MicroRNA-responsive 'sensor' transgenes uncover Hox-like and other developmentally regulated patterns of vertebrate microRNA expression (original) (raw)
So far, miRNA expression studies in mammals have relied on northern blots, tissue-specific RNA cloning and microarrays2,3,4,5. Though powerful, these techniques lack the resolution to show precise spatiotemporal expression patterns. Standard methods of in situ hybridization are problematic owing to the small size of miRNAs, and although a miRNA in situ hybridization method was recently reported for Arabidopsis thaliana6 and maize7, it has proven difficult to adapt it to vertebrate embryos. Promoter fusion constructs have been useful for visualizing miRNA expression patterns in C. elegans8, but this requires previous knowledge of promoter elements.
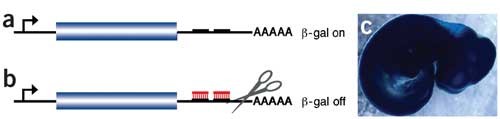
Using an alternative approach previously reported for Drosophila melanogaster9, we generated a series of reporter transgenes ('sensors') designed to detect the presence of specific miRNAs in an embryo. Each sensor contains a constitutively expressed reporter (lacZ) bearing sequences complementary to a given miRNA in the 3′ untranslated region (UTR). In cells lacking the miRNA that the sensor is designed to detect, the transgene RNA is stable and allows reporter expression (Fig. 1a). In contrast, in cells that express the miRNA, its perfect complementarity to sequences in the 3′ UTR of the sensor targets the sensor mRNA to the RNA interference (RNAi) pathway, resulting in an absence of β-galactosidase (β-gal) activity10 (Fig. 1b). Thus, in transgenic embryos carrying a sensor, tissues stained for β-gal are white where the target miRNA is present and blue everywhere else. As a control, we used a sensor lacking known miRNA-complementary sites and found that it drove robust β-gal expression throughout the embryo at embryonic day (E) 9.5 and later (Fig. 1c).
Figure 1: Transgenic sensor design.
(a) A lacZ reporter containing miRNA-complementary sequences in the 3′ UTR constitutively expresses β-gal in cells lacking the complementary miRNA. (b) In cells expressing the miRNA (red), the lacZ message is targeted for degradation by the RNAi pathway. (c) A control sensor lacking any known miRNA complementary sequences drives ubiquitous lacZ expression at E10.5.
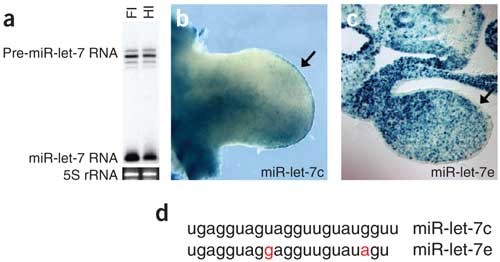
We first focused on two vertebrate homologs of the C. elegans let-7 miRNA. This miRNA regulates several genes in the heterochronic pathway, and its sequence and developmentally regulated expression are well conserved across animal phyla11,12. The mouse genome encodes 11 let-7 family members (including miR-let-7a–miR-let-7f, also known as Mirnlet7a_–_Mirnlet7f in the mouse)1,3. Northern-blot analysis showed that at least four precursor bands, as well as a high level of mature miR-let-7 RNA, were expressed in limbs of developing mice (Fig. 2a). We generated sensors to determine when and where miR-let-7c and miR-let-7e were expressed in the limb and whether different miR-let-7 isoforms were differentially regulated. In embryos carrying either the miR-let-7c or miR-let-7e sensor, β-gal expression was downregulated in limb buds (Fig. 2b,c). In both cases, β-gal expression was more strongly repressed in the distal than the proximal limb, suggesting that miR-let-7c and miR-let-7e had higher expression distally, perhaps relating to the maintenance of an undifferentiated cell population in this region.
Figure 2: Patterns of miR-let-7 expression in transgenic embryos.
