Cryopyrin activates the inflammasome in response to toxins and ATP (original) (raw)
Abstract
A crucial part of the innate immune response is the assembly of the inflammasome, a cytosolic complex of proteins that activates caspase-1 to process the proinflammatory cytokines interleukin (IL)-1β and IL-18. The adaptor protein ASC is essential for inflammasome function1,2, binding directly to caspase-1 (refs 3, 4), but the triggers of this interaction are less clear. ASC also interacts with the adaptor cryopyrin (also known as NALP3 or CIAS1)5,6. Activating mutations in cryopyrin are associated with familial cold autoinflammatory syndrome, Muckle–Wells syndrome and neonatal onset multisystem inflammatory disease, diseases that are characterized by excessive production of IL-1β5,7. Here we show that cryopyrin-deficient macrophages cannot activate caspase-1 in response to Toll-like receptor agonists plus ATP, the latter activating the P2X7 receptor to decrease intracellular K+ levels8,9. The release of IL-1β in response to nigericin, a potassium ionophore, and maitotoxin, a potent marine toxin, was also found to be dependent on cryopyrin. In contrast to _Asc_-/- macrophages, cells deficient in the gene encoding cryopyrin (_Cias1_-/-) activated caspase-1 and secreted normal levels of IL-1β and IL-18 when infected with Gram-negative Salmonella typhimurium or Francisella tularensis. Macrophages exposed to Gram-positive Staphylococcus aureus or Listeria monocytogenes, however, required both ASC and cryopyrin to activate caspase-1 and secrete IL-1β. Therefore, cryopyrin is essential for inflammasome activation in response to signalling pathways triggered specifically by ATP, nigericin, maitotoxin, S. aureus or L. monocytogenes.
Similar content being viewed by others
Main
Cryopyrin-deficient mice (Supplementary Fig. S1) were generated by gene targeting to investigate the role of cryopyrin in inflammatory responses to pathogen-derived molecules. Cryopyrin-deficient (_Cias1_-/-) macrophages stimulated with the Toll-like receptor-4 (TLR4) agonist lipopolysaccharide (LPS) phosphorylated IκBα and ERK normally (Fig. 1a), and they secreted normal amounts of TNF-α (Fig. 1b), IL-12 p40 (Fig. 1c), IL-6 and IL-10 (data not shown). Similar results were obtained using the TLR2 agonists Pam3CSK4 and heat-killed L. monocytogenes (HKLM) (data not shown). Our results show that cryopyrin is dispensable for NF-κB signalling by TLR2 and TLR4 in macrophages.
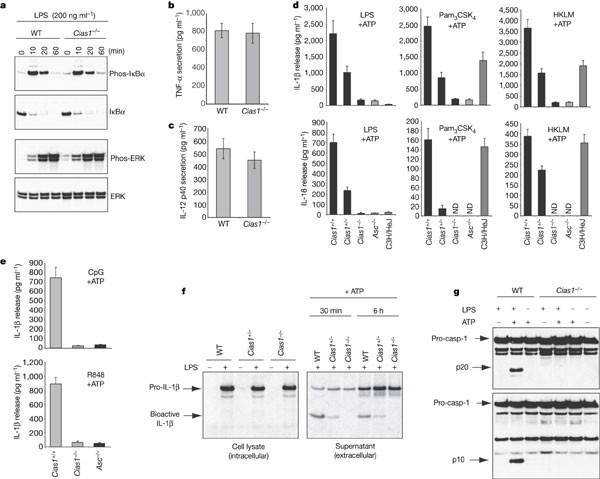
Figure 1: Cryopyrin is essential for caspase-1 activation and IL-1β secretion in response to TLR agonists and ATP.
