Universality of human microbial dynamics (original) (raw)
References
- Cho, I. & Blaser, M. J. The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 13, 260–270 (2012)
Article CAS Google Scholar - Pflughoeft, K. J. & Versalovic, J. Human microbiome in health and disease. Annu. Rev. Pathol. 7, 99–122 (2012)
Article CAS Google Scholar - Lozupone, C. A., Stombaugh, J. I., Gordon, J. I., Jansson, J. K. & Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 489, 220–230 (2012)
Article ADS CAS Google Scholar - Borody, T. J. & Khoruts, A. Fecal microbiota transplantation and emerging applications. Nat. Rev. Gastroenterol. Hepatol. 9, 88–96 (2011)
Article Google Scholar - Aroniadis, O. C. & Brandt, L. J. Fecal microbiota transplantation: past, present and future. Curr. Opin. Gastroenterol. 29, 79–84 (2013)
Article Google Scholar - Gerber, G. K. The dynamic microbiome. FEBS Lett. 588, 4131–4139 (2014)
Article CAS Google Scholar - Costello, E. K., Stagaman, K., Dethlefsen, L., Bohannan, B. J. M. & Relman, D. a. The application of ecological theory toward an understanding of the human microbiome. Science 336, 1255–1262 (2012)
Article ADS CAS Google Scholar - Franzosa, E. A. et al. Identifying personal microbiomes using metagenomic codes. Proc. Natl Acad. Sci. USA 112, E2930–E2938 (2015)
Article CAS Google Scholar - The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012)
- Bucci, V. & Xavier, J. B. Towards predictive models of the human gut microbiome. J. Mol. Biol. 426, 3907–3916 (2014)
Article CAS Google Scholar - David, L. A. et al. Host lifestyle affects human microbiota on daily timescales. Genome Biol. 15, R89 (2014)
Article Google Scholar - Sommer, F. & Backhed, F. The gut microbiota—masters of host development and physiology. Nat. Rev. Microbiol. 11, 227–238 (2013)
Article CAS Google Scholar - Goodrich, J. K. et al. Human genetics shape the gut microbiome. Cell 159, 789–799 (2014)
Article CAS Google Scholar - Walter, J. & Ley, R. The human gut microbiome: ecology and recent evolutionary changes. Annu. Rev. Microbiol. 65, 411–429 (2011)
Article CAS Google Scholar - The Human Microbiome Project Consortium. A framework for human microbiome research. Nature 486, 215–221 (2012)
- Flores, G. E. et al. Temporal variability is a personalized feature of the human microbiome. Genome Biol. 15, 531 (2014)
Article Google Scholar - Youngster, I. et al. Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin. Infect. Dis. 58, 1515–1522 (2014)
Article Google Scholar - Lemon, K. P., Armitage, G. C., Relman, D. a. & Fischbach, M. a. Microbiota-targeted therapies: an ecological perspective. Sci. Transl. Med. 4, 137rv135 (2012)
Article Google Scholar - Levy, R. & Borenstein, E. Metabolic modeling of species interaction in the human microbiome elucidates community-level assembly rules. Proc. Natl Acad. Sci. USA 110, 12804–12809 (2013)
Article ADS CAS Google Scholar - Jumpertz, R. et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 94, 58–65 (2011)
Article CAS Google Scholar - Faust, K. & Raes, J. Microbial interactions: from networks to models. Nat. Rev. Microbiol. 10, 538–550 (2012)
Article CAS Google Scholar - Friedman, J. & Alm, E. J. Inferring correlation networks from genomic survey data. PLoS Comput. Biol. 8, e1002687 (2012)
Article ADS CAS Google Scholar - Koren, O. et al. A guide to enterotypes across the human body: meta-analysis of microbial community structures in human microbiome datasets. PLoS Comput. Biol. 9, e1002863 (2013)
Article CAS Google Scholar - Gibson, T. E., Bashan, A., Cao, H.-T., Weiss, S. T. & Liu, Y.-Y. On the origins and control of community types in the human microbiome. PLOS Comput. Biol. 12, e1004688 (2016)
Article Google Scholar - Stein, R. R. et al. Ecological modeling from time-series inference: insight into dynamics and stability of intestinal microbiota. PLOS Comput. Biol. 9, e1003388 (2013)
