Human chromosome 21 gene expression atlas in the mouse (original) (raw)
So far 178 confirmed genes and 36 predicted genes have been identified on human chromosome 21 (refs 7–11). The mouse syntenic regions (segments of mouse chromosomes 10, 16 and 17) harbour 170 orthologues. We isolated 237 complementary DNA fragments representing 158 mouse orthologues (93%) for in situ hybridization (ISH) experiments and designed primer pairs for 161 genes for polymerase chain reaction with reverse transcription (RT–PCR). To generate the human chromosome 21 gene expression atlas, these orthologues were studied by normalized RT–PCR in 4 developmental stages and 12 adult tissues; whole-mount ISH of embryonic day E9.5 and E10.5 embryos; and ISH on serial sagittal sections of E14.5 embryos (see Supplementary Information for detailed Methods; see also http://www.tigem.it/ch21exp/). For ISH of sections, we developed an ISH robot and an automated microscope that permitted the analysis of about 6,500 tissue sections generated for this atlas12.
Expression was detected for 98% of the tested genes by at least one of the selected methods. The results are compiled in the Supplementary Information and at http://www.tigem.it/ch21exp/, and are also summarized in Fig. 1a–c. Supplementary Information consists of three items: (1) atlas expression map tables, containing a list of the genes ordered by their position on chromosome 21 and all original ISH images, annotation tables and details on probes; (2) some examples of patterned expressions in gut, cerebellum, heart, thymus, pancreas and limbs; and (3) a list of Methods. Previously described expression patterns are in agreement with our data (for example, Sh3bgr13).
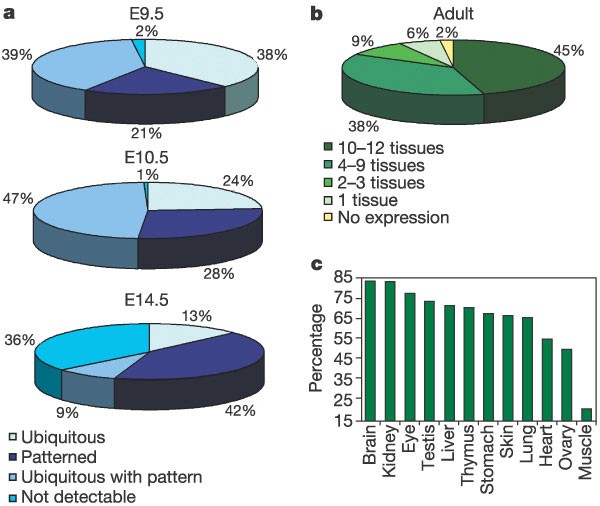
Figure 1: Distribution of expression patterns and transcriptome complexity.
a, Each slice corresponds to the percentage of genes belonging to the four categories of expression pattern observed by ISH at E9.5 (whole mount), E10.5 (whole mount) and E14.5 (sections). b, Each slice represents the percentage of genes expressed in 0, 1, 2–3, 4–9 and 10–12 adult tissues by RT–PCR. c, Percentage of the analysed 161 human chromosome 21 murine orthologues identified in each murine adult tissue.
By ISH, patterned (regional) gene expression was observed for 21% (E9.5), 28% (E10.5) and 42% (E14.5) of genes (see examples in Fig. 2). The highest numbers of genes with a restricted expression pattern were observed in the brain, the eye and the gut at all stages. Ubiquitous expression was observed in 38% (E9.5), 24% (E10.5) and 13% (E14.5) of cases. Genes with both weak ubiquitous expression and strong regional expression were also observed (39% at E9.5, 47% at E10.5, and 9% at E14.5; for example, Pfkl, Fig. 2). No expression was detected for 2% (E9.5), 1% (E10.5) and 36% (E14.5) of genes.