a, Western blot analysis of phosphorylated and total IκBα and ERK in wild-type (WT) and _Cias1_-/- bone-marrow-derived macrophages stimulated with LPS. b, c, TNF (b) and IL-12 p40 (c) secretion by peritoneal macrophages cultured for 16 h with LPS. d, Macrophages primed for 16 h with LPS, Pam3CSK4, or HKLM were then pulsed with ATP. IL-1β or IL-18 released in the next 3 h is shown. ND, not detected. e, IL-1β secretion by macrophages primed with CpG DNA or R848 and then pulsed with ATP. Bars in b–e represent the mean ± s.d. of triplicate wells. Results are representative of four independent experiments. f, Immunoprecipitation of [35S]-labelled pro-IL-1β and IL-1β from macrophages treated with LPS (left panel). Pro-IL-1β and IL-1β secreted after subsequent ATP treatment were immunoprecipitated from culture supernatants (right panel). g, Western blot analysis of caspase-1 in peritoneal macrophages stimulated with LPS for 16 h and then pulsed with ATP for 20 min. All in vitro experiments were performed with ultra-pure LPS.
Because mutant variants of cryopyrin are associated with diseases in which IL-1β is produced in excess5,6,7, we measured IL-1β released from _Cias1_-/- macrophages treated with TLR agonists and ATP (Fig. 1d). TLR agonists induce pro-IL-1β synthesis and ATP stimulates caspase-1-dependent cleavage and secretion of IL-1β10. In contrast to wild-type macrophages, which secreted readily detectable amounts of IL-1β and IL-18 in response to ATP plus ultra-pure LPS, Pam3CSK4, HKLM, R848 (TLR7/8 agonist), or CpG oligonucleotides (TLR9 agonist), _Cias1_-/- macrophages secreted negligible amounts of these cytokines (Fig. 1d, e). As shown previously1,11, _Asc_-/- macrophages exhibited a similar defect in IL-1β and IL-18 production (Fig. 1d, e). Macrophages from heterozygous Cias1+/- mice secreted intermediate amounts of IL-1β and IL-18. C3H/HeJ macrophages expressing a non-functional form of TLR4 (ref. 12) secreted IL-1β and IL-18 in response to ATP plus either Pam3CSK4 or HKLM, but not LPS, demonstrating that our LPS was pure and not contaminated with other TLR agonists (Fig. 1d).
To determine whether IL-1β secretion from _Cias1_-/- macrophages was defective due to impaired pro-IL-1β synthesis and/or impaired caspase-1 activation, we immunoprecipitated [35S]-methionine-labelled pro-IL-1β from LPS-primed macrophages. Wild-type, Cias1+/- and _Cias1_-/- macrophages produced comparable amounts of pro-IL-1β (Fig. 1f, left panel), indicating that defective IL-1β secretion from _Cias1_-/- cells was not due to impaired pro-IL-1β synthesis. Unlike their wild-type counterparts, however, _Cias1_-/- macrophages did not cleave pro-IL-1β after ATP treatment (Fig. 1f, right panel). This finding suggested that cryopyrin is essential for ATP-induced caspase-1 activation. A further indication of caspase-1 activation is its autocatalytic processing into p20 and p10 subunits. Western blotting for caspase-1 after LPS plus ATP treatment revealed the p10 and p20 subunits in wild-type but not _Cias1_-/- macrophages (Fig. 1g). Thus, cryopyrin is essential for activation of caspase-1 in response to LPS plus ATP. Notably, ATP was necessary but not sufficient for caspase-1 activation (Fig. 1g). TLR signalling is probably needed for expression of essential inflammasome components. For example, LPS stimulation of TLR4 increases expression of caspase-11, and analyses of caspase-11-deficient mice and cells demonstrate that caspase-11 is essential for inflammasome function13.