Article Google Scholar - Fisher, C. K. & Mehta, P. Identifying keystone species in the human gut microbiome from metagenomic timeseries using sparse linear regression. PLoS ONE 9, e102451 (2014)
Article ADS Google Scholar - Buffie, C. G. et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 517, 205–208 (2015)
Article ADS CAS Google Scholar - Caporaso, J. G. et al. Moving pictures of the human microbiome. Genome Biol. 12, R50 (2011)
Article Google Scholar - Kassam, Z., Lee, C. H., Yuan, Y. & Hunt, R. H. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am. J. Gastroenterol. 108, 500–508 (2013)
Article Google Scholar - Lozupone, C. A., Hamady, M., Kelley, S. T. & Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 73, 1576–1585 (2007)
Article ADS CAS Google Scholar - Faith, J. J. et al. The long-term stability of the human gut microbiota. Science 341, 1237439 (2013)
Article Google Scholar - Gilbert, J. A. & Alverdy, J. Stool consistency as a major confounding factor affecting microbiota composition: an ignored variable? Gut 65, 1–2 (2016)
Article CAS Google Scholar - Vandeputte, D. et al. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 65, 57–62 (2016)
Article CAS Google Scholar - Lawley, T. D. et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 8, e1002995 (2012)
Article CAS Google Scholar - Faust, K. & Raes, J. Microbial interactions: from networks to models. Nat. Rev. Microbiol. 10, 538–550 (2012)
Article CAS Google Scholar - Goodrich, J. K. et al. Conducting a microbiome study. Cell 158, 250–262 (2014)
Article CAS Google Scholar - Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Statist. Soc. B 57, 289–300 (1995)
MathSciNet MATH Google Scholar - Wu, G. D. et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 334, 105–108 (2011)
Article ADS CAS Google Scholar - Bickel, S. L., Tang, K. W. & Grossart, H.-P. Ciliate epibionts associated with crustacean zooplankton in German lakes: distribution, motility and bacterivory. Front. Microbiol. 3, 243 (2012)
Article Google Scholar
Acknowledgements
We thank E. K. Silverman, G. Weinstock, C. Huttenhower, R. Knight, G. Ackermann, D. Del Vecchio, D. Lauffenburger, G. Abu-Ali, J. Sordillo, M. McGeachie, and J. Gore for discussions. Special thanks to A.-L. Barabási and J. Loscalzo for careful reading of the manuscript. This work was partially supported by the John Templeton Foundation (award number 51977) and National Institutes of Health (R01 HL091528).
Author information
Authors and Affiliations
- Channing Division of Network Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, 02115, Massachusetts, USA
Amir Bashan, Travis E. Gibson, Vincent J. Carey, Scott T. Weiss & Yang-Yu Liu - Department of Physics, Physics of Living Systems, Massachusetts Institute of Technology, Cambridge, 02139, Massachusetts, USA
Jonathan Friedman - Infectious Disease Division, Massachusetts General Hospital and Harvard Medical School, Boston, 02115, Massachusetts, USA
Elizabeth L. Hohmann - Center for Cancer Systems Biology, Dana-Farber Cancer Institute, Boston, 02115, Massachusetts, USA
Yang-Yu Liu
Authors
- Amir Bashan
You can also search for this author inPubMed Google Scholar - Travis E. Gibson
You can also search for this author inPubMed Google Scholar - Jonathan Friedman
You can also search for this author inPubMed Google Scholar - Vincent J. Carey
You can also search for this author inPubMed Google Scholar - Scott T. Weiss
You can also search for this author inPubMed Google Scholar - Elizabeth L. Hohmann
You can also search for this author inPubMed Google Scholar - Yang-Yu Liu
You can also search for this author inPubMed Google Scholar
Contributions
Y.-Y.L. conceived and designed the project. A.B. developed the DOC analysis, performed numerical simulations, and analysed all the real data. A.B. and Y.-Y.L. performed analytical calculations. A.B. and V.J.C. performed statistical tests. All authors analysed the results. A.B. and Y.-Y.L. wrote the manuscript. All authors edited the manuscript.
