Morning hypertension and night non-dipping in patients with diabetes and chronic kidney disease (original) (raw)
Introduction
Blood pressure (BP) is strongly associated with chronic kidney disease (CKD). Elevated BP is a major cause of CKD and aggravates its progression.1, 2 Moreover, it increases the mortality rate in patients with CKD.3 The Kidney Disease: Improving Global Outcomes guidelines recommend strict control of BP in CKD patients, especially in patients with diabetes mellitus (DM) and albuminuria.2 DM increases the risk of cardiovascular disease by a factor of two to three at every level of systolic BP (SBP) and this risk is higher when CKD is present.4
BP control is in practice based mostly on office BP. However, there is growing evidence that diurnal variation is associated with cardiovascular events and stroke.[5](/articles/hr201589#ref-CR5 "Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR . Prognosis of "masked" hypertension and “white-coat” hypertension detected by 24- h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol 2005; 46: 508–515."), 6, 7 BP decreases during sleep and is markedly increased in the morning in healthy individuals. Patients with hypertension (HTN) usually show the same BP pattern, albeit with higher values.8 However, some subjects show loss of nocturnal dipping and rise of morning BP. Morning HTN is a stronger risk factor for stroke than sustained HTN.7 The difference between morning BP and evening BP is associated with left ventricular hypertrophy and diastolic dysfunction.9 In addition, loss of night dipping predicted the development of microalbuminuria10 and was linked to left ventricular hypertrophy, cardiovascular morbidity and mortality in diabetes.[5](/articles/hr201589#ref-CR5 "Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR . Prognosis of "masked" hypertension and “white-coat” hypertension detected by 24- h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol 2005; 46: 508–515."), 6, 11
Although both morning HTN and nocturnal non-dipping (ND) are regarded as important BP patterns in diabetes, little has been reported about these BP patterns in diabetics with advanced CKD. This study aimed to evaluate the relationships of morning HTN and ND with estimated glomerular filtration rate (eGFR) and proteinuria, and to estimate the risk of morning HTN and ND according to the presence of DM and reduced renal function.
Materials and methods
Study design
The Assessment of Blood Pressure Control and Target Organ Damage in Patients with Chronic Kidney Disease and Hypertension (APrODiTe) study was a nationwide cross-sectional study conducted at 21 centers between October 2009 and May 2011. The objectives, inclusion/exclusion criteria and adequacy of the sample size were reported previously.12
Briefly, we included 1312 patients, including 439 diabetics, aged 20–75 years, who had taken antihypertensive medication before participation and had an eGFR of 15–89 ml min−1 per 1.73 m2 based on the modification of diet in renal disease (MDRD) study equation.13 Patients had good compliance with medication and no change in prescriptions 2 weeks before participation. We excluded patients who changed prescriptions regularly according to 24-h ambulatory BP (ABP) monitoring, had a history of hospitalization for acute kidney injury, had a spot urine protein to creatinine ratio (UPCR) of >6.0 g gCr−1, had undergone kidney transplantation or had uncontrolled arrhythmia, uncontrolled bronchial asthma, chronic obstructive pulmonary disease or a primary endocrinologic disease other than DM. Pregnant and lactating females and nightshift workers were also excluded.
The study protocol was approved by the Institutional Review Boards of the participating centers. Various data were collected: anthropometric measures, history of DM, cardiovascular diseases such as coronary artery disease and stable angina, smoking, alcohol, exercise and prescribed medications. Laboratory data included serum creatinine, eGFR and morning UPCR.
BP measurement
Office BP was measured using an oscillometric OMRON IA-2 automatic BP device (IntelliSense, Omron, Kyoto, Japan). Three consecutive seated BP readings were recorded at intervals of 1–2 min and clinic BP was calculated as the mean of the last two readings. Twenty-four-hour ABP was measured every 30 min using an oscillometric TM-2430 monitor (A&D, Seoul, Korea).