(a) Northern-blot analysis of total RNA from forelimb (Fl) and hindlimb (Hl) of an E11.5 mouse embryo probed for miR-let-7a. At least four miR-let-7 precursor (pre-miR-let-7) bands are expressed, and processed miR-let-7 is strongly expressed in both limbs. (b) Whole-mount forelimb from an E11.0 embryo bearing a miR-let-7c sensor. β-gal expression is downregulated in the anterior, distal region of the limb, but not in the posterior region, suggestive of an anterior concentration of miR-let-7c. miR-let-7c is absent from the apical ectodermal ridge, as shown by strong β-gal expression (arrow). (c) Transverse section through the forelimb of an embryo bearing a miR-let-7e sensor. β-gal activity is slightly downregulated throughout the distal portion of the forelimb mesenchyme and is absent (suggestive of high miR-let-7e expression) in the ectoderm (arrow). (d) miR-let-7c and miR-let-7e differ by two nucleotides. The different expression patterns shown by miR-let-7c and miR-let-7e sensors suggest that their regulation by the corresponding miRNAs is sequence-specific.
Notably, in other respects, the limb expression patterns of these two miR-let-7 miRNAs, which differ in sequence by only two nucleotides (Fig. 2d)3, were very different. miR-let-7c was expressed highly in the anterior limb bud but comparatively weakly in the posterior limb and in the chondrogenic core (Fig. 2a and data not shown). miR-let-7c was absent from the apical ectodermal ridge, as evidenced by high β-gal activity in this tissue (Fig. 2a). In contrast, miR-let-7e was expressed weakly in the limb mesenchyme and robustly throughout the limb ectoderm (Fig. 2b).
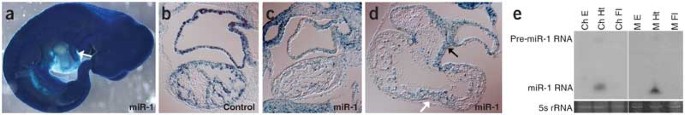
Data from mouse-organ total RNA libraries identified another miRNA, miR-1, that is highly and specifically expressed in the adult heart3. Consistent with this finding, and in contrast to controls, embryos transiently transgenic with respect to an miR-1-responsive sensor showed a marked reduction in β-gal expression specifically in the heart (Figs. 1c and 3a,b). Notably, β-gal activity was lowest (suggestive of highest miR-1 expression) in the chamber myocardium, particularly the ventricles, and was somewhat higher in the atrioventricular canal, which gives rise to the valves, and in the future ventricular septum and outflow tract (Fig. 3a,c,d). Northern-blot analysis confirmed that miR-1 was present specifically in RNA extracted from heart in both mouse and chick embryos (Fig. 3e).
Figure 3: miR-1 is expressed in the heart.
(a) lacZ expression from a sensor containing miR-1 complementary sequences is specifically downregulated in the heart of an E10.5 embryo. Downregulation (corresponding to expression of miR-1) seems limited to the chambers and not the outflow tract (arrow). (b) Sagittal section through the heart of an E10.5 embryo bearing a control sensor. lacZ is highly expressed in both the atrium (upper chamber) and ventricle (lower chamber). (c) Sagittal section through the heart of an E10.5 embryo bearing an miR-1 sensor. β-gal activity is downregulated in both chambers, suggestive of high miR-1 expression. (d) Frontal section through the heart of an E10.5 embryo bearing an miR-1 sensor, showing a four-chambered view. β-gal expression is downregulated in the chambers, particularly the ventricles (lower chambers), and is less downregulated (suggestive of lower levels of miR-1 expression) in the atrioventricular canal (black arrow) and in cells that give rise to the ventricular septum (white arrow). (e) A northern blot of forelimb (Fl), heart (Ht) and whole-embryo (E) RNA from Hamburger-Hamilton stage-25 chick embryos (Ch)27 and E10.5 mouse embryos (M) probed with miR-1 indicates that miR-1 is specifically expressed in the heart in embryos from both species.