Corresponding author
Correspondence toYang-Yu Liu.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Reviewer Information Nature thanks F. He, P. Rohani and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Extended data figures and tables
Extended Data Figure 1 Displacement of normalized _N_-dimensional random walks.
a, Trajectory of a two-dimensional random-walk represents the absolute abundance of two species  . The initial state is marked by a red circle and the first 100 steps are shown. The solid black line is the one-dimensional simplex upon which the locations are projected to obtain the relative abundances
. The initial state is marked by a red circle and the first 100 steps are shown. The solid black line is the one-dimensional simplex upon which the locations are projected to obtain the relative abundances 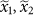 . The dotted lines starting at the origin represent the projection process: all the points in a dotted line have the same relative abundances and they are all projected to the intersection of the dotted line and the simplex (for example, the solid red and green circles are projected to the red and green open circles, respectively). We define a new coordinate
. The dotted lines starting at the origin represent the projection process: all the points in a dotted line have the same relative abundances and they are all projected to the intersection of the dotted line and the simplex (for example, the solid red and green circles are projected to the red and green open circles, respectively). We define a new coordinate 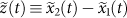 for the location of normalized relative abundance on the simplex. The displacement of the normalized random walk after t steps is then
for the location of normalized relative abundance on the simplex. The displacement of the normalized random walk after t steps is then  , where
, where  is the projected location of the initial state (see, as an example, the distance between the green and the red open circles in a). b, Distributions of displacement of an ensemble of 1,000 random walks after t steps (t = 1, 5, 10, 100, 1,000). For small t, the displacement distributions depend on t, while for large t (t = 100, 1,000) the distributions are the same. c, Symbols represent the average displacement of 1,000 _N_-dimensional normalized random walks (here we set N = 50), measured as _D_rJSD, and the error bars represent the s.d. Each random walk is forced to stay on the positive orthant, that is, if
is the projected location of the initial state (see, as an example, the distance between the green and the red open circles in a). b, Distributions of displacement of an ensemble of 1,000 random walks after t steps (t = 1, 5, 10, 100, 1,000). For small t, the displacement distributions depend on t, while for large t (t = 100, 1,000) the distributions are the same. c, Symbols represent the average displacement of 1,000 _N_-dimensional normalized random walks (here we set N = 50), measured as _D_rJSD, and the error bars represent the s.d. Each random walk is forced to stay on the positive orthant, that is, if  we set
we set 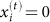 . _D_rJSD was calculated using all N coordinates, setting
. _D_rJSD was calculated using all N coordinates, setting  as a pseudo count for
as a pseudo count for  . Where t is small, the distance grows with increasing t; however, the distance saturates for large t. The dashed red and green lines represent the average distance between two random locations (green) and between the final locations (
. Where t is small, the distance grows with increasing t; however, the distance saturates for large t. The dashed red and green lines represent the average distance between two random locations (green) and between the final locations ( ) of the random walks (red).
) of the random walks (red).
Extended Data Figure 2 Detection of group dynamics using an ordination technique.
a–h, In each row, 500 synthetic samples were generated. Samples in the same group were taken from the steady states of the same GLV model of 100 species. The initial species assemblages were determined in two scenarios: at random (a–d) or on the basis of the group (e–h). In the latter scenario, in each group the species were first randomly ordered and then in each of the samples the first f species were selected and the other been removed (f is randomly chosen from a uniform distribution  ). In columns a and e, a standard ordination technique, that is, principal coordinate analysis (PCoA), was applied. All 500 samples were shown in the plane of the first two principal coordinates (using rJSD as the distance metric) and coloured according to their group. In b and f, only the samples that have high overlap (>0.95) with at least one other sample were shown. Panels c and g show the dissimilarity distributions P(rJSD) between the high-overlap sample pairs. Panels d and h show the DOCs. The ordination technique successfully detects the existence of group dynamics (especially when the number of groups is small). We anticipate that the group dynamics can also be detected by classical clustering analysis. In the scenario of random collections, the PCoA of high-overlap samples, that is, samples that have high overlap (>0.95) with at least one other sample, is doing better than the PCoA of all samples to detect group dynamics, especially for a small number (~2–10) of groups. Moreover, for a small number of groups, the dissimilarity distributions P(rJSD) can distinguish between the two scenarios of initial assemblage selection: random or group-based. The ordination technique cannot distinguish between the cases of 500 groups (individual dynamics) and single group (universal dynamics). Those cases can be distinguished by the DOC analysis.