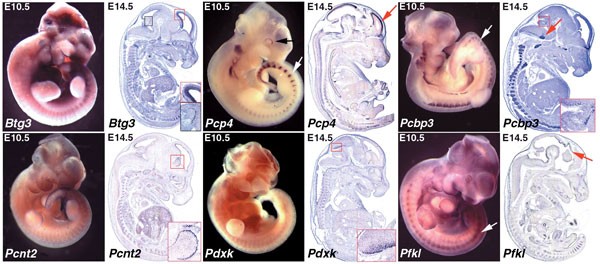
Figure 2: Representative examples of in situ hybridization data of E10.5 (whole mount) and E14.5 (sections) embryos.
See supplementary Information for the list of genes studied and images of their expression patterns. Btg3 messenger RNA is ubiquitously expressed at E10.5, with higher abundance in brain and gut. At E14.5 expression in the brain is restricted to the ventricular zone (red insert) and to a group of cells migrating into the developing cerebellum (black insert). Pcp4 is transcribed in brain, eye (black arrow) and dorsal root ganglia (white arrow) at E10.5. At E14.5 cells transcribing this gene are found in numerous tissues including the cortical plate (red arrow), midbrain, cerebellum, spinal chord, intestine, heart and dorsal root ganglia. Pcbp3 transcripts are restricted to the central and peripheral nervous systems both at E10.5 and E14.5 (white arrow, dorsal root ganglia; red arrow, facial nerve nucleus). Insert shows a group of neuroblasts migrating from the midbrain into the cerebellar anlage. Expression of Pcnt2 at E10.5 is ubiquitous but stronger in brain, eye, limbs, branchial arches and gut. At E14.5 Pcnt2 brain expression is restricted to proliferating cells of the ventricular zone (red insert). At E10.5 Pdxk mRNA is ubiquitous but is more strongly expressed in brain and eye. At E14.5 a group of cells in the inferior colliculus express Pdxk (red insert). Pfkl transcripts are found throughout the E10.5 embryo with higher levels in brain, eye and dorsal root ganglia (white arrow). At E14.5 this transcript is widely expressed but exhibits regional upregulation in the ventricular zone (red arrow), axial and cranial cartilage, thymus, gut and lung.
In addition to ISH, we carried out 2,576 RT–PCR reactions covering 95% of the human chromosome 21 murine orthologues (RT–PCR results are documented on Supplementary Information and http://www.tigem.it/ch21exp/). The 161 orthologues analysed were found to be expressed on average in 8 of 12 adult tissues tested (s.d. = 3.6). The transcriptomes of brain and kidney showed the highest complexity, each tissue expressing 85% of the 161 genes. Other tissues are less complex (muscle, 21%; heart, 56%; ovary, 51%; lung 67%; skin, 68%; stomach, 69%; thymus, 72%; liver, 73%; testis 75%; and eye, 79%; Fig. 1c). Forty-five per cent of all genes were widely expressed (>9 tissues out of 12 tissues were positive; Fig. 1b). Thirty-eight per cent of the genes show expression in 4–9 tissues. Restricted expression (2–3 tissues) and single-tissue expression was observed in 9% and 6% of the genes, respectively. Only 2% of the genes were not detectable by RT–PCR.
There is good concordance between the whole-mount data at E9.5 and E10.5 (Fig. 1a). It is, however difficult to compare these results with the data obtained by the two other methods, (that is, sections at E14.5 and RT–PCR on adult tissues). This is due to differences in sensitivity (RT–PCR is more sensitive), resolution (sections have a cellular resolution), and ascertaining background signal compared with weak ubiquitous expression (easier in whole mounts). Moreover, E14.5 has a more complex tissue architecture than earlier stages. A longitudinal analysis of 147 of the analysed genes at E9.5, E10.5 and E14.5 revealed that 59% of the genes retain their expression pattern during these developmental stages, whereas 17% acquire new, specific expression at E14.5. For the remaining 24%, we are uncertain owing to low expression in some stages.