Definitions
Daytime SBP was defined as the mean SBP between 1000 and 2000 h, and nighttime SBP was the mean SBP between 0000 and 0600 h. Morning highest was defined as the average of three SBP readings centered on the highest morning (0600–1200 h) reading (the highest reading plus the readings immediately before and after). Evening lowest was defined as the average of three SBP readings centered on the lowest evening reading (2000–0000 h). Morning–evening difference was defined as morning highest minus evening lowest. Night lowest was defined as the average of three SBP readings centered on the lowest nighttime reading.7, 14 ND was defined as a ratio of average nighttime SBP to average daytime SBP of >0.9.15 Morning HTN was defined as an average of morning highest and evening lowest of ⩾135 mm Hg and a morning–evening difference of ⩾20 mm Hg.7, 14
Controlled clinical BP was defined as a BP of <140/90 mm Hg. ABP was considered normal if the daytime value was <135/85 mm Hg and the nighttime value was <120/70 mm Hg. True controlled HTN was defined as controlled clinic, daytime and nighttime BP. Masked HTN was defined as controlled clinic BP and elevated daytime/nighttime ABP. Sustained uncontrolled BP was defined as uncontrolled clinic BP and ABP.
Statistical analyses
All analyses were performed using SPSS version 18.0 for Windows (Chicago, IL, USA). Data are presented as means±s.d. for continuous variables and as proportions for categorical variables. Differences were analyzed using the _χ_2-test for categorical variables and analysis of variance or Student’s _t_-test for non-categorical variables. Participants were divided into four groups according to presence of DM and CKD stage: CKD2 only (no DM and eGFR 60–89 ml min−1 per 1.73 m2), DMCKD2 (DM and eGFR 60–89 ml min−1 per 1.73 m2), CKD3–4 only (no DM and eGFR 15–59 ml min−1 per 1.73 m2) and DMCKD3–4 (DM and eGFR 15–59 ml min−1 per 1.73 m2). Unadjusted relative risks for morning HTN and ND were calculated using logistic regression and adjustments were made for age, gender and other variables with _P_-values <0.05 in univariate analyses. P<0.05 was considered to indicate statistical significance.
Results
Baseline characteristics
The mean age of patients was 56.6 years and 63.0% were males. The mean duration of HTN was 8.3 years. Mean eGFR was 48.9 ml min−1 per 1.73 m2 and mean UPCR was 1.2 g gCr−1. The 38% of patients took three or more anti-hypertensive medications. In patients with CKD stage 2, age, body mass index and waist circumference were higher in diabetics. Hematuria, history of alcohol consumption and use of diuretics were more frequent in diabetics (P<0.05). In patients with stage 3–4 CKD, age, body mass index, waist circumference and UPCR were significantly higher, and hemoglobin, eGFR and uric acid lower, in diabetics. Hematuria, coronary artery disease, stable angina and use of β-blockers, calcium channel blockers and diuretics were significantly more frequent in diabetics (P<0.05) (Table 1).
Table 1 Baseline characteristics of patients
BP patterns
There was no significant difference in office BP between diabetics and non-diabetics with stage 2 CKD. However, office BP was significantly higher in diabetics compared with non-diabetics in individuals with stage 3–4 CKD (P<0.05).
For 24-h ABP, daytime, nighttime, morning highest, evening lowest, morning–evening difference and night lowest BP values were higher in diabetics compared with non-diabetics with stage 2 CKD. For stage 3–4 CKD, diabetics showed higher values for 24-h, day, night, morning highest, evening lowest and night lowest BP (P<0.05). Furthermore, the difference in BP between morning and evening was significantly greater in diabetics compared with non-diabetics (P<0.05) (Table 2).
Table 2 SBP patterns according to diabetes and CKD stage
In patients with stage 2 CKD, the rate of true controlled HTN was less frequent in diabetics and the rate of masked HTN was more frequent in diabetics (P<0.05).
In patients with stage 3–4 CKD, BP was significantly uncontrolled in diabetics: 51.3% of these patients showed sustained HTN (Table 2). There was no difference in masked HTN or white coat HTN between diabetics and non-diabetics (Table 2).
