Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know (original) (raw)
Different generations of vaccines
Vaccines that have been developed for a range of different infectious diseases and the approaches for identifying immune correlates of protection have undergone substantial changes since the inception of the field of vaccinology. First-generation vaccines were exclusively live, attenuated pathogens. Because of safety concerns, second-generation vaccines—chemically or physically inactivated pathogens—were later developed. Purified or synthetic proteins represent a third generation, and recent advances in molecular biology and genetic engineering have led to the development of the fourth vaccine generation, which includes DNA- and virus vector–based vaccines.
Until recently, experimental approaches to vaccine development have been mostly empirical. Most vaccine candidates have entered clinical trials in the context of a limited knowledge about their ability to stimulate immune responses and a poor understanding of the types of immune responses that they might elicit to confer protection. More recently, delineation of the immune correlates of protection during natural infection and after vaccination has become a fundamental step in the process of vaccine development. This has been possible thanks to a better understanding of the mechanisms underlying the generation of immune responses and the development of powerful technologies to monitor the vaccine-induced immune response.
Vaccines and immune responses
Vaccine-induced immune responses can vary substantially depending on the nature of the immunogen. The immune response generated by live-attenuated vaccines is generally very similar to that elicited by natural infection and thus includes both antibodies able to prevent infection of target cells (neutralizing antibodies) and cell-mediated immunity. Killed virus vaccines and purified synthetic proteins preferentially elicit neutralizing antibodies and CD4+ T-cell responses but not CD8+ cytotoxic T lymphocytes (CTLs). Replication-defective virus-based vectors alone, and to a greater extent in combination with DNA, predominantly stimulate CTLs and CD4+ T-cell responses but are less efficient at eliciting antibodies. A number of live attenuated virus, killed virus and protein-based vaccines are clearly effective against certain human viruses (Table 1). These vaccines provide protection through the generation of neutralizing antibodies, cell-mediated virus-specific immune responses or both1,2,3,4,5, indicating that these responses represent the immune correlates of protection against a range of human viruses. Notably, all of the vaccines able to prevent chronicity during natural infection are associated with the generation of virus-specific neutralizing antibodies. Indeed, there is wide consensus that antibodies are crucial for preventing chronic infection, whereas cell-mediated responses can potentially control the infection in instances where chronicity is not abrogated. That antibodies may also have beneficial effects against disease progression cannot be ruled out, particularly as recent studies (discussed later) suggest a protective effect of antibodies during chronic infection.
Table 1 Correlates of immune protection in different virus infections
Virus biology and vaccine protection
Knowledge of pathogen biology may aid in understanding why certain viruses are more effective at establishing chronic infection and why different viruses have different susceptibilities to the components of the immune response. Two classes of virus can be distinguished: those that are normally cleared during natural infection (acute viruses) such as smallpox, polio, measles, mumps, rubella and influenza, and those that normally establish chronic infection, such as Epstein-Barr virus (EBV), cytomegalovirus (CMV), hepatitis (B and C), herpes simplex virus (HSV) and HIV-1. Neutralizing antibodies can prevent infection with both acute and chronic viruses. Currently available vaccines are mostly effective against acute viruses, and protection is indeed conferred through neutralizing antibodies. However, there is strong evidence that cell-mediated immunity is crucial in the control of established chronic virus infections, such as CMV6 and EBV infection7. Vaccine-induced cell-mediated immune responses have also been shown to control chronic disease in the simian immunodeficiency virus (SIV) model of AIDS8,9,10,11,12.