). In columns a and e, a standard ordination technique, that is, principal coordinate analysis (PCoA), was applied. All 500 samples were shown in the plane of the first two principal coordinates (using rJSD as the distance metric) and coloured according to their group. In b and f, only the samples that have high overlap (>0.95) with at least one other sample were shown. Panels c and g show the dissimilarity distributions P(rJSD) between the high-overlap sample pairs. Panels d and h show the DOCs. The ordination technique successfully detects the existence of group dynamics (especially when the number of groups is small). We anticipate that the group dynamics can also be detected by classical clustering analysis. In the scenario of random collections, the PCoA of high-overlap samples, that is, samples that have high overlap (>0.95) with at least one other sample, is doing better than the PCoA of all samples to detect group dynamics, especially for a small number (~2–10) of groups. Moreover, for a small number of groups, the dissimilarity distributions P(rJSD) can distinguish between the two scenarios of initial assemblage selection: random or group-based. The ordination technique cannot distinguish between the cases of 500 groups (individual dynamics) and single group (universal dynamics). Those cases can be distinguished by the DOC analysis.
Extended Data Figure 3 Detecting universality in population dynamics models.
Synthetic microbial samples were calculated as steady states of GLV models (see Methods). The GLV models are generated as cohorts (100 models in each cohort) with different levels of (i) inter-species interaction strength; and (ii) universality, tuned by the parameters 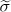 and
and  , respectively (see Methods). In each of the 100 models, a random fraction f of the species (
, respectively (see Methods). In each of the 100 models, a random fraction f of the species ( ) was initially removed, and the remaining species were initiated with random abundance
) was initially removed, and the remaining species were initiated with random abundance  ). The dissimilarity–overlap points of sample pairs in each cohort and of the corresponding randomized samples are shown in light blue and yellow, respectively. The solid curves represent the DOCs calculated using the robust LOWESS method. The DOC of cohorts generated by GLV models without inter-species interactions (a1, a4, a7) is flat even in the high-overlap region. This is because, without inter-species interactions, for any sample pair the presence or absence of unique (that is, non-shared) species has no effect on the shared ones. A flat DOC is also observed in the case of individual dynamics (a7, a8, a9), where a higher overlap between sample pairs does not lead to more similar abundance profiles. However, in the case of universal dynamics with strong inter-species interactions (for example, a3), the DOC displays a clear negative slope in the high-overlap region.
). The dissimilarity–overlap points of sample pairs in each cohort and of the corresponding randomized samples are shown in light blue and yellow, respectively. The solid curves represent the DOCs calculated using the robust LOWESS method. The DOC of cohorts generated by GLV models without inter-species interactions (a1, a4, a7) is flat even in the high-overlap region. This is because, without inter-species interactions, for any sample pair the presence or absence of unique (that is, non-shared) species has no effect on the shared ones. A flat DOC is also observed in the case of individual dynamics (a7, a8, a9), where a higher overlap between sample pairs does not lead to more similar abundance profiles. However, in the case of universal dynamics with strong inter-species interactions (for example, a3), the DOC displays a clear negative slope in the high-overlap region.
