Cancer website
Supplementary information
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Weide, B., Elsässer, M., Büttner, P. et al. Serum markers lactate dehydrogenase and S100B predict independently disease outcome in melanoma patients with distant metastasis.Br J Cancer 107, 422–428 (2012). https://doi.org/10.1038/bjc.2012.306
- Received: 25 January 2012
- Revised: 16 May 2012
- Accepted: 18 June 2012
- Published: 10 July 2012
- Issue Date: 24 July 2012
- DOI: https://doi.org/10.1038/bjc.2012.306