Correlates of protection in HIV-1 infection
The susceptibility of HIV-1 to the components of the antiviral immune response is shown in Figure 1. HIV-1-specific cell-mediated immunity is generally unable to adequately control virus replication. A series of studies have helped to characterize CD4+ and CD8+ HIV-1-specific responses and to understand why these responses may be poorly effective. Despite the loss of HIV-1-specific helper CD4+ T cells with the capacity to proliferate, HIV-1-specific CD4+ T cells that secrete IFN-γ remain abundant13. Therefore, the problem is not a lack of HIV-1-specific CD4+ T cells but rather a skewing toward one functional population of CD4+ T cells. The functional heterogeneity of antiviral memory T-cell responses against different virus infections such as HIV-1, EBV and CMV14,15,16,17 has recently been delineated. As EBV and CMV infections are effectively controlled and cell-mediated immunity seems to play the predominant role in this control, it has been assumed that the EBV- and CMV-specific T cells are prototypes of effective immune responses, whereas HIV-1-specific T cells represent ineffective immune responses. EBV- and CMV-specific CD4+ T-cell responses are characterized by the presence of three functionally distinct populations: cells that secrete IL-2 but not IFN-γ, cells that secrete both IL-2 and IFN-γ, and cells that secrete IFN-γ but not IL-2 (ref. 16). These functionally distinct cell populations are associated with different conditions of antigen persistence and antigen load. The single IL-2 response is typical of antigen clearance, the single IFN-γ response is typical of antigen persistence and high antigen load, and the polyfunctional IL-2 plus IFN-γ response is typical of protracted antigen exposure and low antigen load. Notably, the HIV-1-specific CD4+ T-cell responses in subjects with nonprogressive disease were polyfunctional, similar to the EBV- and CMV-specific CD4+ T-cell responses16.
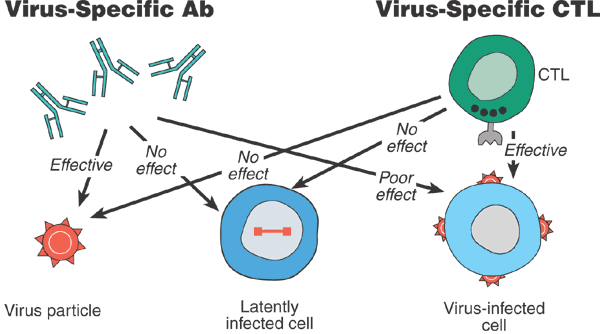
Figure 1: Schematic representation of the effectiveness of the components of the antiviral immune response against different forms of HIV-1.
Neutralizing antibodies are efficient in blocking virus particles but poorly effective against cell-associated virus, such as virus-infected cells. Some CTLs are effective against virus-infected cells but not against free virus particles. Neither antibodies nor CTLs are effective against latently infected cells.
Similar considerations can be made for HIV-specific CD8+ T-cell responses. Lack of viral control occurs despite high frequencies of HIV-1-specific IFN-γ-secreting CD8+ T cells18 and the recognition of multiple epitopes within virus proteins19. Substantial qualitative differences have been found in the type of antigen-specific CD8+ T cells involved in the response against HIV-1 as compared to CMV and EBV. In HIV-1 infection there is a skewing of memory CD8+ T cells toward those that secrete IFN-γ, whereas there is no evidence of virus-specific CD8+ T cells with the capacity to proliferate20 or to secrete IL-2 (G.P. and A. Harari., unpublished observations). The presence of virus-specific CD8+ T cells that can proliferate and secrete IL-2 seems to be associated with low levels of antigen load and virus control, as in CMV and EBV infections and in HIV-1 infection in individuals with nonprogressive disease.
Overall, chronic, progressive HIV-1 infection is associated with a monofunctional T-cell response characterized by high frequencies of virus-specific CD4+ and CD8+ T cells that secrete IFN-γ15,16. In contrast, EBV and CMV infections and nonprogressive HIV-1 infection are characterized by polyfunctional (IL-2 plus IFN-γ secreting) memory CD4+ and CD8+ T-cell responses reflecting the presence of memory T cells at different stages of differentiation16. These data suggest that the effectiveness of virus-specific immune responses is not based exclusively on the quantity but rather on the quality of CD4+ and CD8+ T cells.
Several observations support this hypothesis. (i) Virus-specific CD8+ T-cell responses have been detected in individuals who were exposed to HIV-1 but remained uninfected21,22,23. (ii) The preservation of HIV-1-specific helper CD4+ T-cell responses is associated with virus control after interruption of antiviral therapy in HIV-1-infected individuals treated early during primary infection24. (iii) Depletion of CD8+ T cells results in a loss of virus control in monkeys infected with SIV, and SIV replication is rapidly controlled after CD8+ T-cell responses are restored25,26. (iv) A small percentage of HIV-1 infected individuals do not experience disease progression in the absence of treatment—the so-called long-term nonprogressors (LTNPs). (v) HIV-1-specific CD4+ and CD8+ T-cell responses are preserved in LTNPs and, more importantly, the memory T-cell responses are qualitatively very similar (polyfunctional) to the EBV- and CMV-specific immune responses16. Therefore, under certain conditions HIV-1-specific cell-mediated immunity may effectively control virus replication during established chronic infection.