Extended Data Figure 4 DOC analysis of gut microbiome samples from longitudinal studies.
a–d, Sample pairs are selected from four different subjects, with number of samples: _M_a = 299, _M_b = 180, _M_c = 336, _M_d = 131, respectively. The mean DOCs (calculated from 100 bootstrap realizations using the robust LOWESS method) of each subject and the corresponding randomized samples are shown in dark blue and yellow, respectively. The shaded area indicates the range of the 94% confidence intervals. The overlap distributions are shown in red. For all the four subjects, a clear negative slope of the DOC is observed at the high-overlap region, indicating largely time-invariant or universal dynamics for each subject throughout the measurement period. This is in marked contrast with the flat DOC of the null model (see Supplementary Information section 1.3). The secondary peak of lower-overlap samples in b (overlap of ~0.8) is of sample pairs from two different periods, before and after a Salmonella infection, which represent two distinct microbial steady states and thus exhibit a flat DOC. This is consistent with our assumption of time-invariant microbial dynamics for a given healthy individual.
Extended Data Figure 5 DOC analysis of gut microbiome samples is consistent across different studies and different dissimilarity measures.
For two microbiome samples, the dissimilarity of their abundance profiles over shared species can be evaluated by different measures. Weighted measures, such as rJSD, Bray–Curtis (BC) dissimilarity and Yue–Clayton (YC) dissimilarity should be applied to the renormalized abundance profiles, to ensure mathematical independence between the overlap and the dissimilarity measures. Rank-based dissimilarity measures, for example, negative Spearman correlation (nSC), can be directly applied without renormalization. We used the four dissimilarity measures (rJSD, BC, YC and nSC) to calculate the DOC (using robust LOWESS) of gut microbiome samples from two studies: HMP and SMP. In all cases, we observed a pronounced negative slope in the DOC (dark-blue curve) of real sample pairs (light-blue points) and a flat DOC (orange curve) for the pairs of randomized samples (yellow points).
Extended Data Figure 6 Quantifying the universality of human microbial dynamics in different body sites.
a, The fraction (_f_ns) of data for which a negative slope is observed in Fig. 3. Note that for overlap values close to zero (for example, Fig. 3d, f1) a positive slope occurs as the artefact of dissimilarity between relative abundance profiles with small number of species (see Supplementary Information section 1.1.3). For gut and mouth, a negative slope of DOC is observed in the two data sets for a broad range of overlap, indicating a significant universality of microbial dynamics in those habitats. By contrast, the negative slope of DOC in the hand’s skin microbiome is observed only for a small part of the sample pairs. b, Box plot of the slope of DOC calculated from 200 bootstrap realizations. The slope is calculated by fitting a linear mixed-effects model for data points with overlap larger than the median. We report one-tailed P values, calculated as the fraction of bootstrap realizations with a non-negative slope, adjusted for multiple comparisons by the procedure of Benjamini and Hochberg. The null hypothesis of non-negative slope is rejected for all body sites (P < 1 × 10−2) except four skin sites: forehead (P = 0.099), palm (P = 0.377) in the SMP study and left/right antecubital fossa in the HMP study (P = 0.099 and P = 0.495).
Extended Data Figure 7 Effects of various host factors on the DOC analysis.
a, The effect of body mass index (BMI) on the DOC analysis. a1, DOC analysis of all gut microbiome sample pairs among 190 subjects from the HMP study. Red points represent samples pairs associated with at least one obese subject (with BMI > 30). a2, Same as in a1, but 13 obese subjects with BMI > 30 were excluded. a3, Blue points represent the gut microbiome samples’ overlap and ΔBMI. The red curve is the average (error bars represent the s.e.m.). a4, Dissimilarity versus ΔBMI. a5, Distribution of ΔBMI values, divided into four groups of equal number of pairs. a6–a9, DOC analysis of the sample pairs in each group. b, The effect of diet on the DOC analysis. b1, Diet difference (Δdiet) between two subjects is defined as the Euclidean distance between their associated diet scores in the two leading principal components PC1 and PC2. In total there are M = 97 healthy subjects in the COMBO study38. b2, Overlap versus Δdiet. Blue points represent the overlap and Δdiet of all gut microbiome pairs among the 97 subjects from the COMBO study. The red curve is the average (error bars represent the s.e.m.). b3, Dissimilarity versus Δdiet. b4, Distribution of Δdiet values, divided into four groups of equal number of pairs. b5–b8, DOC analysis of the pairs in each group. c, The effect of age on the DOC analysis. c1, Overlap versus Δdiet. Blue points represent the overlap and Δage of all gut microbiome samples pairs between the 190 subjects from the HMP study. The red curve is the