Figure 2 presents examples of high-resolution expression patterns in the developing nervous system. Some of the brain-expressed genes may contribute to the Down's syndrome cognitive defects. At E14.5 Btg3, Pcnt2 and Pfkl transcripts are detected in the ventricular zone, whereas Pcp4 staining is observed in the cortical plate and the mantel layer of the midbrain. Btg3 and Pcbp3 staining was also observed in neuronal precursors that migrate from the inferior colliculus into the cerebellum. At the same developmental stage, Pdxk is expressed in a group of cells in the inferior colliculus. The KH-domain (maxi-K Homology domain) encoding Pcbp3 transcript is restricted to the central and peripheral nervous systems both at E10.5 and E14.5. Finally, Pcp4, Pcbp3 and Pfkl are strongly expressed in dorsal root ganglia, as visible in whole-mount ISH at E10.5.
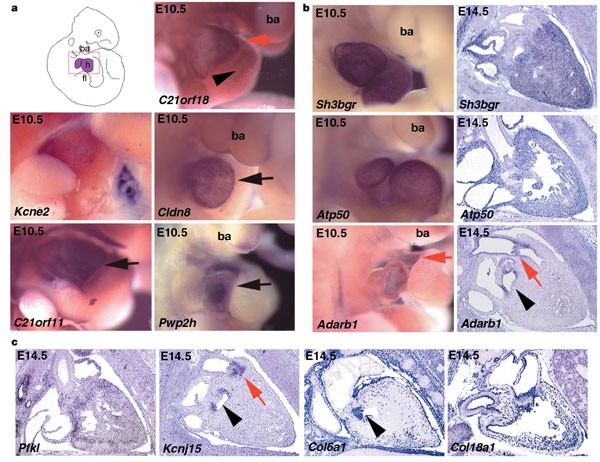
Down's syndrome is associated with congenital heart disease, and provides an important model to link individual genes to pathways controlling heart development. In trisomy 21 the most frequent and specific heart abnormalities are atrioventricular canal and atrial septal defects. Pwp2h and C21orf11 show elevated expression in the developing atria (Fig. 3), and Pfkl is strongly expressed in the ventricular wall and atrium. Two other heart-specific expression patterns are noteworthy: Adarb1 and C21orf18 are both expressed in E10.5 aortic sac; the precursor of the ascending aorta and the pulmonary artery. Furthermore, C21orf18 is expressed in the bulbus cordis, which develops into the right ventricle. At E14.5, Kcnj15 and Adarb1 are expressed in the aortic valve and trunk, while Kcnj15 is also expressed along the outflow track of the heart and in the superior vena cava. Atp50 and Sh3bgr transcripts are detected throughout the heart, whereas Cldn8 is regionally expressed in the primitive ventricle. The human genes COL6A1, COL6A2, COL18A1 and KCNE2 are mutated in Bethlem myopathy, Ullrich's disease, long QT6, and Knobloch syndrome, respectively14,15,16,17,18. We found that Kcne2 is expressed in the entire developing heart including its vessels, whereas Col18a1 is detected in cardiac vessels only. Col6a1 and Col6a2 are strongly expressed in the mitral valve and along the pericardium16,18 (Fig. 3).
Figure 3: Expression analysis in the developing heart.
E10.5 whole embryos and E14.5 sections were probed for Adarb1, Atp50, C21orf11, C21orf18, Col6a1, Col18a1, Cldn8, Kcnj15, Kcne2, Pfkl, Pwp2h and Sh3bgr genes. a, The region portrayed from the whole-mount embryos is schematically represented in the red box of the top left panel. h, heart; ba, branchial arch; fl, forelimb. At E10.5, C21orf18 transcripts are present in the aortic sac (red arrow) and the bulbus cordis (black arrowhead). Kcne2 is expressed in atria and ventricles. mRNA for C21orf11 and Pwp2h is restricted to the atria (black arrows), and Cldn8 transcripts are restricted to the primitive ventricle (black arrow). b, Sh3bgr and Atp50 transcripts are detected throughout the heart. Adarb1 mRNA is present in the aortic sac (red arrow) at E10.5, and is restricted to the mitral valve (black arrowhead), aortic valve (red arrow) and the endothelium of the aortic trunk at E14.5. c, At E14.5 Pfkl is expressed throughout the heart. Kcnj15 transcripts are found in the aortic and mitral valve (red arrow and black arrowhead, respectively). Col6a1 is strongly expressed in the mitral valve (black arrowhead) and along the pericardium, whereas Col18a1 expression is seen in the vessels of the heart.