Morning HTN and ND according to eGFR and proteinuria
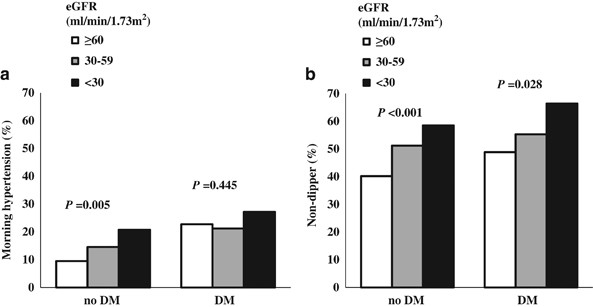
The rates of morning HTN and ND were significantly higher in diabetics compared with non-diabetics: 23.2% vs. 13.6%, P<0.001 and 58.2% vs. 48.2%, _P_=0.002, respectively. Regarding the relationships of eGFR level with morning HTN and ND according to DM, morning HTN was negatively correlated with eGFR in non-diabetics (_P_=0.005). In diabetics, there was no significant association between eGFR and morning HTN (_P_=0.445) (Figure 1a). ND was significantly correlated with eGFR, irrespective of DM (P<0.05) (Figure 1b). These results suggest that morning HTN may be related to diabetes, rather than to CKD stage.
Figure 1
Morning HTN and nocturnal ND according to eGFR. (a) The rate of morning HTN differed significantly according to eGFR in non-diabetics, but not in diabetics. (b) The rate of ND differed according to eGFR in diabetics and non-diabetics. DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; ND, nocturnal non-dipping.
Level of proteinuria was positively correlated with morning HTN in diabetics (_P_=0.001) but not in non-diabetics (Supplementary Figure S1A). Proteinuria level was significantly correlated with ND in patients with and without DM (P<0.05) (Supplementary Figure S1B).
Morning HTN according to diabetes and CKD stage
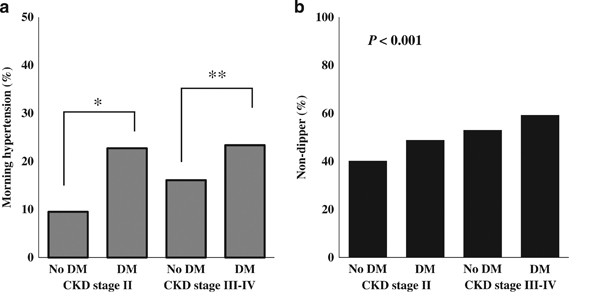
Two hundred and twenty-one patients (16.8%) had morning HTN. The rate of morning HTN was higher in patients in the DMCKD2 and DMCKD3–4 groups (22.7% and 23.4%, respectively), and lower in the CKD2-only and CKD3-4-only groups (9.5% and 16.1%, respectively) (P<0.001; Figure 2a).
Figure 2
Rates of morning HTN and nocturnal ND according to diabetes and CKD stage. (a) The rate of morning HTN was significantly different between diabetics and non-diabetics. In stage 2 CKD, the rate of morning HTN was higher in diabetics than in non-diabetics (22.7% vs. 9.5%, _P_=0.001). In stage 3–4 CKD, the rate of morning HTN was also higher in diabetics than in non-diabetics (23.4% vs. 16.1%, _P_=0.007). However, there were no differences between CKD2 and CKD3–4 in diabetics. Morning HTN may be affected mainly by DM, rather than by CKD stage. (b) The rate of ND was associated with DM and CKD stage (CKD2 only, 40.2%; DMCKD2, 48.9%; CKD3–4 only, 53.0%; DMCKD3–4, 59.3%; P<0.001). CKD, chronic kidney disease; DM, diabetes mellitus; ND, nocturnal non-dipping. *P<0.05, CKD2 only vs. DMCKD2; **P <0.05, CKD3–4 only vs. DMCKD3–4.
The risk of morning HTN was determined after adjustment for age, gender, body mass index, proteinuria, coronary artery disease, angiotensin-converting enzyme inhibitor and angiotensin receptor blocker use, diuretics and number of drugs taken per patient. Compared with the CKD2-only group, the DMCKD2, CKD3–4-only and DMCKD3–4 groups showed significantly higher risks of morning HTN. The risk of morning HTN was 2.093 (95% confidence interval (95% CI): 1.070–4.094) in the DMCKD2 group (_P_=0.031), 1.634 (95% CI: 1.044–2.557) in the CKD3–4-only group (_P_=0.032) and 2.236 (95% CI: 1.401–3.570) in the DMCKD3–4 group (_P_=0.001).