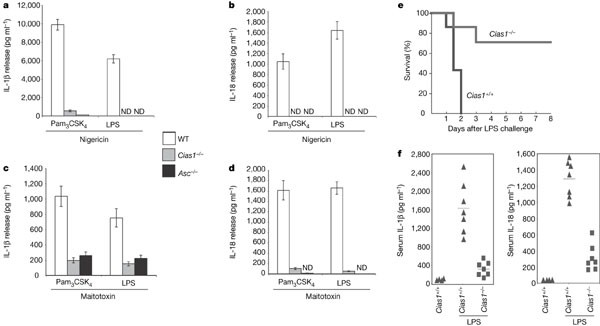
To test whether the role of ATP in cryopyrin- and ASC-dependent caspase-1 activation relates to its ability to stimulate the P2X7 receptor9 and thereby reduce intracellular K+ (ref. 8), we treated TLR-primed macrophages from wild-type, _Asc_-/- and _Cias1_-/- mice with nigericin or maitotoxin to deplete cytosolic K+ (refs 14, 15). Wild-type macrophages primed with LPS or Pam3CSK4 secreted IL-1β and IL-18 in response to nigericin (Fig. 2a, b) or maitotoxin (Fig. 2c, d). By contrast, neither _Asc_-/- nor _Cias1_-/- macrophages released significant IL-1β or IL-18. Our data therefore show that ASC and cryopyrin are essential for IL-1β and IL-18 production by TLR-primed macrophages treated with agents that deplete intracellular K+. Neither nigericin nor maitotoxin alone induced IL-1β release (Supplementary Fig. S2). Again, TLR signalling is probably required not only for the induction of pro-IL-1β (Fig. 1f) but also for expression of other proteins that are essential for inflammasome function.
Figure 2: Cryopyrin is essential for caspase-1 activation and IL-1β secretion in response to TLR agonists plus nigericin or maitotoxin.
a–d, Wild-type, _Cias1_-/- and _Asc_-/- macrophages were primed with Pam3CSK4 or ultra-pure LPS and then treated with nigericin (a, b) or maitotoxin (c, d). IL-1β (a, c) and IL-18 (b, d) release was measured by ELISA. Bars in a–d represent the mean ± s.d. of triplicate wells. Results are representative of three independent experiments. ND, not detected. e, Survival of 8-week-old wild-type (n = 7) or _Cias1_-/- (n = 7) female mice injected intraperitoneally with crude LPS (40 mg kg-1 of body weight) on day 0. f, Levels of IL-1β and IL-18 in the serum of the mice in e at 3 h after injection. Lines indicate the mean serum level (IL-1β: WT, 1,581 pg ml-1; _Cias1_-/-, 304 pg ml-1, P = 0.001; IL-18: WT, 1,321 pg ml-1; _Cias1_-/-, 329 pg ml-1, P = 0.0004).
To determine whether cryopyrin is essential for inflammation in vivo, wild-type and _Cias1_-/- mice were injected with a lethal dose of LPS to induce caspase-1-dependent endotoxic shock16. All of the wild-type mice died within 48 h, whereas only ∼30% of the _Cias1_-/- mice had died after 72 h (Fig. 2e). Correlating with their enhanced survival, _Cias1_-/- mice had markedly less serum IL-1β and IL-18 than the wild-type mice (Fig. 2f). It is unclear why exogenous ATP is not required for IL-1β and IL-18 secretion in vivo. We speculate that other cell types impacted by LPS in vivo provide the ATP needed to engage the P2X7 receptor. Our results demonstrate that cryopyrin, like ASC, is also an important mediator of LPS-induced endotoxic shock1.