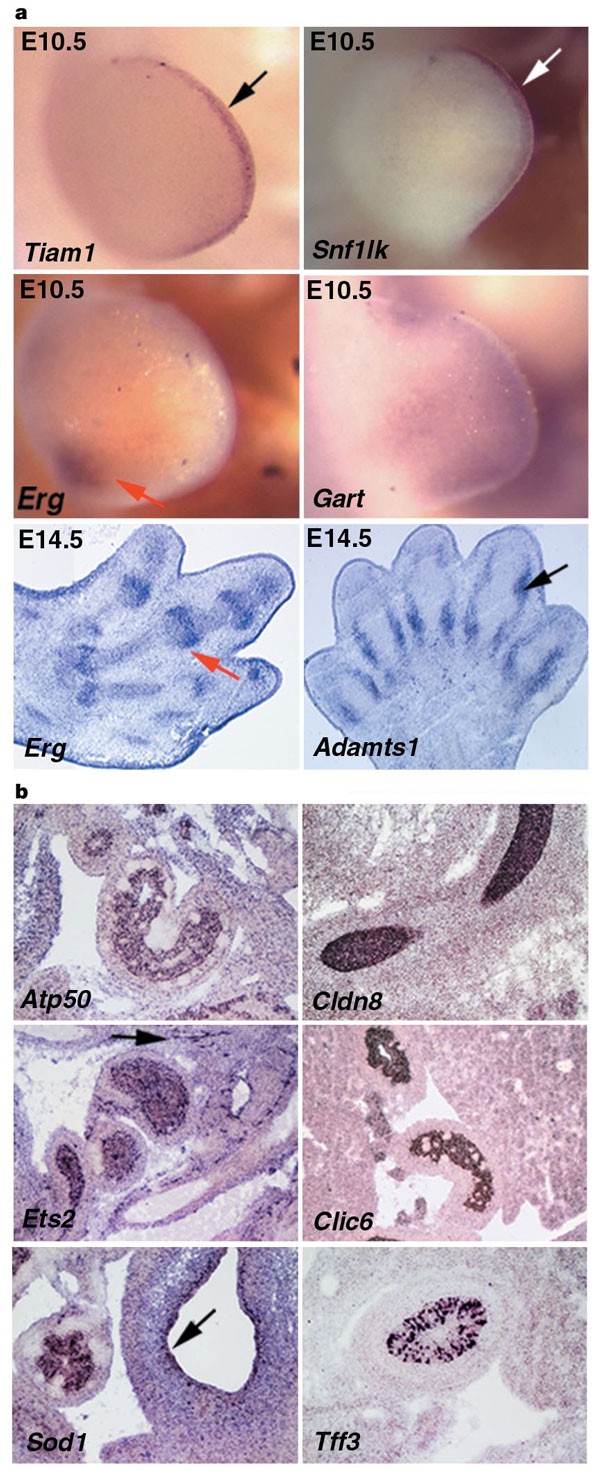
Down's syndrome fetuses exhibit reduced growth rate of long limb bones during the third trimester of pregnancy. Furthermore, a non-ossified or hypoplastic middle phalanx of the fifth digit is present in these fetuses6. Numerous genes are expressed in the limb buds (Fig. 4a). Tiam1 and Snf1lk transcripts were found specifically in the apical ectodermal ridge; a specialized ectodermal thickening regulating proliferation of the underlying mesoderm (Fig. 4a). Erg showed strong expression in the posterior proximal mesoderm at E10.5 and in joints at E14.5. Finally, Adamts1 was expressed at E14.5 in the perichondrium of developing bones.