In addition, proteinuria was a significant risk factor for morning HTN (1.591, 95% CI: 1.100–2.300). The significant low risk of morning HTN was shown in patients using angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (0.401, 95% CI: 0.263–0.611) (P<0.001). By contrast, higher risk of morning HTN was noticed in patients using diuretics (1.678, 95% CI: 1.226–2.295) (_P_=0.001; Table 3). These results suggest that morning HTN is affected mainly by diabetes and proteinuria, rather than by renal function.
Table 3 Risk of morning HTN according to diabetes and CKD stage
Nocturnal ND according to diabetes and CKD stages
ND was documented in 51.2% of the study patients. The prevalence of ND was highest in the DMCKD3–4 group (59.3%) and lowest in the CKD2-only group (40.2%) (P<0.001). The DMCKD2 group and CKD3–4-only group had ND prevalences of 48.9% and 53.0%, respectively (Figure 2b).
The risk of ND was evaluated according to DM and CKD stage, with adjustment for age, gender, body mass index, proteinuria, hematocrit, duration of HTN, stable angina, β-blocker use, calcium channel blocker use and number of drugs taken per patient. The DMCKD2 group showed no significant risk elevation compared with the CKD2-only group (1.400, 95% CI: 0.851–2.303). However, the CKD3–4-only group had a 1.581-fold higher risk of ND compared with the CKD2-only group (95% CI: 1.180–2.120) (_P_=0.002); the DMCKD3–4 group showed a 1.872-fold risk elevation (95% CI: 1.348–2.601) (P<0.001; Table 4). The ND risk in diabetics was significant only in patients with advanced CKD (stage 3–4).
Table 4 Risk of non-dipping according to diabetes and CKD stage
Discussion
The present study indicates that diabetics had different BP patterns compared with non-diabetics. In addition to office HTN, diabetics showed higher prevalence of sustained uncontrolled HTN, morning HTN and ND. Notably, morning HTN was prominent in diabetics with proteinuria, regardless of renal function, and ND was significant in diabetics with advanced kidney damage.
Previous studies that included fewer CKD patients noted that the morning BP surge (morning SBP minus lowest SBP during nighttime) was significantly higher in diabetics.16, 17 However, in a study of a large population of CKD patients, the morning surge was smaller in diabetics than in non-diabetics, and eGFR and proteinuria were not significantly associated with morning BP surge.18 The authors explained that various antihypertensive drugs might influence BP patterns in CKD patients; however, the type and number of antihypertensive medications were not investigated.18 In our study, DM, renal function and proteinuria were significant risk factors for morning HTN in CKD patients. Notably, morning HTN was more frequent in diabetics and was related to proteinuria, rather than eGFR, in diabetes. There are several possible reasons for these different results. First, we selected morning HTN for measurement of morning BP, because morning BP surge could include normotensive subjects with BP variation.19 In our study, the group of morning BP surge include 53.1% of hypertensive patients and 46.9% of normotensive patients (_P_=0.644). The definition of morning surge can only evaluate the ‘delta’ value between morning and night BP, and cannot determine the absolute value. According to the renal function decline, dipping is blunted and sustained elevation of BP is observed during sleep.20, 21 Morning HTN in CKD patients was reported as the sustained type than the surge type.22 Although that can lower the difference of morning and night BP, BP variability could be more frequent among CKD patients with high BP. We adopted the definition of morning HTN (an average of morning highest and evening lowest of ⩾135 mm Hg and a morning–evening difference of ⩾20 mm Hg), because this concept consider both high BP and BP variability. In addition, we defined morning HTN based on the highest BP in the morning and lowest BP in the evening, because the waking and sleeping times were arbitrary in this study. Second, we analyzed the type and number of anti-hypertensive medications in a multivariate analysis. Third, proteinuria was measured by UPCR in our study and by urine albumin–creatinine ratio in previous studies.