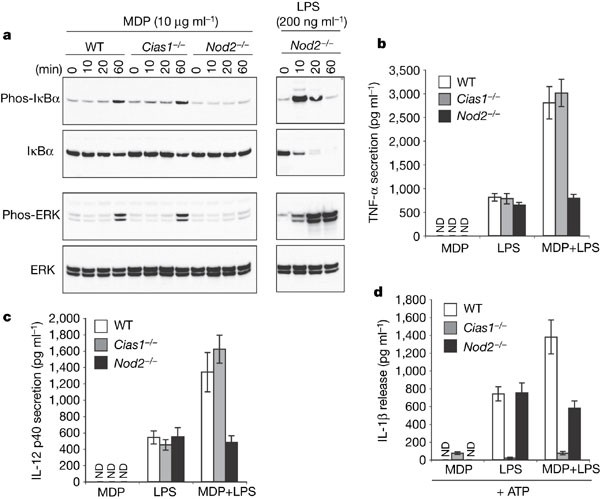
A previous study suggested that bacterial muramyl dipeptide (MDP) can activate a cryopyrin-containing inflammasome17. MDP is also the ligand for NOD2, which resembles cryopyrin in that it possesses a nucleotide binding domain and leucine-rich repeats7,18,19,20. Mutated NOD2 has been linked to Crohn's disease7. MDP induced phosphorylation of IκBα and ERK in wild-type and _Cias1_-/- macrophages but, in agreement with published results19, _Nod2_-/- macrophages were unresponsive (Fig. 3a). NOD2-deficiency, however, did not impact on LPS-induced phosphorylation of IκBα and ERK, or subsequent IκBα degradation (Fig. 3a). In terms of cytokine production, MDP enhanced LPS-induced secretion of TNF and IL-12 p40 by wild-type and _Cias1_-/- macrophages but not _Nod2_-/- macrophages (Fig. 3b, c). Because cryopyrin was essential for LPS- plus ATP-induced IL-1β secretion, we could not assess whether MDP increased IL-1β production in wild-type cells by engaging cryopyrin (Fig. 3d). Our data indicate that cryopyrin is dispensable for MDP-induced activation of NF-κB and ERK, and confirm a crucial role for cryopyrin in ATP-induced activation of the macrophage inflammasome.
Figure 3: MDP triggers NOD2-dependent, cryopyrin-independent NF-κB and ERK signalling.
a, Western blot analysis of phosphorylated or total IκBα and ERK in wild-type, _Cias1_-/- and _Nod2_-/- macrophages stimulated with MDP or LPS. b–d, Secretion of TNF (b), IL-12 p40 (c), or IL-1β (d) by bone-marrow-derived macrophages treated with MDP and/or LPS. Macrophages in d were also pulsed with ATP. ND, not detected. Bars represent the mean ± s.d. of triplicate wells. Results are representative of three independent experiments.
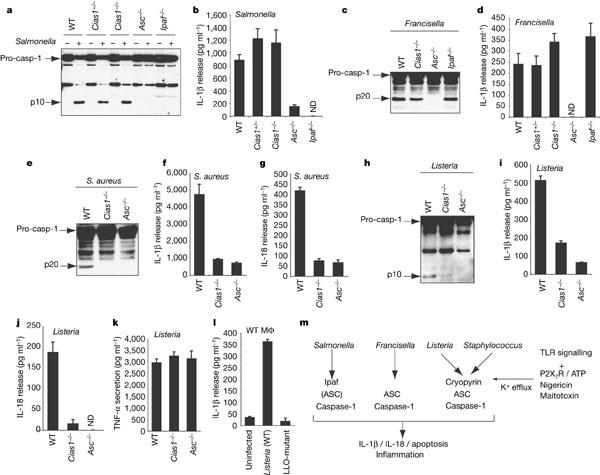
Next we determined whether cryopyrin is required for caspase-1 activation and IL-1β release when macrophages are infected by specific bacterial pathogens. Unlike ASC, cryopyrin was dispensable for normal caspase-1 activation, IL-1β secretion and macrophage cell death in response to Gram-negative S. typhimurium (Fig. 4a, b) or F. tularensis (Fig. 4c, d) (Supplementary Fig. S4). As shown previously1,21, macrophages infected by S. typhimurium also require the adaptor protein Ipaf to activate caspase-1 (Fig. 4a). ASC and cryopyrin were essential for caspase-1 activation and secretion of IL-1β or IL-18 when macrophages were cultured with Gram-positive S. aureus (Fig. 4e–g) or L. monocytogenes (Fig. 4h–l), but NOD2 was dispensable (data not shown). ASC or cryopyrin deficiency caused a specific defect in IL-1β and IL-18 release because _Asc_-/- and _Cias1_-/- macrophages infected by L. monocytogenes and S. aureus yielded similar amounts of TNF to wild-type macrophages (Fig. 4k and data not shown). Macrophages cultured with L. monocytogenes deficient for the toxin listeriolysin O did not secrete IL-1β (Fig. 4l), so we speculate that listeriolysin O perturbs intracellular K+ levels similar to extracellular ATP, nigericin and maitotoxin. Furthermore, live bacteria seem to be required because heat-killed Listeria monocytogenes (HKLM) induced very little IL-1β secretion (data not shown). S. aureus deficient in alpha-, beta- or gamma-toxin induced comparable IL-1β secretion to wild-type S. aureus (Supplementary Fig. S5), indicating that other S. aureus toxins may contribute to caspase-1 activation.
