iPSC lines that do not silence the expression of the ectopic reprogramming factors may display enhanced propensity to genomic instability (original) (raw)
Induced pluripotent stem cell (iPSC) technology, which uses defined transcription factors to reprogram somatic cells to become pluripotent cells, represents a simple method to generate donor/patient-specific pluripotent stem cells with reduced ethical concerns 1, 2. Thus, iPSC technology provides unprecedented opportunities in biomedical research and regenerative medicine. Human iPSCs have been derived from different cell types 3, 4, 5, 6, 7. However, still very little information is available about iPSC safety, better ways to direct a specific reprogramming process and the reprogramming mechanisms, which remain a mystery. Based on previous work, it is hypothesized that the transformation resulting from c-myc and Klf4 is followed by a pluripotent cell induction process mediated by ESC-associated transcription factors such as Oct4, Sox2 and Lin28 8.
Reprogramming of differentiated cells to iPSCs is a stochastic process, but those cells that reach the pluripotency state seem to progress through several milestones in an orderly fashion 9, 10. Accordingly, live cell imaging studies have very recently suggested that the current experimental approaches commonly used to generate iPSCs may eventually give rise to either bona fide, fully reprogrammed human iPSCs or just partially reprogrammed cells (pseudo-iPSCs) 11.
Similar to what has been extensively reported for human hESCs, human iPSCs are expected to be genetically stable, since karyotypic/genomic alterations represent a pitfall for potential future downstream applications. Several studies have analyzed the genomic stability of several hESC lines maintained after prolonged in vitro culture 12, 13, 14. However, data on the genomic stability of iPSCs after short, medium and long-term culture remain scarce.
We have very recently derived four human iPSC lines using a lentiviral construct that expresses the reprogramming factors Oct4, Klf4, Sox2 and c-myc upon insertion (Figure 1A). Out of the four lines, two (termed iAND-4 and iMSUH) were generated from human fibroblasts and the other two (termed CB-CD34+ iPSC#1 and CB-CD34+ iPSC#2) were generated from cord blood-derived CD34+ hematopoietic progenitor cells. These lines have been fully characterized and deposited according to the Spanish Legislation in the Spanish Stem Cell Bank (http://www.isciii.es/htdocs/terapia/terapia_lineas.jsp). These iPSC lines show ESC-like morphology, express robust levels of Nanog, Oct4, Rex1 and Sox2, as well as the surface markers SSEA-3, SSEA-4, Tra-1-60 and Tra-1–81. Furthermore, the four iPSC lines display pluripotency potential, giving rise to tissues representing the three germ layers in vitro (by embryoid body formation) and in vivo (by teratoma formation) (data not shown). Besides, they proved to be bacteria-, fungi- and mycoplasma-free and STR and HLA typing analyses revealed that each iPSC line fully matched the original parental cell line 15, 16.
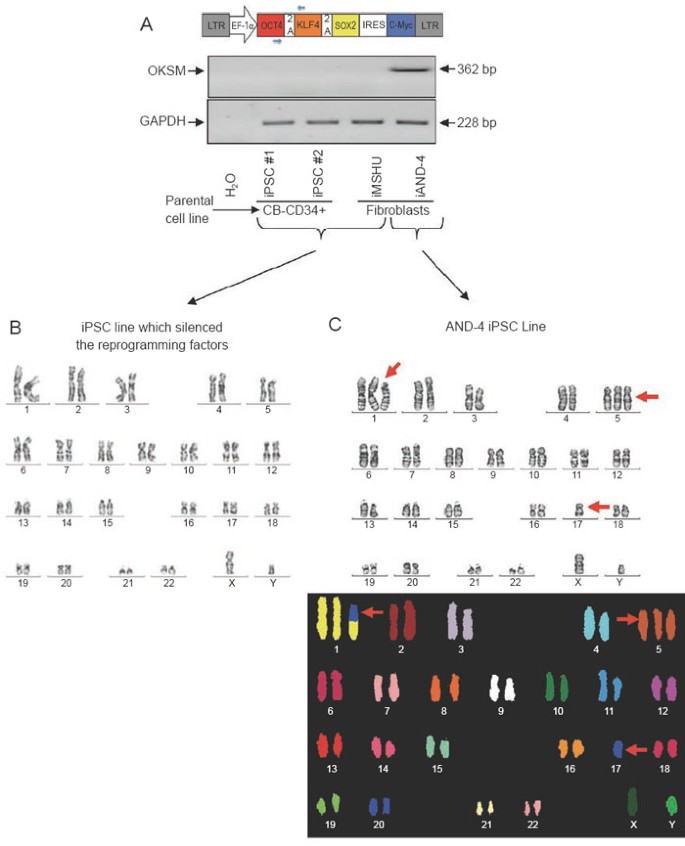
Figure 1