The pathogenesis of morning HTN in diabetes is unclear. Activation of the sympathetic nervous system and renin–angiotensin–aldosterone system is involved in morning HTN. Morning HTN leads to a direct load on the vascular wall, an increase in shear stress, vascular endothelial cell dysfunction, vascular spasm and rupture of plaques.23 Endothelial dysfunction in the early morning was previously documented in patients with HTN and diabetes.24, 25 The abnormal elevation of morning BP may affect the kidneys, brain and heart, leading to increased renal vascular resistance and albuminuria.26 Morning–evening difference was a significant predictor of left ventricular hypertrophy and higher brain natriuretic peptide.27, 28 The risk of stroke was significantly higher in patients with morning HTN than in patients with sustained HTN.7 Therefore, morning HTN could be a specific type of HTN to detect target organ damage and estimate cardiovascular risk.
As other studies reported, prevalence of ND was relatively high in patients with DM or reduced renal function.20, 29 We noted significant associations among ND, CKD stage and level of proteinuria. The risk of ND was increased in advanced CKD, irrespective of DM. This association is compatible with other findings.18, 20, 29 ND was closely associated with the renal damage markers, eGFR and proteinuria. Increased extracellular volume, predominance of sympathetic activity and autonomic neuropathy are thought to be the mechanisms of ND in diabetes.29 ND is an abnormal elevation of nocturnal BP compared with daytime BP. In contrast, morning HTN is a concept that includes high average BP and high BP variability during daytime. The patients with ND showed much lower BP level of day–night difference and morning–evening difference: day–night difference: 1.7±9.7 vs. 24.9±9.1 mm Hg, ND vs. dipping, P<0.001; morning–evening difference: 13.3±20.2 vs. 20.8±23.0 mm Hg, ND vs. dipping, P<0.001. In contrast, there was no difference in day–night difference between patients with and without morning HTN. Therefore, patients with ND and morning HTN showed different BP patterns of day–night difference and morning–evening difference.
This study had several limitations. First, the prescribed dose frequency of anti-hypertensive medications was not evaluated. However, we evaluated the type and number of anti-hypertensive medications, and adjusted the risks of morning HTN and ND for type and number of anti-hypertensive drugs taken. Second, glycemic control status was not determined in this study. Third, ABP was measured only once. Fourth, we estimated GFR based on a single creatinine measurement. Finally, this study was cross-sectional and basic characteristics differed between the groups. We adjusted for these factors in a multivariate analysis to estimate the risk of abnormal BP patterns. In spite of these limitations, the study had several strengths. It was a multicenter study with a large number of subjects in whom ABP was measured using identical methods. The study subjects were homogenous and were relatively evenly distributed among CKD stages 2, 3 and 4. Furthermore, markers of renal damage such as eGFR and UPCR were measured.
In conclusion, diabetics showed higher rates of morning HTN, ND and uncontrolled sustained HTN compared with non-diabetics. These patterns of HTN were prominent in diabetics with advanced CKD or proteinuria. Morning HTN and ND should be monitored closely in diabetics with CKD. Further studies are required to examine the underlying mechanisms and prognosis of morning HTN and ND in diabetics with CKD.
References
- Bakris GL, Wiliams M, Dworkin L, Elliott WJ, Epstein M, Toto R, Tuttle K, Douglas J, Hsueh W, Sowers J . Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis 2000; 36: 646–661.
Article CAS Google Scholar - Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int Suppl 2012; 2: 337–414.
Article Google Scholar - Kovesdy CP, Bleyer AJ, Molnar MZ, Ma JZ, Sim JJ, Cushman WC, Quarles LD, Kalantar-Zadeh K . Blood pressure and mortality in U.S. veterans with chronic kidney disease: a cohort study. Ann Intern Med 2013; 159: 233–242.
Article Google Scholar - Stamler J, Vaccaro O, Neaton JD, Wentworth D . Diabetes, other risk factors, and 12- yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care 1993; 16: 434–444.
Article CAS Google Scholar - Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR . Prognosis of "masked" hypertension and “white-coat” hypertension detected by 24- h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol 2005; 46: 508–515.
Article Google Scholar - Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y . Non-dipping is a potent predictor of cardiovascular mortality and is associated with autonomic dysfunction in haemodialysis patients. Nephrol Dial Transplant 2003; 18: 563–569.