Figure 4: L. monocytogenes and S. aureus induce cryopyrin-dependent caspase-1 activation and IL-1β secretion.
a–k, Wild-type, _Cias1_-/-, _Asc_-/- and _Ipaf_-/- macrophages pre-treated with LPS were infected with S. typhimurium (a, b), F. tularensis (c, d), S. aureus (e–g) or L. monocytogenes (h–k) and immunoblotted for the p10 or p20 subunit of caspase-1 (a, c, e, h). IL-1β (b, d, f, i), IL-18 (g, j) or TNF (k) secretion was measured by ELISA. ND, not detected. l, IL-1β released from wild-type macrophages pre-treated with LPS and infected with wild-type L. monocytogenes or a listeriolysin O (LLO) mutant. All data are representative of 2–4 independent experiments. Bars represent the mean ± s.d. of triplicate wells. LPS pre-treatment was not essential for IL-1β release (Supplementary Fig. 6). Cell viability after infection is shown in Supplementary Fig. 4. m, Model for the differential activation of the caspase-1 inflammasome by various pathogens and toxins.
Our results suggest that the caspase-1 inflammasome is a dynamic entity that is assembled from different adaptor proteins in a stimulus-dependent manner (Fig. 4m). We show that cryopyrin is essential for inflammasome activation in response to signalling pathways triggered by specific bacterial infections and to treatments that deplete intracellular K+. Ipaf and perhaps other members of the large NALP family of proteins might substitute for cryopyrin upon infection with Gram-negative bacteria such as S. typhimurium and F. tularensis. The cryopyrin-dependent response to extracellular ATP may represent a physiological response that occurs when ATP is released by dying cells and degranulating platelets. Future studies will need to address how activating mutations in cryopyrin circumvent the normal regulation of the inflammasome to produce inflammatory diseases such as familial cold autoinflammatory syndrome, Muckle–Wells syndrome and neonatal onset multisystem inflammatory disease22,23,24,25. An intriguing possibility is that low level bacterial infection coupled with a dysregulated cryopyrin response triggers the symptoms associated with these diseases.
Methods
Cias1, Nod2, Asc and Ipaf mutant cells and mice
_Asc_-/- and _Ipaf_-/- mice have been described1. _Cias1_-/- and _Nod2_-/- mice are described in Supplementary Figs 1 and 3. Macrophage and bacterial cultures are described in detail in the Supplementary Methods. Briefly, macrophages primed overnight with 50 ng ml-1 ultra-pure LPS were infected at a multiplicity of infection of 50 (30 for F. tularensis) for 1 h (S. typhimurium), 2.5 h (L. monocytogenes), 3 h (S. aureus) or 5 h (F. tularensis).
Immunoprecipitation, western blotting and pulse–chase analysis
IL-1β was immunoprecipitated with goat anti-mouse IL-1β (clone AF-401-NA; R&D Systems) and blotted with hamster anti-mouse IL-1β (clone B122; Becton Dickinson). For pulse–chase analyses, macrophages were treated with 1 µg ml-1 ultra-pure LPS for 3 h and then labelled with 200 µCi ml-1 [35S]-methionine (ICN) for 45 min. Labelled cells were treated with 5 mM ATP (Sigma) for 30 min and then placed in fresh medium. Caspase-1 was blotted with rat anti-mouse caspase-1 (clone 4B4; Genentech) and rabbit anti-caspase-1 (sc-514; Santa Cruz Biotechnology). Phospho-IκBα (Ser32), IκBα, phospho-ERK and ERK antibodies were from Cell Signalling Technology.