With regard to the role of humoral immunity in HIV-1 infection, the demonstration that passive immunization with neutralizing antibody can prevent establishment of chronic infection in chimpanzees acutely inoculated with HIV-1 dates back to 1992 (refs. 27,28). It is still unclear, however, whether neutralizing antibodies have a substantial role in the control of chronic, established HIV-1 infection, although there is some evidence from the SIV model that neutralizing antibodies may influence chronic steady-state viremia31. The limited effect may be related to the very rapid escape from neutralizing antibodies that occurs in infected individuals29,30.
However, the correlates of protection involved in preventing disease progression during natural infection with HIV-1 do not necessarily reflect those that would be protective in the presence of pre-existing vaccine-induced immunity. In the absence of clinical trials, it is difficult to predict exactly how pre-existing immunity would affect the outcome of HIV-1 infection.
HIV-1 vaccine candidates
Most of the first candidate HIV-1 vaccines were based entirely or partially on the use of recombinant envelope proteins, with the intent of stimulating neutralizing antibodies. Despite the discovery that these candidates were unable to elicit antibodies that could neutralize primary isolates of HIV-1 (the proposed immune mechanism of protection) during phase 1 and 2 testing, one candidate vaccine was pushed into phase 3 efficacy testing. Not surprisingly, this vaccine did not show efficacy in large-scale clinical trials32. Over the last ten years, it has become clear that HIV-1 evades neutralizing antibodies through a variety of mechanisms29,30,33,34, and there is active research aimed at discovering ways to overcome the apparent difficulty in stimulating a broad neutralizing antibody response to HIV-1.
Most vaccines currently in the development pipeline are designed to stimulate strong cell-mediated immune responses to HIV-136,37,38,39. These include several different viral vectors with or without a DNA prime or a recombinant antigen boost40,41,42. Because several of the candidate vaccines have already, in phase 1 and 2 testing, shown an ability to stimulate an immune response that is a reasonable correlate of protection, there is a rational justification for proceeding with some of these candidates to phase 3 efficacy and correlates trials44,45,46,47,48.
It is likely that not every vaccine that stimulates HIV-1-specific T-cell immunity will prove equally efficacious or even have the same correlate of protection. Although stimulating HIV-1-specific CD4+ T-cell responses with a vaccine would seem important (these responses would help with both B-cell and CD8+ T-cell responses), the fact that HIV-1-specific CD4+ T cells are preferentially infected during natural HIV-1 infection suggests that their existence at the time of initial infection with HIV could be detrimental by providing the optimal substrate for HIV-1 replication49. It will therefore be important to carefully dissect the positive and negative correlates of immune protection in future trials of T cell–stimulating vaccines.
Immunologic monitoring
With regard to the humoral immune response, the fundamental requisite of a vaccine that is capable of 'sterilizing immunity' (prevention of infection) is that it elicits both high titers of neutralizing antibodies, and antibodies with broad neutralizing activity.
For cell-mediated immunity, the situation is more complex. The observations supporting the protective role of cell-mediated immunity against disease progression remain predominantly indirect21,22,23,24,25,26. There is still no direct experimental evidence (as has been provided, in the case of neutralizing antibodies, by passive immunization studies27) that HIV-1-specific cellular immunity prevents disease progression. However, if we accept the assumption that the EBV- and CMV-specific and the HIV-1-specific immune responses observed in subjects with nonprogressive disease are protective, a vaccine should stimulate polyfunctional (IL-2 plus IFN-γ) CD4+ and CD8+ T-cell responses to be effective against disease progression (Pantaleo, G. et al, unpublished observations (G.P., S. Zimmerli and A. Harari)16.