Article Google Scholar - Kario K, Ishikawa J, Pickering TG, Hoshide S, Eguchi K, Morinari M, Hoshide Y, Kuroda T, Shimada K . Morning hypertension: the strongest independent risk factor for stroke in elderly hypertensive patients. Hypertens Res 2006; 29: 581–587.
Article Google Scholar - Pickering TG, Shimbo D, Haas D . Ambulatory blood-pressure monitoring. N Engl J Med 2006; 354: 2368–2374.
Article CAS Google Scholar - Matsui Y, Eguchi K, Shibasaki S, Shimizu M, Ishikawa J, Shimada K, Kario K . Association between the morning-evening difference in home blood pressure and cardiac damage in untreated hypertensive patients. J Hypertens 2009; 27: 712–720.
Article CAS Google Scholar - Lurbe E, Redon J, Kesani A, Pascual JM, Tacons J, Alvarez V, Batlle D . Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med 2002; 347: 797–805.
Article CAS Google Scholar - Rahman M, Griffin V, Heyka R, Hoit B . Diurnal variation of blood pressure; reproducibility and association with left ventricular hypertrophy in hemodialysis patients. Blood Press Monit 2005; 10: 25–32.
Article Google Scholar - Cha RH, Kim S, Ae Yoon S, Ryu DR, Eun Oh J, Han SY, Young Lee E, Ki Kim D, Kim YS . Association between blood pressure and target organ damage in patients with chronic kidney disease and hypertension: results of the APrODiTe study. Hypertens Res 2014; 37: 172–178.
Article Google Scholar - Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254.
Article CAS Google Scholar - Kario K . Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension 2010; 56: 765–773.
Article CAS Google Scholar - Fagard R, Brguljan J, Thijs L, Staessen J . Prediction of the actual awake and asleep blood pressures by various methods of 24 h pressure analysis. J Hypertens 1996; 14: 557–563.
Article CAS Google Scholar - Afsar B, Elsurer R . The relationship between central hemodynamics, morning blood pressure surge, glycemic control and sodium intake in patients with type 2 diabetes and essential hypertension. Diabetes Res Clin Pract 2014; 104: 420–426.
Article CAS Google Scholar - Toyama M, Watanabe S, Miyauchi T, Kuroda Y, Ojima E, Sato A, Seo Y, Aonuma K . Diabetes and obesity are significant risk factors for morning hypertension: from Ibaraki Hypertension Assessment Trial (I-HAT). Life Sci 2014; 104: 32–37.
Article CAS Google Scholar - Iimuro S, Imai E, Watanabe T, Nitta K, Akizawa T, Matsuo S, Makino H, Ohashi Y, Hishida A, Chronic Kidney Disease Japan Cohort Study Group. Clinical correlates of ambulatory BP monitoring among patients with CKD. Clin J Am Soc Nephrol 2013; 8: 721–730.
Article Google Scholar - Hoshide S, Kario K . Early morning hypertension: a narrative review. Blood Press Monit 2013; 18: 291–296.
Article Google Scholar - Thompson AM, Pickering TG . The role of ambulatory blood pressure monitoring in chronic and end-stage renal disease. Kidney Int 2006; 70: 1000–1007.
Article CAS Google Scholar - Agarwal R, Kariyanna SS, Light RP . Circadian blood pressure classification scheme and the health of patients with chronic kidney disease. Am J Nephrol 2009; 30: 536–546.
Article Google Scholar - Mizuno M, Fukuda M, Miura T, Wakamatsu T, Naito T, Sato R, Togawa H, Sasakawa Y, Tomonari T, Ono M, Kato Y, Ichikawa T, Shirasawa Y, Ito A, Yoshida A, Kimura G . Morning hypertension in chronic kidney disease is sustained type, but not surge type. Blood Press Monit 2012; 17: 20–23.
Article Google Scholar - Kario K, Shimada K, Pickering TG . Clinical implication of morning blood pressure surge in hypertension. J Cardiovasc Pharmacol 2003; 42 (Suppl1): S87–S91.
Article CAS Google Scholar - Kollias GE, Stamatelopoulos KS, Papaioannou TG, Zakopoulos NA, Alevizaki M, Alexopoulos GP, Kontoyannis DA, Karga H, Koroboki E, Lekakis JP, Papamichael CM . Diurnal variation of endothelial function and arterial stiffness in hypertension. J Hum Hypertens 2009; 23: 597–604.