Cytokine ELISAs
TNF and IL-12 p40 secretion were measured by enzyme-linked immunosorbent assay (ELISA; R&D Systems) after 16 h of stimulation with 500 ng ml-1 ultra-pure LPS (List Biologicals), 100 ng ml-1 Pam3CSK4 (Invivogen), 108 bacteria per ml HKLM (Invivogen), 5 µM CpG oligonucleotide (Invivogen ODN1826), 100 ng ml-1 R848 (Invivogen) or 10 µg ml-1 MDP (Invivogen). The macrophages then were pulsed for 20 min with 5 mM ATP, 20 µM nigericin (Calbiochem), or 0.5 nM maitotoxin (Dako) and cultured an additional 3 h for IL-1β and IL-18 secretion. Serum levels of IL-1β and IL-18 were determined 3 h after intraperitoneal injection with 40 mg kg-1 crude LPS (Escherichia coli serotype 0111:B4; Sigma).
References
- Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004)
Article ADS CAS Google Scholar - Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002)
Article CAS Google Scholar - Srinivasula, S. M. et al. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J. Biol. Chem. 277, 21119–21122 (2002)
Article CAS Google Scholar - Wang, L. et al. PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κB and caspase-1-dependent cytokine processing. J. Biol. Chem. 277, 29874–29880 (2002)
Article CAS Google Scholar - Agostini, L. et al. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20, 319–325 (2004)
Article CAS Google Scholar - Dowds, T. A., Masumoto, J., Zhu, L., Inohara, N. & Nunez, G. Cryopyrin-induced interleukin 1β secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J. Biol. Chem. 279, 21924–21928 (2004)
Article CAS Google Scholar - Ting, J. P. & Davis, B. K. CATERPILLER: a novel gene family important in immunity, cell death, and diseases. Annu. Rev. Immunol. 23, 387–414 (2005)
CAS Google Scholar - Perregaux, D. & Gabel, C. A. Interleukin-1β maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 269, 15195–15203 (1994)
CAS PubMed Google Scholar - Solle, M. et al. Altered cytokine production in mice lacking P2X7 receptors. J. Biol. Chem. 276, 125–132 (2001)
Article CAS Google Scholar - Hogquist, K. A., Nett, M. A., Unanue, E. R. & Chaplin, D. D. Interleukin 1 is processed and released during apoptosis. Proc. Natl Acad. Sci. USA 88, 8485–8489 (1991)
Article ADS CAS Google Scholar - Yamamoto, M. et al. ASC is essential for LPS-induced activation of procaspase-1 independently of TLR-associated signal adaptor molecules. Genes Cells 9, 1055–1067 (2004)
Article CAS Google Scholar - Poltorak, A. et al. Defective LPS signalling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998)
Article ADS CAS Google Scholar - Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998)
Article CAS Google Scholar - Schilling, W. P., Wasylyna, T., Dubyak, G. R., Humphreys, B. D. & Sinkins, W. G. Maitotoxin and P2Z/P2X7 purinergic receptor stimulation activate a common cytolytic pore. Am. J. Physiol. 277, C766–C776 (1999)
Article CAS Google Scholar - Verhoef, P. A., Kertesy, S. B., Estacion, M., Schilling, W. P. & Dubyak, G. R. Maitotoxin induces biphasic interleukin-1β secretion and membrane blebbing in murine macrophages. Mol. Pharmacol. 66, 909–920 (2004)
CAS PubMed Google Scholar - Li, P. et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell 80, 401–411 (1995)
Article CAS Google Scholar - Martinon, F., Agostini, L., Meylan, E. & Tschopp, J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr. Biol. 14, 1929–1934 (2004)
Article CAS Google Scholar - Girardin, S. E. et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278, 8869–8872 (2003)
Article CAS Google Scholar - Kobayashi, K. S. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005)