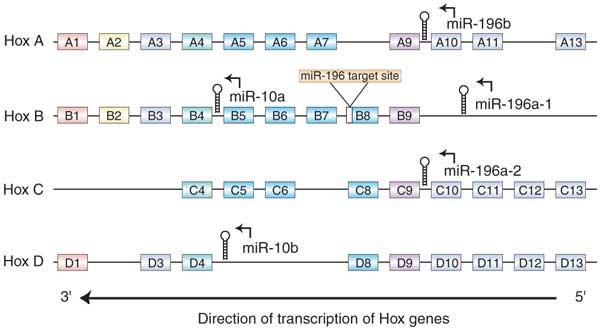
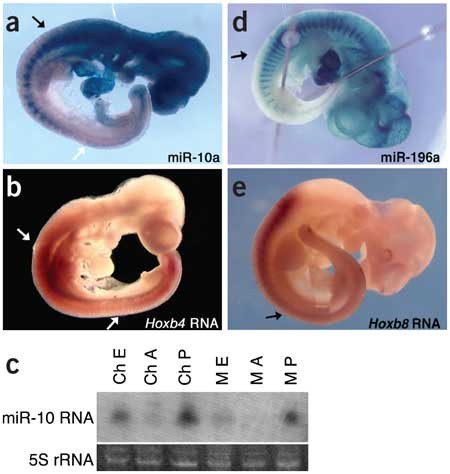
Finally, we assayed the expression of two miRNAs embedded in the Hox clusters, miR-10a and miR-196a13,14,15 (Fig. 4). In the mouse, miR-10a and miR-10b are embedded in equivalent locations in the Hoxb and Hoxd clusters, respectively, and this chromosomal location is conserved in invertebrates13. We constructed a sensor for miR-10a, which lies upstream of Hoxb4, and found that their expression patterns were markedly similar. The miR-10a sensor directed high levels of β-gal activity in the head and anterior trunk but was downregulated in the posterior trunk, with an anterior expression limit similar to that of Hoxb4 (Fig. 5a,b)16. miR-10a was most highly expressed (as indicated by absence of β-gal activity) in a restricted region of the posterior trunk surrounding the hindlimb buds. Notably, Hoxb4 RNA is downregulated by a post-transcriptional mechanism in the same specific region (Fig. 5a,b)17, although obvious miR-10a binding sites were not detected in the Hoxb4 transcript. Nevertheless, the similarities between the expression patterns of miR-10a and Hoxb4 suggest that miR-10a may have a role in establishing or maintaining rostrocaudal cell fates in conjunction with the Hox-Hom complex.
Figure 4: Two families of miRNAs, miR-10 and miR-196, are embedded in the mouse Hox clusters.
As shown below, the Hoxb8 3′ UTR contains a site complementary to miR-196a.
Figure 5: miR-10a and miR-196a are expressed in Hox-like patterns.
(a) lacZ expression from a sensor containing miR-10a complementary sequences is downregulated in the trunk of an E10.0 embryo. The anterior limit of miR-10a expression is similar to that of the flanking gene, Hoxb4, at the border of mesodermal expression (black arrow). In the posterior part of the embryo, miR-10a expression is complementary to that of Hoxb4. It is most highly expressed in a region posterior to the limb buds but anterior to the tail (white arrow), where Hoxb4 is downregulated by a post-transcriptional mechanism. (b) An in situ hybridization for Hoxb4 shows the previously reported anterior expression limit (top arrow) of Hoxb4 RNA. The transcript is not detected in the posterior trunk (bottom arrow) and is expressed in the tail. (c) A northern blot of Hamburger-Hamilton stage-25 chick (Ch) and E10.5 mouse (M) embryos, bisected at the anterior limit of miR-10a suggested by the sensor. The blot was probed for miR-10a, confirming that in both species expression is high in the posterior (P) and very low in the anterior (A) of the embryo (whole embryo; E). (d) Like miR-10a, miR-196a is expressed in a Hox-like pattern. An miR-196a sensor is downregulated in the posterior trunk and tail, with an anterior expression limit similar to the posterior limit of expression for Hoxb8 RNA. (e) In situ hybridization for Hoxb8, showing previously reported downregulation in the posterior trunk and tail.