Article CAS Google Scholar - Yoda K, Inaba M, Hamamoto K, Yoda M, Tsuda A, Mori K, Yamada S, Emoto M, Koyama H, Imanishi Y . Association between glycemic control and morning blood pressure surge with vascular endothelial dysfunction in type 2 diabetic patients. Diabetes Care 2014; 37: 644–650.
Article CAS Google Scholar - Yano Y, Kario K . Nocturnal blood pressure, morning blood pressure surge, and cerebrovascular events. Curr Hypertens Rep 2012; 14: 219–227.
Article Google Scholar - Ikeda T, Gomi T, Shibuya Y, Matsuo K, Kosugi T, Oku N, Uetake Y, Kinugasa S, Furutera R . Morning rise in blood pressure is a predictor of left ventricular hypertrophy in treated hypertensive patients. Hypertens Res 2004; 27: 939–946.
Article Google Scholar - Ishikawa J, Hoshide S, Shibasaki S, Matsui Y, Kabutoya T, Eguchi K, Ishikawa S, Pickering TG, Shimada K, Kario K, JMS-1 Study Group. Relationship between morning hypertension identified by home blood pressure monitoring and brain natriuretic peptide and estimated glomerular filtration rate: the Japan Morning Surge 1 (JMS-1) Study. J Clin Hypertens (Greenwich) 2008; 10: 34–42.
Article CAS Google Scholar - Pecis M, Azevedo MJ, Moraes RS, Ferlin EL, Gross JL . Autonomic dysfunction and urinary albumin excretion rate are associated with an abnormal blood pressure pattern in normotensive normoalbuminuric type 1 diabetic patients. Diabetes Care 2000; 23: 989–993.
Article CAS Google Scholar
Acknowledgements
A special acknowledgment is extended to the APrODiTe study participants (Shin Wook Kang, Nam Ho Kim, Sung Gyun Kim, Ki Young Na, Hyunjin Nho, Cheol Whee Park, Hyung Chon Park, Sun Hee Park, Eun Young Sung, Sung Jun Shin, Chung Sik Lee, Eun Sil Jeon, Dong Chan Jin and Byoung Geun Han) for their time and commitment. This work was sponsored by Sanofi Korea.
Author information
Authors and Affiliations
- Department of Internal Medicine, Inje University College of Medicine, Ilsan-Paik Hospital, Goyang, Korea
Se Won Oh, Sang Youb Han & Kum Hyun Han - Department of Internal Medicine, National Medical Center, Seoul, Korea
Ran-hui Cha - Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
Sejoong Kim - Department of Internal Medicine, Catholic University College of Medicine, Uijeongbu, Korea
Sun Ae Yoon - Department of Internal Medicine, School of Medicine, Ewha Womans University, Seoul, Korea
Dong-Ryeol Rhu - Department of Internal Medicine, Hallym University College of Medicine, Seoul, Korea
Jieun Oh - Department of Internal Medicine, Soonchunhyang University College of Medicine, Cheonan, Korea
Eun Young Lee - Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
Dong Ki Kim & Yon Su Kim - Department of Medical Science, Seoul National University College of Medicine, Seoul, Korea
Yon Su Kim
Authors
- Se Won Oh
- Sang Youb Han
- Kum Hyun Han
- Ran-hui Cha
- Sejoong Kim
- Sun Ae Yoon
- Dong-Ryeol Rhu
- Jieun Oh
- Eun Young Lee
- Dong Ki Kim
- Yon Su Kim
Consortia
on behalf of the APrODiTe investigators
Corresponding author
Correspondence toSang Youb Han.
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Hypertension Research website
Supplementary information
Rights and permissions
About this article
Cite this article
Oh, S., Han, S., Han, K. et al. Morning hypertension and night non-dipping in patients with diabetes and chronic kidney disease.Hypertens Res 38, 889–894 (2015). https://doi.org/10.1038/hr.2015.89
- Received: 20 December 2014
- Revised: 16 June 2015
- Accepted: 26 June 2015
- Published: 27 August 2015
- Issue date: December 2015
- DOI: https://doi.org/10.1038/hr.2015.89