Article ADS CAS Google Scholar - Maeda, S. et al. Nod2 mutation in Crohn's disease potentiates NF-κB activity and IL-1beta processing. Science 307, 734–738 (2005)
Article ADS CAS Google Scholar - Damiano, J. S., Newman, R. M. & Reed, J. C. Multiple roles of CLAN (caspase-associated recruitment domain, leucine-rich repeat, and NAIP CIIA HET-E, and TP1-containing protein) in the mammalian innate immune response. J. Immunol. 173, 6338–6345 (2004)
Article CAS Google Scholar - Hoffman, H. M., Mueller, J. L., Broide, D. H., Wanderer, A. A. & Kolodner, R. D. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nature Genet. 29, 301–305 (2001)
Article CAS Google Scholar - McDermott, M. F. Genetic clues to understanding periodic fevers, and possible therapies. Trends Mol. Med. 8, 550–554 (2002)
Article CAS Google Scholar - Feldmann, J. et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am. J. Hum. Genet. 71, 198–203 (2002)
Article CAS Google Scholar - Aganna, E. et al. Association of mutations in the NALP3/CIAS1/PYPAF1 gene with a broad phenotype including recurrent fever, cold sensitivity, sensorineural deafness, and AA amyloidosis. Arthritis Rheum. 46, 2445–2452 (2002)
Article CAS Google Scholar
Acknowledgements
We thank members of the Dixit laboratory for discussions, and M. Bauer, J. Starks, C. Olsson, M. Osborn, J. Hongo, M. Bever, J. Cupp and H. Maecker for technical assistance. S. aureus strains were provided by T. Foster. L. monocytogenes LLO mutant was provided by D. Portnoy. This work was supported by NIH grants awarded to D.M.M. and a fellowship from the Giannini Family Foundation awarded to D.S.W.
Author information
Authors and Affiliations
- Molecular Oncology Department,
Sanjeev Mariathasan, Kim Newton, Karen O'Rourke & Vishva M. Dixit - Physiology Department,
Meron Roose-Girma - Immunology Department, Genentech Inc, 1 DNA Way, South San, California, 94080, Francisco, USA
Jacqueline McBride & Wyne P. Lee - Departments of Microbiology and Immunology, Stanford University School of Medicine, Stanford, California, 94305, USA
David S. Weiss & Denise M. Monack - Department of Microbiology, New York University School of Medicine, New York, New York, 10016, USA
Yvette Weinrauch
Authors
- Sanjeev Mariathasan
You can also search for this author inPubMed Google Scholar - David S. Weiss
You can also search for this author inPubMed Google Scholar - Kim Newton
You can also search for this author inPubMed Google Scholar - Jacqueline McBride
You can also search for this author inPubMed Google Scholar - Karen O'Rourke
You can also search for this author inPubMed Google Scholar - Meron Roose-Girma
You can also search for this author inPubMed Google Scholar - Wyne P. Lee
You can also search for this author inPubMed Google Scholar - Yvette Weinrauch
You can also search for this author inPubMed Google Scholar - Denise M. Monack
You can also search for this author inPubMed Google Scholar - Vishva M. Dixit
You can also search for this author inPubMed Google Scholar
Corresponding author
Correspondence toVishva M. Dixit.
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Methods and Supplementary Figures 1–6. (PDF 1354 kb)
Rights and permissions
About this article
Cite this article
Mariathasan, S., Weiss, D., Newton, K. et al. Cryopyrin activates the inflammasome in response to toxins and ATP.Nature 440, 228–232 (2006). https://doi.org/10.1038/nature04515
- Received: 07 October 2005
- Accepted: 12 December 2005
- Published: 11 January 2006
- Issue Date: 09 March 2006
- DOI: https://doi.org/10.1038/nature04515