Figure 4: Expression analysis in the developing limb and gastrointestinal tract.
a, At E10.5 Tiam1 and Snf1lk are expressed specifically in the apical ectodermal ridge, which is a specialized ectodermal thickening regulating proliferation of the underlying mesoderm (arrows). A more diffuse distribution in the limb bud mesoderm was found for Gart. Erg transcripts are present in the posterior proximal mesoderm at E10.5 and in the joints (red arrows) at E14.5. Adamts1 showed expression in the perichondrium of the developing digits (black arrow). b, Atp50, Cldn8, Ets2 and Clic6 are expressed within the duodenum endoderm. Ets2 transcripts are also detected in the vasculature surrounding the duodenum (arrow). Sod1 mRNA is present in the midgut endoderm and in the stomach endothelium (arrow). Tff3 is transcribed by a subgroup of cells within the gastroduodenal junction region of the pyloric sphincter.
Gastrointestinal abnormalities such as duodenal stenosis, Hirschsprung's disease, gastro-oesophageal reflux and imperforate anus are frequent in Down's syndrome patients6. At E14.5 the digestive tract is well differentiated and the expression of many genes can be detected. Atp50, Cldn8, Clic6 and Ets2 are expressed within the endothelium of the duodenum (Fig. 4b). Interestingly, the latter is also expressed in the skeletal system and previously found to result in skeletal abnormalities reminiscent of Down's syndrome when overexpressed in transgenic mice19. Tff3 and Sod1 transcripts are present in a subgroup of cells within the gastroduodenal junction region of the pyloric sphincter, and in the endothelium of the stomach and the intraperitoneal portion of the midgut, respectively.
Previous studies suggested the presence of genomic regions containing clusters of genes with similar expression patterns20,21,22,23,24. To search for such clusters on chromosome 21, all RT–PCR expression data underwent a test for clustering25 (see Methods in Supplementary Information). In four regions we found significant clustering of genes showing absence of expression in specific tissues. These include genes not expressed in heart (from B3galt5 to Hsf2bp; 3.9 Megabases (Mb), 31 genes, P < 0.001), lung (from Dscam to Slc37a1; 2.7 Mb, 19 genes, P = 0.007), testis (from C21orf25 to Pde9a; 1.0 Mb, 12 genes, P = 0.028), and muscle (from Hsf2bp to Hrmt1l1; 3.4 Mb, 43 genes, P = 0.02). The following duplicated genes were found: Mx1 and Mx2; Tff1, Tff2 and Tff3; Ifnar1 and Ifnar2; and Col6a1 and Col6a2. Notably, each of these regions is fully contained within a syntenic block on the mouse chromosomes. Consistent clustering results were obtained when the cluster analysis was extended to expression data collected by ISH. In addition, E14.5 ISH data suggested a cluster for presence of expression within fore-, mid- and hindbrain, which extended from Hunk to Atp50 (22 genes, P = 0.004). Significant clustering was observed even when applying two additional statistical analyses: the Bonferoni correction and Fisher's combined probability (see Supplementary Information). Albeit preliminary, these data suggest that regions containing either co-silenced or co-expressed genes exist on chromosome 21, and their presence should be considered in future expression studies of large genomic regions.
The combination of gene mapping with expression analysis is a helpful tool in the identification of positional candidate genes for human diseases26. Thus the human chromosome 21 expression atlas provides a rich resource for candidate genes for both monogenic and multifactorial diseases mapping to chromosome 21. We anticipate, however, that the greatest impact of the atlas lies in assessing the contribution of specific genes to Down's syndrome traits and phenotypes. The correlation of the atlas expression patterns with specific features of Down's syndrome may lead to the identification of candidate genes for these features of the disease. In particular, ADARB1, KCNJ15, PFKL, PWP2H and SH3BGR are promising candidate genes for Down's syndrome heart defects, as they map to the Down's syndrome congenital heart disease critical region27, and because of the expression patterns of their murine orthologues in the developing heart. Similarly, the human orthologues of the gut-expressed genes Atp50, Cldn8, Clic6, Ets2, Hmgn1, Sh3bgr, Sod1 and Wrb may have a role in the Down's syndrome gastrointestinal abnormalities6. Mapping gene expression of an entire chromosome at high resolution defines a new level of gene annotation, which is anticipated to advance our knowledge on gene function and regulation, and our understanding of human aneuploidies, such as Down's syndrome.