(A) Cartoon of the lentiviral vector structure used for iPSC generation (top) and RT-PCR demonstrating that the ectopic reprogramming factors (OKSM) have been silenced after 6-8 passages in the three iPSC lines karyotypically euploid, while remaining robustly expressed in the iAND-4 iPSC line that is karyotypically aneuploid (bottom). Primers used are depicted as small blue arrows in the cartoon showing the lentiviral structure. Primers used were: forward (on Oct-4): 5′-GCCTTTCCCCCTGTCTCCGTC-3′ and reverse (on Klf-4): 5′-GGCTCCGCCGCTCTCCAGGTCTG-3′. The PCR conditions were as follows: 2 min at 95 °C, 35 cycles of 30 s at 94 °C followed by 30 s at 60 °C and 60 s at 72 °C, and a final extension of 5 min at 72 °C. GAPDH was used as a housekeeping control 17, 18. (B) Representative euploid karyotype (G-banding) of several iPSCs (iPSC #1, iPSC #2, iMSUH), which did silence the reprogramming factors. (C) Representative aneuploid (47,XY, +1, +5, -17) karyotype of iAND-4 iPSC line (top panel), which did not silence the reprogramming factors. The bottom panel shows a SKY-FISH confirmation of the chromosomal abnormalities. Twenty metaphases were consistently analyzed as previously described 19.
However, regarding karyotypic/genomic stability we observed an interesting result. The iPSC lines CB-CD34+ iPSC#1, CB-CD34+ iPSC#2 and iMSUH all showed a euploid karyotype after 8-10 passages in vitro (Figure 1B). Notably, ectopic reprogramming factors were found to be silenced in these three iPSC lines (Figure 1A). However, the iAND-4 iPSC line consistently displayed an aneuploid karyotype 47,XY,+1,+5,-17 (analyzed at passage 3 and passage 7; Figure 1C). These chromosomal abnormalities were subsequently confirmed by SKY-FISH (Figure 1C). The intriguing result, however, was that this karyotypically unstable iPSC line did not silence the expression of the reprogramming factors from the proviral insertion. All the original parental somatic cells used for reprogramming (CD34+ cells and fibroblasts) were karyotypically euploid (data not shown), ruling out the possibility that the aneuploid DNA content in the iAND-4 iPSC line was present in the parental cells. To the best of our knowledge, this is the first evidence showing preliminary data highlighting a potential link between lack of proviral silencing and genomic instability in human iPSCs. We therefore hypothesize that the absence of proviral silencing may make iPSCs more prone to genomic instability. This hypothesis may be supported by the fact that two of the reprogramming genes are oncogenes (c-myc and Klf4), whose continuous ectopic expression may make the cells more vulnerable to acquire further oncogenic events and eventually gross chromosomal abnormalities. We envision that epigenetic mechanisms, proviral insertion site and other properties associated with non-viral strategies commonly employed to direct a specific reprogramming process may also play a key role that still demands further exhaustive studies.
Because cell reprogramming is becoming the 'flavor of the month' and many donor-, patient- and tissue-specific iPSC lines are being generated worldwide for downstream applications such as drug screening and specific cell differentiation towards lineages with potential future clinical applications, our preliminary data highlight the need to prospectively further analyze proviral silencing and genomic stability over time using both conventional and molecular cytogenetic technologies. We hope our observation based on four iPSC lines will encourage the stem cell scientific community to undertake rigorous characterization and standardization of much larger cohorts of iPSC lines generated in different laboratories, from different somatic cells and using distinct reprogramming methods including integrative and non-integrative strategies in order to further confirm or refute our proposal and set up the criteria to define putative fully reprogrammed safe and stable iPSCs.