We confirmed the posteriorly restricted pattern of expression of miR-10a by northern-blot analysis; this expression pattern was also conserved in chick embryos (Fig. 5c). Because mouse miR-10a and miR-10b differ by a single nucleotide, they are expected to cross-hybridize with the northern-blot probe; thus, both paralogs probably are restricted to the posterior of the embryo. In at least one case (miR-let-7c and miR-let-7e), we found that sensor transgenes could discriminate a difference of two nucleotides, but we do not know whether this is true for a single-nucleotide difference. Therefore, the expression pattern of the miR-10a sensor may be a composite of the expression patterns of miR-10a and miR-10b.
The similarity between the expression patterns of miR-10a and Hoxb4 suggests that transcription of miR-10a may be regulated by cis elements that also regulate Hox genes. Whereas miR-10a and miR-10b lie upstream of HOXB4 and HOXD4 in humans, miR-10b is located in the last intron of Hoxd4 in the mouse, further supporting this hypothesis13.
We also constructed a sensor transgene for the Hox complex–embedded miRNA miR-196a. This sensor was expected to uncover a composite pattern of expression of miR-196a1 and miR-196a2, which have identical mature sequences and are located upstream of Hoxb9 and Hoxc9, respectively14,15. In embryos transgenic with respect to this sensor, β-gal activity was high in the head and anterior trunk and was downregulated in the posterior trunk (Fig. 5d). This pattern is reminiscent of that of Hox gene expression, suggesting that miR-196a may be influenced by regulatory controls imposed on the Hox clusters. But Hox9-group genes, located immediately upstream of miR-196, all have more anterior expression limits than miR-196 (ref. 18), suggesting that miR-196 family members are not regulated simply by control elements shared with the nearest Hox gene.
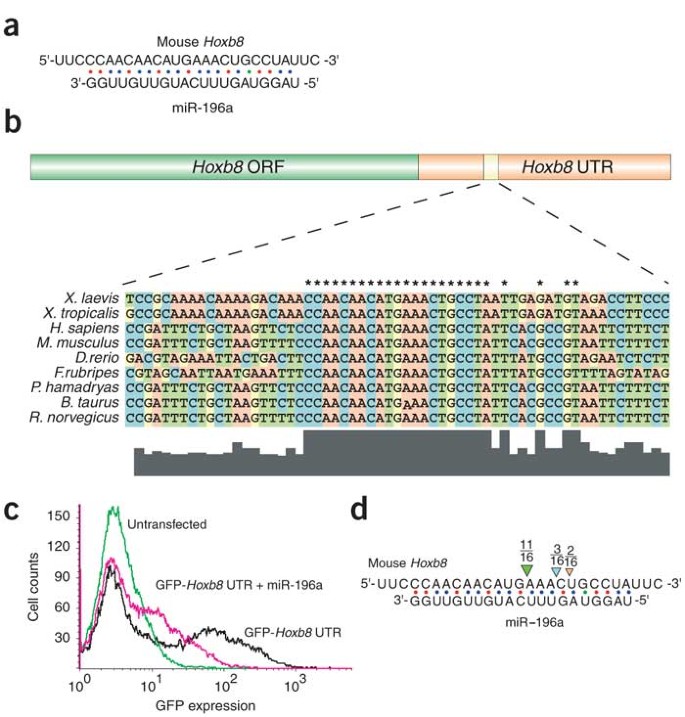
We did note that the posterior limit of Hoxb8 expression was similar to the anterior limit of miR-196a expression identified by the sensor (Fig. 5e)19. This is notable because we identified, using a new miRNA target prediction program to be described elsewhere, a site in the Hoxb8 3′ UTR containing 21 nucleotides of complementarity (including one G–U base pair) to miR-196a (Fig. 6a). This site is highly conserved across vertebrate species (Fig. 6b).
Figure 6: miR-196a downregulates Hoxb8 by mediating RNA cleavage.
(a) Complementarity between miR-196a and the Hoxb8 3′ UTR was predicted by a new miRNA target prediction program, FASTH (J.S., M.T.M., P.A.S. and M.Z., unpublished data). (b) The miR-196a putative binding site is conserved in Hoxb8 orthologs from multiple species. Asterisks indicate conserved nucleotides. (c) miR-196a downregulates a GFP reporter bearing the miR-196 complementary site from the Hoxb8 3′ UTR in cell culture. (d) Hoxb8 RNA is a target of RISC-mediated RNA cleavage in vivo. RNA was extracted from E11.5 mouse embryos and subjected to a modified form of 5′ RACE. Most (11 of 16) sequenced Hoxb8 clones were cleaved at the expected site. The remaining clones may represent artifacts owing to 5′ exonuclease activity or may be indicative of alternative processing of miR-196a isoforms. Our data does not distinguish between these possibilities.