Recent advances in delineating the functional complexity of both CD4+ and CD8+ T cells should prompt a re-evaluation of strategies currently used to monitor vaccine-specific immune responses. Although measuring IFN-γ-secreting cells after antigen-specific stimulation using ELISpot assays can provide information on the immunogenicity of a vaccine, it may be insufficient for defining an immune correlate—determining the functional diversity of the vaccine-induced immune response may be necessary. Furthermore, the detection of IFN-γ-secreting cells may provide limited information on the durability of the vaccine-stimulated immune response. In fact, most current vaccine strategies should have a limited duration of vaccine antigen expression. Secretion of IFN-γ is typical of the early (effector) phase of immune response generation but will decrease with antigen clearance. In contrast, IL-2 secretion is typical of the long-term memory (predominantly CD4+) T-cell response and thus it may be an important cytokine signature in long-term memory T cells50. Finally, we cannot exclude the possibility that certain vaccines will be poor inducers of IFN-γ responses while stimulating dominant IL-2 responses. Therefore, the assessment of vaccine-specific responses must be extended to detect IL-2-secreting cells. Advances in the development of multiparameter flow cytometry now allow for simultaneous assessment of multiple cytokines produced from antigen-specific CD4+ and CD8+ T cells. Thus, although ELISpot assays may be suitable for initial screening of the immunogenicity of large numbers of samples from vaccine trials, multiparameter flow cytometry may be needed to fully characterize vaccine-induced memory T-cell responses.
Design of phase 3 vaccine trials
Initial phase 3 trials of new vaccine products can be designed to determine efficacy or a combination of efficacy and the correlate of immune protection. Obviously, there is no correlate in the absence of demonstrated efficacy, but inherent in these two trial designs is the fact that efficacy/correlates trials may require more subjects than straight efficacy trials. How many more depends, to some extent, on the ability of the vaccine to stimulate a measurable immune response. It will take many more patients to determine a correlate of protection if an immune response can only be measured in 30% of vaccine recipients than if it can be measured in 90% of recipients. Therefore, a combination of a potent vaccine and a sensitive and specific assay to measure even low levels of a vaccine-induced immune response will combine to lessen the number of subjects needed to prove an immune correlate of protection, potentially avoiding any need to add subjects to the efficacy trial.
There are compelling reasons to design initial phase 3 trials to determine the correlates of protection, some theoretical and others practical in nature. For one thing, despite the potential increased size and complexity of a correlates trial, including the power to determine a correlate does not result in any loss in the ability to determine efficacy. A disappointing result would be that a vaccine could be proven to be efficacious while the correlate was unknown. This is no different from the situation that existed during the early days of vaccinology. A more academic consideration is that determining the correlate of immune protection provides insight into the mechanism of protection, although it certainly does not prove cause and effect. Inherent in the fact that an immune response only 'correlates' with protection is the fact that the measured immune response may be a surrogate for some other (unmeasured) immune response (or genetic factor) that provides the true mechanism for protection. This said, the initial identification of a correlate can lead to further investigation that could ultimately determine the mechanism and lead to further rational vaccine design.
The most practical reason for determining a correlate of protection, however, is that it allows future trials to proceed more readily. If a strong correlate is found, then future trials of the vaccine can use that correlate as the endpoint, streamlining the testing process
Final considerations
Current knowledge about the correlates of protection is summarized in Box 1. Whether or not there are sufficient scientific data to support the testing of the current vaccines in large phase 2 or phase 3 efficacy trials has recently been the subject of extensive and sometimes harsh debate. The lack of understanding of some crucial scientific questions (such as how to generate neutralizing antibodies), the fact that current HIV vaccine candidates may not protect from infection, and the absence of definitive experimental evidence that certain types of immune response are indeed immune correlates of protection all favor the view that more basic research is needed before current vaccine candidates can be moved into large efficacy trials. However, it is also unclear what data from which animal model of HIV-1 infection are most relevant to human infection and vaccine protection. Notwithstanding the importance of supporting and reinforcing basic science studies to inform the direction of HIV vaccine development, it would be inappropriate to conclude that no current vaccine strategies will provide any data of importance to future vaccine development. Therefore, large phase 2 or phase 3 clinical trails designed to address the issues discussed above are crucial to hasten the future development of HIV vaccines. Identification of the correlates of protection within the vaccine trials will certainly depend upon the quality of the candidate vaccines. For example, weak vaccines are unlikely to provide protection, and as such will not generate any immune correlate. In contrast, cellular responses stimulated by potent vaccines are more likely to provide protection and a measurable correlate. Therefore, a detailed qualitative and functional characterization, rather than the simple detection of the vaccine-specific immune response, will be required to determine the immune correlates of protection against HIV.