average (error bars represent the s.e.m.). c2, Dissimilarity versus Δage. c3, Distribution of Δage values, divided into four groups of equal number of pairs. c4–c7, DOC analysis of the pairs in each group. d, The effect of stool consistency on the DOC analysis. d1, DOC analysis of all sample pairs. In this data set the subjects have BSS values between 1 and 6. The points (sample pairs) associated with subjects with BSS = 6 (at least one subject has BSS = 6) are coloured in red. The black line is the DOC. d2, DOC analysis of all subjects with BSS < 6. d3, d4, Among all subjects with 1 ≤ BSS ≤ 5, the overlap and the dissimilarity are independent of ΔBSS. d5, Distribution of ΔBSS values for the 46 subjects with 1 ≤ BSS ≤ 5. d6, d7, DOC analysis of the pairs with similar BSS values, 0 ≤ ΔBSS ≤ 1 (d6), and pairs with more different BSS values, 2 ≤ ΔBSS ≤ 4 (d7). In both cases, a clear negative slope of the DOC is observed. e, The effect of race on the DOC analysis. e1, All subjects (M = 190). e2, e3, White subjects (M = 153) (e2) and Asian subjects (M = 25) (e3). Note that in the HMP study, stool samples were collected from 153 white subjects, 10 black subjects, 25 Asian subjects, and 2 subjects from other races.
Extended Data Figure 8 DOC analysis under special conditions.
a, The effect of strongly interacting species. A comparison of two GLV models of 100 species with random inter-species interactions. The system parameters were fixed for all the simulated samples (M = 100), representing maximal universality. In a1, all species have the same characteristic interaction strength, while in a2, the inter-species interactions of one species are markedly stronger than all other species, representing a strongly interacting species. The presence/absence of the strongly interacting species markedly affects (either directly or indirectly) the abundance profile of many other species, leading to a pronounced secondary cloud of points in the dissimilarity–overlap plane (a4). The effect is the most pronounced in the region of high-overlap (top 5%) pairs, and can be detected by looking at their dissimilarity distributions (a5, a6). b, DOC behaves the same for samples with uniform or skewed abundance distribution. b1, b2, Samples were generated from the steady states of the GLV model with largely uniform abundance distribution (determined mainly by the species growth rates). In the presence of inter-species interactions (b1), a negative slope of the DOC is observed. By contrast, in the absence of inter-species interactions (b2), a flat DOC is observed. b3, Real samples from the gut (from the HMP study, genus level) exhibit a high level of alpha-diversity and a very skewed abundance distribution. A negative slope of the DOC in the high-overlap region is observed. b4, The randomized samples preserve the abundance distribution of the real samples but the effect of inter-species interactions is removed, leading to a flat DOC. c, Effect of core species and non-interacting periphery species. c1, Samples were generated as steady states of the GLV model with N =100 species. The parameters of the GLV model were fixed for all the samples, representing maximal universality. The initial species assemblages were chosen as follows: 30 species were present in all the samples, representing a set of ‘core species’, and the other 70 ‘peripheral’ species were present with lower probability (mean 0.18, min 0.12, and max 0.24). c2, Presence probability of real gut microbial samples, from the HMP at the genus level. Only one genus (Bacterioides) is present in all the samples. c3, Species presence probability in a GLV model where all species are present with average probability 0.6. c4, The effect of the interactions of the peripheral species. In the GLV model, the inter-species interactions among the core species (core–core) has a characteristic strength _σ_core = 0.15, and both the periphery–periphery and the periphery–core interactions have a characteristic strength _σ_p. When _σ_p = 0, that is, the peripheral species do not interact with the core species, the DOC is flat. When _σ_p > 0, the DOC has a negative slope. c5, c6, In the case of real gut microbiome samples as well as the GLV model without core species, the DOC has a negative slope in the high-overlap region. d, The effect of sequencing depth on the DOC analysis. d1, Richness (number of present OTUs) versus sequencing depth of 190 HMP gut samples. 12 subjects with fewer than 1,300 reads per sample were excluded and the remaining 178 were assigned into two groups of n = 89 subjects, with average sequencing depth 3,019 and 8,640 reads per sample. d2, d3, The characteristic overlap between samples of group 1 is smaller than between samples of group 2. However, DOC analysis of each group shows a clear negative slope. d4–d6, Samples of each group were rarefied before analysis with minimal community size of 1,317 and 4,333 in group 1 and 2, respectively, as represented by the black dashed lines in d4. d7–d9, Samples of both groups were rarefied before analysis with the same minimal community size of 1,317, as represented by the black dashed line in d7.