To determine whether this miR-196 complementary site could function as a target for miR-196 regulation, we cloned it downstream of a green fluorescent protein (GFP) reporter and assayed GFP expression in cultured cells in the presence or absence of miR-196a. Reporter expression was six to ten times lower in the presence of miR-196a, indicating that miR-196a can interact with the Hoxb8 3′ UTR (Fig. 6c).
In all previously reported cases in animals, miRNAs bind their targets with incomplete complementarity, resulting in translational repression; however, it has been shown that the degree of complementarity between the miRNA and its target determines whether the target RNA will be translationally repressed or degraded through the RNAi pathway10,20. The perfect complementarity between miR-196a and the Hoxb8 3′ UTR suggests that miR-196a could mediate degradation rather than translational repression of Hoxb8 mRNA. A recent study using a modified form of 5′ RACE showed this to be the case15. We also tested this possibility using the 5′ RACE assay, which is designed to detect RNA-induced silencing complex (RISC)-mediated RNA cleavage products21,22. We detected Hoxb8 RNA cleavage products in total RNA extracted from E11.5 mouse embryos and found that the transcript was cleaved at the expected site in the region of miR-196a complementarity (Fig. 6d). Our results confirm the previous results15 and indicate that miR-196 directs Hoxb8 cleavage through a small interfering RNA mechanism. The previous study, moreover, used reporter assays to show that mRNA cleavage is the primary mechanism by which miR-196 regulates Hoxb8 gene activity15.
Our results show that a miRNA-responsive transgene can be used to glean detailed information about the expression of various miRNAs during mouse embryonic development. Although our approach relied on transient transgenics, in which each embryo represented an independent insertion of the transgene, our results were reproducible. For example, four of four embryos transgenic with respect to the miR-1 sensor, three of three embryos transgenic with respect to the miR-let-7c sensor and three of four embryos transgenic with respect to the miR-10a sensor showed identical β-gal-negative domains in their hearts, limbs and axes, respectively. The expression patterns of miR-let-7c and miR-let-7e were identical in all four limbs of the embryos analyzed. In addition to being reproducible, within the parameters of our study, this approach was specific. Only two nucleotides separate the miR-let-7c and miR-let-7e sequences, yet their corresponding sensors showed different patterns of β-gal activity. We have not, however, tested whether single-base differences can be differentially detected by our reporter system, or whether all two-base differences can be distinguished by sensors. Nonetheless, the reproducible and distinct patterns uncovered by the miR-let-7c and miR-let-7e sensors showed that miRNAs have a high degree of specificity in vivo and suggested that closely related miRNA family members may have distinct targets.
We found that miR-10a is expressed in a pattern similar to that of the neighboring gene Hoxb4, possibly indicative of similar regulatory mechanisms or functions. We observed a Hox-like expression pattern for miR-196a, with anterior limits that differed from those of its nearest neighbors, possibly suggestive of a different regulatory mechanism. Nevertheless, the expression pattern of miR-196a is the inverse of that of Hoxb8 RNA, and Hoxb8 RNA contains a miR-196a complementary site. In agreement with recent findings15, we found that miR-196a bound to the Hoxb8 3′ UTR in tissue culture and that RISC-mediated Hoxb8 RNA cleavage products could be detected in vivo. Taken together, these data indicate that miR-196a represses Hoxb8 expression in the posterior trunk and tail and predict that the restriction of miR-196a to this posterior region is important for proper embryonic patterning.
Understanding the spatial and temporal expression patterns of vertebrate miRNAs is an important first step towards understanding their functions. Expression data will be particularly crucial for designing misexpression studies in redundant miRNA families, where knockouts might be uninformative. The method we describe is rapid when done in transient transgenics, and it should allow efficient screening of hundreds of miRNAs for those involved in a particular process or organ system of interest.