Extended Data Figure 9 DOC analysis of longitudinal microbiome data from six lakes in Germany39.
Data downloaded from http://qiita.microbio.me, study ID 945. a, Stechlin (M = 440). b, Haus (M = 26). c, Tiefwaren (M = 164). d, Melzer (M = 68). e, Breiter Luzin (M = 89). f, Fuchskuhle (M = 355). Blue points represent the dissimilarity–overlap values of sample pairs from the same lake. The DOCs of real samples from each lake and that from the corresponding randomized samples are calculated using robust LOWESS and shown in red and yellow, respectively. For all the six lakes, a clear negative slope is observed for the DOCs of real samples, suggesting universal or time-invariant microbial dynamics for each lake. Differences in the DOC shapes (for example, the moderate DOC slope in b, c and d, in contrast with the steep DOC in a, e and f) deserve a systematic study of those microbial ecosystems. This example clear demonstrates the applicability of DOC analysis to general microbial ecosystems, for example, soil, ocean, rizosphere/phyllosphere and fermenters.
Extended Data Figure 10 Average dissimilarity between two normalized random vectors.
Two independent vectors x, y of  elements randomly chosen from the uniform distribution
elements randomly chosen from the uniform distribution  were generated and then normalized
were generated and then normalized  and
and  . (Note that in practice all
. (Note that in practice all  elements are always shared in x and y, since zeros are very unlikely.) The dissimilarity
elements are always shared in x and y, since zeros are very unlikely.) The dissimilarity  is then calculated using the five dissimilarity measures (_D_JSD, _D_rJSD, _D_BC, _D_YC and _D_nSC). Average dissimilarity and standard deviations of 1,000 pairs are shown in a1, b1, c1, d1 and e1, for the different measures. The horizontal black dashed line represents the average dissimilarity for n = 100. For all the measures here, the dissimilarity displays no _n-_dependence for n > 15, while _D_nSC is _n_-independent for any n > 0. Similar analysis was performed for vectors whose elements were chosen from power-law distributions
is then calculated using the five dissimilarity measures (_D_JSD, _D_rJSD, _D_BC, _D_YC and _D_nSC). Average dissimilarity and standard deviations of 1,000 pairs are shown in a1, b1, c1, d1 and e1, for the different measures. The horizontal black dashed line represents the average dissimilarity for n = 100. For all the measures here, the dissimilarity displays no _n-_dependence for n > 15, while _D_nSC is _n_-independent for any n > 0. Similar analysis was performed for vectors whose elements were chosen from power-law distributions  with α = 3 (a2, b2, c2, d2 and e2) and
with α = 3 (a2, b2, c2, d2 and e2) and  with α = 2 (a3, b3, c3, d3 and e3).
with α = 2 (a3, b3, c3, d3 and e3).
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Tables 1-4 and additional references. (PDF 929 kb)
Supplementary Data
This zipped file contains the source code of the DOC method. (ZIP 329 kb)
PowerPoint slides
Source data
Rights and permissions
About this article
Cite this article
Bashan, A., Gibson, T., Friedman, J. et al. Universality of human microbial dynamics.Nature 534, 259–262 (2016). https://doi.org/10.1038/nature18301
- Received: 24 August 2015
- Accepted: 09 May 2016
- Published: 08 June 2016
- Issue Date: 09 June 2016
- DOI: https://doi.org/10.1038/nature18301